Abstract
To investigate the toxicity of cadmium (Cd) on female reproduction in birds, this study was conducted to determine the changes in biochemical parameters of serum and ovary tissue caused by dietary cadmium in hens. Ninety 50-day-old hyline white hens were randomly divided into three groups (30 hens per group): a control group was fed with basal diet, a low dose group was fed with basal diet containing 140 mg/kg CdCl2 and a high dose group was fed with basal diet containing 210 mg/kg CdCl2. After being treated with Cd for 20, 40 and 60 days, ovary and serum samples were collected and examined for Cd content, histological evaluations, malondialdehyde (MDA) content, glutathione peroxidase (GPx) content, activities of superoxide dismutase (SOD), nitric oxide (NO) content, nitric oxide synthase (NOS) activity, and serum estradiol and progestogen levels. The results showed that the content of Cd, MDA, NO and the activity of NOS in ovary and serum were increased (P < 0.05), while the level of GPx and the activity of SOD were decreased (P < 0.05) in low dose and high dose groups. A time- and dose-dependent correlation was observed between serum and ovary tissue cadmium levels. The number of apoptotic cells in the ovary was increased in the Cd treatment group (P < 0.05). Extensive damage was observed in the ovary. The level of estradiol and progestogen in the serum of low dose and high dose groups was decreased significantly (P < 0.05). It indicated that Cd exposure resulted in oxidative damage of hens' ovary tissue by altering antioxidant defense enzyme systems, lipid peroxidation, apoptosis and endocrine disturbance which may be possible underlying reproductive toxicity mechanisms induced by Cd.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heavy metal cadmium (Cd) is one of the most common environmental pollutants associated with many modern industrial processes. Exposure to Cd is usually the result of environmental contamination by waste from human activities such as the residues found in waste, those released by the combustion of fossil fuels and industry, and the runoff from agricultural land [1, 2]. Relatively large quantities of Cd are found in commercial phosphate fertilizer, thus the increase of Cd contents in soil and plant may result in increase in dietary Cd. So, it is a common finding that poultry industry is affected by Cd toxicity. Body burdens of Cd already approach values considered as critical for the functional impairment of the target organs.
It has been shown that the maximum tolerable dietary Cd level for domestic animals is 0.5 ppm. Dietary concentrations of 1 ppm result in undesirable effects, while 5 ppm cause adverse health effects [3]. Uyanik et al. also found that 50, 75 and 100 mg/kg CdCl2 had adverse effects on biochemical parameters and resulted in tissue accumulation in broilers. It was revealed that cadmium has the potential to induce hepatotoxicity and nephrotoxicity, and supplementation with E. officinalis, vitamin E and polyherbal formulation (stressroak) has a beneficial role in preventing the adverse effects in broilers [4]. Cd is a powerful inducer of oxidative stress. It causes ventral body wall defects in chick embryos treated at Hamburger–Hamilton stages 16–17 [5] and testicular toxicity induced by dietary cadmium in cocks [6].
Many researchers have investigated the reproductive toxicity induced by Cd in mammals. Recent research indicates that Cd is an endocrine disrupter (ED) with detrimental effects on mammalian reproduction [7, 8]. Cd exposure can cause changes in gonadal tissue histopathology, increase oxidative stress, endocrine disruption and apoptosis in rodents, rabbit, sheep and humans [9–11]. Moreover, high doses of Cd could cause rapid and long lasting damage in the testes of male rats [12]. Amara [13] found that Cd treatment led to a decrease in testicular and plasma testosterone levels. Increasing evidence demonstrates that Cd has an apoptotic effect in germ cells of mammals or rodents. Acute Cd exposure resulted in germ cell CdCl2 (2.0 mg/kg) apoptosis in testes [14].
Cd administration could affect the reproductive system in female mammals. Cd administration to rats alters ovarian steroidogenesis, associated with a reduction in progesterone secretion in a dose- and age-dependent manner [9]. Similarly, exposure of cultured rat and human ovarian granulosa cells to Cd caused a reduction in progesterone production [15, 16]. Cd, at concentrations as low as 1 μM, significantly decreased the germ cell density in human fetal ovaries, and Cd increased female germ cell apoptosis [17].
Although many researchers have investigated the toxicity of Cd on mammals and birds, few attempts have been performed on the toxicity of reproduction in hens. Therefore, this research aimed to investigate ovarian toxicity induced by Cd in hens after a subchronic exposure to Cd by a dietary route. The changes in histopathology, oxidative stress, endocrine disruptors and apoptosis were evaluated.
Materials and Methods
Animal Model and Tissue Collection
All procedures used in this experiment were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University. The dietary regimes and the design of dosing have been described previously [18]. Ninety 50-day-old hyline white hens were randomly divided into three groups (30 hens per group): a control group was fed with basal diet, a low dose group was fed with basal diet containing 140 mg/kg CdCl2 and a high dose group was fed with basal diet containing 210 mg/kg CdCl2. The hens were given free access to standard feed and water which were in line with the national standards for feed hygiene (GB 13078-2001 (Cd content in feeds ≤ 0.201 mg/kg, Cd content in water ≤ 0.002 μg/ml)). Ten hens were selected from each group and were killed by decapitation, respectively, at the 20th, 40th and 60th days after Cd exposure. Then, the ovary tissue and serum were collected, respectively, for the detection.
The Content of Cd Assay in Serum and Ovary
The content of Cd in serum and ovary was detected by flame atomic absorption spectrophotometry (FAAS, Shanghai Huipu Analytical Instruments Company). The optimal operating conditions were: wave length 228.8 λ/nm, slit 0.2 nm, burner height 5.0 mm, light current 2.0 I/mA, acetylene discharge 1.5 l/min and air discharge 6.0 l/min. The wet tissue samples were cut into small pieces (1.0 g) with a stainless steel knife and were transferred into beakers. In the digestion procedures, the beakers were added concentrated HNO3/HCl (4:1) and warmed in a low temperature electric hot plate to solution transparence. The samples were metered volume to 10 ml by 0.5% HNO3 and measured by FAAS. The content of Cd was calculated by a drawn standard curve.
Histological and Ultrastructural Observations
Specimens were fixed in 10% buffered neutral formalin, processed for paraffin wax sectioning of about 5 μm thickness and stained with hematoxylin and eosin for light microscopy. For the ultrastructural examination, the ovary tissues (size: 1.0 mm × 1.0 mm × 1.0 mm) were fixed immediately in 2.5% glutaraldehyde phosphate buffer saline (v/v, pH 7.2), postfixed in 1% osmium tetroxide (v/v) and stained with 4.8% uranyl acetate following dehydration. The samples were washed in propylene oxide and impregnated with epoxy resins. The semifine sections were contrasted with uranyl acetate and lead citrate for study via microscopy. The microphotographs were taken with a transmission electron microscope (TEM).
Determination of Apoptosis in Ovary
Apoptotic nuclei in tissue sections were identified using the in situ terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) biotin nick-end labeling (TUNEL) technique that identifies DNA strand breaks by labeling their free 3′-OH termini. We used an in situ cell death detection kit (Roche Diagnostics GmbH, Mannheim, Germany). The method distinguishes apoptotic cells from those undergoing necrosis because damaged DNA in the former leads to a different distribution of staining and nuclear morphology. Paraffin wax-embedded tissue sections were treated with proteinase K and the endogenous peroxidase activity was blocked with hydrogen peroxide. The sections were incubated at 37°C with the terminal TdT nucleotide mixture for 1 h. Then, the reaction was stopped and the slides were rinsed with phosphate-buffered saline. Nuclear labeling was developed with horseradish peroxidase and diaminobenzidine. Hematoxylin was used for counterstaining. Quantitative evaluation of the apoptotic index was performed by manual counting of positively stained nuclei at 400× magnification.
Apoptosis was determined in five ovaries from each group of hen by counting at least 1,000 cells from five to six sections of each ovary. The results are expressed as the percentage of TUNEL-positive cells among the total number of cells counted.
Measurement of Oxidative Stress
The content of malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione peroxidase (GPx) was assayed by using kits (Nanjing Jiancheng Bioengineering Institute, PR China) according to the manufacturer's protocol.
Determination of NO and NOS in Serum and Ovary
The nitric oxide (NO) and nitric oxide synthase (NOS) levels of ovary and serum were measured using the commercial kits (Jiancheng Institute of Biotechnology, Nanjing, China). NO scontent were expressed as micromole per milligram of protein. Data were expressed as units of NOS activity per milligram of protein.
Determination of Estradiol and Progestogen Level in Serum
Serum estradiol and progestogen were determined by radioimmunoassay (RIA) using estradiol and progestogen 125I RIA kit, respectively, (Beijing North Institute of Biological Technology, PR China) according to the manufacturer's protocol. Radioactivity was determined using an automatic gamma counter. All samples were run in duplicate in a single assay to avoid interassay variation.
Statistical Analysis
Statistical analysis of all data was performed with SPSS 13.0 computer software and all data were assessed by one-way ANOVA. All data were expressed as means ± SD. P < 0.05 was considered significant difference.
Results
The Content of Cd
Cd content in the ovary tissue and serum were shown in Table 1. A time- and dose-dependent correlation was observed between serum and ovary tissue cadmium levels after treatment with Cd for 20, 40 and 60 days. Compared with the corresponding control group, Cd content in low dose and high dose groups increased significantly (P < 0.05) in ovary tissue and serum at each time point. Compared with the corresponding low dose group, Cd content in the high dose group increased significantly (P < 0.05) in ovary tissue and serum at each time points.
Histopathology and Ultrastructure
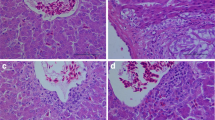
Histology of the ovary in control hens showed normal ovarian structure with regular morphology and normal oogenesis (Fig. 1 a1, a2). Hens treated with Cd showed ovarian lesions with severe necrosis and degeneration of ovarian follicle and interstitial cell (Fig. 1 a3, a4, a5, a6). In addition, ovarian damage increased with the treatment of Cd, especially the high concentration of Cd.
Histology (a hematoxylin and eosin staining) and TUNEL staining (b counterstained with hematoxylin) of the ovarial tissues of hen in different groups at the end of the 60 days of treatment with Cd. a1 Section of ovaries from a hen in the control group. a2 Section of ovaries from a hen in the control group. a3 Growing follicle of a hen ovaries from the low dose group. a4 Section of ovaries from a hen in the low dose group. a5 Growing follicle of a hen ovaries from the high dose group showing a high level of atretic follicle. a6 Section of ovaries from a hen in the high dose group. Many interstitial cells were analosis, but are undergoing degeneration along with loss of oogenesis. b1 Normal ovaries in the control groups. b2 Granular cell of a hen's ovaries from the low dose group showing a high level of apoptosis (arrows). b3 Interstitial cell of a hen's ovaries from the low dose group showing increased numbers of apoptotic cells (arrows). b4 Granular cell of a hen's ovaries from the high dose group showing a high level of apoptosis (arrows). b5 Interstitial cell of a hen's ovaries from the high dose group showing increased numbers of apoptotic cells (arrows). GF growing follicle, AF atretic follicle, GC granular cell, IC interstitial cell. a1 × 100, a2 × 400, a3 × 100, a4 × 400, a5 × 100, a6 × 400, b1 × 400, b2 × 400, b3 × 400, b4 × 400, b5 × 400
Electron microscopy showed normal ovarian ultrastructure in the control group (Fig. 2 a1, b1). Cd treatment caused extensive ovarian tissue damage. Growing follicle displayed morphological characteristics of apoptosis including markedly swollen mitochondria with degeneration or loss of cristae, dilated cisternae of the smooth endoplasmic reticulum (SER), blebbing of the membranes with cytoplasmic vacuolation, cell shrinkage and chromatin condensation (Fig. 2 a2,a4). Chromatin condensation showed as crescentic bodies with a clear boundary of the nuclear membrane (Fig. 2 a3). Mitochondria were markedly swollen with cristae degeneration, or fuzzy and dilated cisternae of the SER were observed in interstitial cells (Fig. 2 b3, b4). Interstitial cells showed chromatin condensation and margination (Fig. 2 b2, b4).
a Transmission electron microscopy of ovarial tissues. a1 The control showed normal granular cell. a2 Granular cells in the low dose group had markedly swollen mitochondria with degenerated or missing cristae, dilated smooth endoplasmic reticulum (SER) cisternae and nuclear envelope resolved. a3 Granular cells in the low dose group displayed morphological characteristics of apoptosis including cell shrinkage and chromatin condensation showing as crescentic bodies with a clear boundary under the nuclear envelope. a4 Granular cells in the high dose group had markedly swollen mitochondria with degenerated or missing cristae, dilated SER cisternae and nuclear chromatin concentrated under the caryotheca. b Transmission electron microscopy of ovarial tissues. b1 The control showed normal interstitial cells. b2 Interstitial cells in the low dose group had markedly swollen mitochondria with loss of cristae and nuclear chromatin condensation. b3 Interstitial cells in the low dose group showed slightly dilated SER cisternae, mitochondria with degenerated or missing cristae and nuclear chromatin condensation. b4 Interstitial cells in the high dose group had markedly swollen mitochondria with loss of cristae and nuclear chromatin concentrated under the caryotheca. SER smooth endoplasmic reticulum, MI mitochondria, NU nucleus. a1 × 8,000, a2 × 10,000, a3 × 15,000, a4 × 10,000, b1 × 2,000, b2 × 8,000, b3 × 12,000, b4 × 10,000
Apoptosis in the Ovary
The number of apoptotic cells in the ovary was significantly increased in low dose and high dose groups compared with the corresponding control group. Interestingly, most of the apoptotic cells in the ovary were found in the granulosa cells and interstitial cells of ovarian region (Fig. 1 b2, b3, b4, b5). Effects of Cd treatment on the apoptosis index in the ovary were presented in Fig. 3. The number of apoptotic cells in the Cd group significantly increased compared with the corresponding control group (P < 0.05). In addition, the apoptosis index increased with the treatment of Cd, which was higher in the low dose group at the 40th and the 60th days than in the high dose group.
Effects on ovary cell apoptosis in Cd-treated hens. Each value is the mean ± SD of at least five hens. Statistically significant differences: means with different uppercase within the same group at different time points are significantly different (p < 0.05) and means with different lowercase in different groups at the same time point are significantly different (p < 0.05)
Variation in Antioxidant Activity
As shown in Table 2, the MDA level in the ovary of low dose and high dose groups was significantly higher than in the corresponding control group (P < 0.05). The MDA level of the ovary increased with the treatment of Cd. The MDA level in the serum of low dose and high dose groups was significantly higher than the corresponding control group (P < 0.05), and the MDA level in the high dose group increased more obviously than the low dose group (P < 0.05).
The SOD and GPx activities in the ovary and serum were shown in Table 2. The activities of SOD and GPX decreased compared with the corresponding control group. A more significant decrease of SOD and GPx activities could be observed the longer the treatment and the higher the concentration of Cd. Especially at the end of the 40th and the 60th days, the differences were significant in both ovary and serum (P < 0.05). The SOD and GPx activities in the high dose group decreased more obviously than the low dose group (P < 0.05).
Variation of NO Metabolism
The NO levels and NOS activity in the ovary and serum were shown in Table 3. NO levels and NOS activity significantly increased in the ovary and serum (at 20 days, 40 days and 60 days) of Cd treatment groups compared with the corresponding control groups (P < 0.05). NO levels and NOS activity increased with the treatment of Cd, especially in the high dose group.
Effects of Cd on Estradiol and Progestogen Levels in Serum
Effects of Cd on estradiol and progestogen levels in serum were showed in Table 4. The concentrations of both estradiol and progestogen increased in the control group. At the end of the 20th, 40th and 60th days, significant decreases were observed in the concentrations of both estradiol and progestogen in the Cd group compared with the corresponding control group (P < 0.05). The more Cd added in the feed, the lower was the concentration of both estradiol and progestogen in the serum, and there is a time- and dose-dependent correlation.
Discussion
In this study, we showed that the content of Cd in the ovary and serum was increased after treatment with Cd. Tissue Cd content was consistent with serum Cd, but the increase of ovary tissue Cd content was lower compared with serum Cd. Therefore, it showed that Cd did not preferentially accumulate in ovary tissue in hens. The toxicity of Cd on the genitical gland varied in different animals mainly because of the distinction of its accumulation in different tissues. Cd has a long biological half-life and accumulates over time in kidney, liver and cerebellum as well as in reproductive organs including placenta, testis and ovaries [19, 20]. Administration of Cd could induce histological changes in the increased occurrence of primary atretic follicles that indicated alterations in the membrane structures and organelles of oocytes and in the follicular cells of the stratum granulosum in sheep ovarian [11]. Cd has also been shown to increase oocyte degeneration rate and impair oocyte maturation in sheep [21] and pigs [10]. Similarly, adult male rats have been shown to develop gonadal damage following administration of Cd [12]. Our results demonstrated that Cd exposure led to morphological changes in ovary of hens (atrophy, necrosis and degeneration of ovarian follicle and interstitial cell) (Fig. 1 a3–6), which was in accordance with the results in mammals and rodents [11, 19].
Various damages of Cd on germ cell have been described, but definitive conclusions about its actions depend on the experimental model and the dosage [22]. Cd exposure increase the numbers of apoptotic cells in the testes of cocks, as determined by terminal dUTP nick-end labeling (TUNEL) staining [6]. A most recent single dose (sc) of Cd chloride treatment study in rat found that Cd-induced apoptosis of germ cell at low doses of Cd (0.13 and 0.15 mg/100 g body weight [BW]), while high doses of Cd (0.2 and 0.3 mg/100 g BW) caused more necrosis than apoptosis [22]. Our results showed that the number of apoptotic cells in low dose and high dose groups significantly increased compared with the control group. The apoptosis index increased with the treatment of Cd, which was higher in the low dose group at the 40th and 60th days than in the high dose group. The apoptosis was found mainly occurring in ovarian tissue in the low dose group (140 mg/kg) which was consistent with previous studies [22].
Various effects of Cd on reproductive endocrinology have been described, but definitive conclusions about its actions on target tissues vary depending on the experimental model and the dosage employed [23, 24]. In vitro studies showed that Cd treatment impaired testosterone production in isolated Leydig cells and reduced the biosynthesis of progesterone in cultured human trophoblast cells and ovaries in ovarian granular cells [7, 15, 25, 26]. The disruption of steroidogenesis is likely to be an initial target of Cd toxicity as an endocrine modulator [8, 9, 25]. The present study revealed that Cd exposure decreased serum estradiol and progesterone levels in hens which could be explained by the obvious pathological changes and apoptosis in the ovarian cell components (e.g., growing follicles, ovaries granule cells and interstitial cells). These results suggested that the toxicity of Cd on the reproductive system in birds was in consistence with mammals and rodents.
The molecular mechanism about the toxic effect of Cd is not well understood. Various studies connect Cd with oxidative stress, since this metal can alter the antioxidant defense system and caused marked disturbances of the antioxidant defense system both in vivo and in vitro [27–30]. In the present study, we found that the activities of SOD and GPx in the ovary and serum exposed to Cd decreased, while the MDA content increased significantly compared to the control group in a time- and dose-dependent pattern which suggested that Cd exposure disrupted oxidative stress in hen ovary and serum. The overproduction of ROS was one of the possible mechanisms of the oxidative stress induced by Cd treatment. Our results were in agreement with the previous studies which clearly demonstrated that Cd exposure increases MDA and suppressed the antioxidant defense mechanisms in the ovary and serum, with significant reduction in ovarian function and hormone secretion of genitical gland [11].
In conclusion, our study demonstrated that dietary Cd exposure caused histopathological changes, oxidative stress, endocrine disorder and apoptosis in the ovary of hens. There were significant reductions in ovary function and secretion. Moreover, ovarian damage and atretic follicle increased with the treatment of Cd. However, further investigations are needed to clarify the relationship between Cd toxication and breeding function of birds.
References
Martelli A, Rousselet E, Dycke C et al (2006) Cadmium toxicity in animal cells by interference with essential metals. Biochimie 88(11):1807–1814
Nordberg M, Jin T, Nordberg GF (1992) Cadmium, metallothionein and renal tubular toxicity. IARC Sci Publ 118:293–297
McDowell LR (1992) Minerals in animal and human nutrition. Academic Press, New York, pp 359–361
Swapna G, Reddy AG (2011) Effect of cadmium on organ biomarkers and evaluation of certain adaptogens in broilers. Toxicol Int 18:47–49
Thompson J, Doi T, Power E et al (2010) Evidence against a direct role for oxidative stress in cadmium-induced axial malformation in the chick embryo. Toxicol Appl Pharmacol 243(3):390–398
Li JinLong, Gao R, Li S et al (2010) Testicular toxicity induced by dietary cadmium in cocks and ameliorative effect by selenium. Biometals 23:695–705
Lee CK, Lee JT, Yu SJ et al (2009) Effects of cadmium on the expression of placental lactogens and Pit-1 genes in the rat placental trophoblast cells. Mol Cell Endocrinol 298(1–2):11–18
Thompson J, Bannigan J (2008) Cadmium: toxic effects on the reproductive system and the embryo. Reprod Toxicol 25(3):304–315
Zhang W, Pang F, Huang Y (2008) Cadmium exerts toxic effects on ovarian steroid hormone release in rats. Toxicol Lett 182(3):18–23
Vrsanska S, Nagyova E, Mlynarcikova A et al (2003) Components of cigarette smoke inhibit expansion of oocyte–cumulus complexes from porcine follicles. Physiol Res 52(3):383–387
Bires J, Maracek I, Bartko P et al (1995) Accumulation of trace elements in sheep and the effects upon qualitative and quantitative ovarian changes. Vet Hum Toxicol 37:349–356
El-Ashmawy IM, Youssef SA (1999) The antagonistic effect of chlorpromazine on cadmium toxicity. Toxicol Appl Pharmacol 161(1):34–39
Amara S, Abdelmelek H, Garrel C et al (2008) Preventive effect of zinc against cadmium induced oxidative stress in the rat testis. J Reprod Dev 54(2):129–134
Ji YL, Wang H, Meng C et al (2011) Melatonin alleviates cadmium-induced cellular stress and germ cell apoptosis in testes. J Pineal Res. doi:10.1111/j.1600-079X.2011.00921.x
Zhang W, Jia H (2007) Effect and mechanism of cadmium on the progesterone synthesis of ovaries. Toxicology 239(3):204–212
Paksy K, Varga B, Lázár P (1997) Zinc protection against cadmium-induced infertility in female rats. Effect of zinc and cadmium on the progesterone production of cultured granulosa cells. Biometals 10(1):27–35
Angenard G, Muczynski V, Coffigny H et al (2010) Cadmium increases human fetal germ cell apoptosis. Environ Health Perspect 118(3):331–337
Yang SH, He JB, Li JL et al (2008) The effect of cadmium on the level of FSHRmRNA and LHRmRNA expression in hen ovaries. Acta Scientiae Circumstantiae 28(1):138–141
Souad H, Bekheet M (2011) Comparative effects of repeated administration of cadmium chloride during pregnancy and lactation and selenium protection against cadmium toxicity on some organs in immature rats' offsprings. Biol Trace Elem Res. doi:s/s12011-011-9084-z
Piasek M, Blanusa M, Kostial K et al (2001) Placental cadmium and progesterone concentrations in cigarette smokers. Reprod Toxicol 15:673–681
Leoni G, Bogliolo L, Deiana G et al (2002) Influence of cadmium exposure on in vitro ovine gamete dysfunction. Reprod Toxicol 16(4):371–377
Sen Gupta R, Kim J, Gomes C et al (2004) Effect of ascorbic acid supplementation on testicular steroidogenesis and germ cell death in cadmium-treated male rats. Mol Cell Endocrinol 221:57–66
Smida AD, Cadmium XP (2004) Cadmium stimulates transcription of the cytochrome P450 side chain cleavage gene in genetically modified stable porcine granulose cells. Biol Reprod 70(1):25–31
Casalino E, Sblano C, Landriscina C (1997) Enzyme activity alteration by cadmium administration to rats: the possibility of iron involvement. Arch Biochem Biophys 346:171–179
Yang JM, Arnush M, Chen QY et al (2003) Cadmium-induced damage to primary cultures of rat Leydig cells. Reprod Toxicol 17(5):553–560
Yang K, Julan L, Rubio F (2006) Cadmium reduces 11β-hydroxysteroid dehydrogenase type 2 activity and expression in human placental trophoblast cells. Am J Physiol Endocm 290:135–142
Fouad AA, Qureshi HA, Al-Sultan AI et al (2009) Protective effect of hemin against cadmium induced testicular damage in rats. Toxicology 257(3):153–160
Chen L, Liu L, Huang S (2008) Cadmium activates the mitogen-activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic Biol Med 45(7):1035–1044
Liu J, Qian SY, Guo Q et al (2008) Cadmium generates reactive oxygenand carbon-centered radical species in rats: insights from in vivo spin-trapping studies. Free Radic Biol Med 45(4):475–481
Kara H, Cevik A, Konar V et al (2007) Protective effects of antioxidants against cadmium induced oxidative damage in rat testes. Biol Trace Elem Res 120(1–3):205–211
Acknowledgments
This work was supported by a grant from the Bureau of Education of Heilongjiang Province (No. 10551038). We thank the members of the veterinary internal medicine laboratory in the College of Veterinary Medicine, Northeast Agricultural University, and special thanks to the members of the group for the help they supplied in the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, S., Zhang, Z., He, J. et al. Ovarian Toxicity Induced by Dietary Cadmium in Hen. Biol Trace Elem Res 148, 53–60 (2012). https://doi.org/10.1007/s12011-012-9343-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9343-7