Abstract
The study population included employees of metal works, with significant exposure to lead (Pb) for about 20 years (mean blood lead level PbB = 43 μg/dl), divided into four groups: normotensive (Pb-normotensive), high-normotensive, first (HT-1), and second degree (HT-2) of hypertension. The control group comprised of 30 office workers with normal blood pressure and no history of occupational exposure to lead. In erythrocytes, the activity of antioxidant enzymes and lipid peroxidation (measured as concentration of malondialdehyde (MDA)) was estimated. MDA concentration, glutathione peroxide (GPx), and superoxide dimutase (SOD) activities were significantly higher in Pb-normotensive group when compared to the normotensive control. Body mass index, age, duration of exposure to lead, and PbB were higher in both hypertensive groups than in Pb-normotensive or high-normotensive groups. MDA increased in HT-1 group by 48% and in HT-2 by 72%, and the activity of GPx decreased significantly in HT-1 group, by 30% and in HT-2 by 43%. No significant differences were observed in their activity of SOD, catalase, and glutathione reductase in erythrocytes. Arterial blood pressure (both systolic and diastolic) positively correlated with body mass index (BMI), age, lead exposure duration, PbB, MDA, and negatively correlated with GPx. There was no significant correlation between BMI and MDA, BMI and GPx, age and MDA, AND age and GPx. In conclusion: (1) lead increases erythrocyte MDA concentration and the activity of GPx as well as SOD in normotensive subjects. (2) Among individuals exposed to lead, with arterial hypertension diagnosed, higher body mass index, age, values of blood lead level, and prolonged exposure to lead have been noticed, accompanied by intensified oxidative stress and the decrease in the activity of glutathione peroxidase in erythrocytes. The reasons for increase of blood pressure in lead exposure remain unrecognized.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Much of the undertaken research have shown that lead (Pb) may significantly increase arterial blood pressure [1–3]. Among the mechanisms possibly leading to arterial hypertension, there are hormonal disturbances (the renin-angiotensin and the sympathetic systems), changes in signal transmission in cells, increased inactivation of nitrogen oxide, as well as direct or indirect influence of reactive oxygen species (ROS) on the metabolism of endothelium and other cells of the circulatory system [4, 5]. The experimental studies have revealed an intensified production of free radicals (superoxide radical O2 •–and hydroxyl radical •OH) [1]. ROS may increase blood pressure directly or indirectly by increasing the concentration of Ca2+ in endothelial cells or by inactivating nitrogen oxide [6]. The release of ROS increases the arterial blood pressure and induces atherosclerotic changes in blood vessels. In consequence, it may lead to arterial hypertension and coronary heart disease [1, 2].
Our earlier studies [7–9] have proven that the exposure of humans to lead compounds results in increased peroxidation of lipids in blood, due to changes in the activity of antioxidant enzymes. Among individuals substantially exposed to lead (concentration of lead in blood—PbB—ranged between 25 and 65 μg/dl), we have noted increased activity of the superoxide dismutase (SOD) in blood. The activity of glutathione peroxidase (GPx) increased in lower (PbB = 25–40 μg/dl) and decreased in higher exposure to lead (PbB > 40 μg/dl). The activated SOD coupled with simultaneous reduction of GPx activity and absence of changes in the activity of catalase caused the increase in hydrogen peroxide (H2O2) formation. GPx and catalase removes hydrogen peroxide. As a result, an increased concentration of malondialdehyde (MDA)—a product of lipid peroxidation in comparison with control—was observed.
Intensified synthesis of ROS caused by the lead has been confirmed by many studies carried out on animals, both in blood and in numerous organs: the brain, kidneys, liver, lungs, eyeball lens, as well as in cell cultures [3, 4, 10, 11]. Similarly, clinical studies concerning individuals exposed to lead revealed the intensified peroxidation of lipids in blood due to ROS [12, 13].
Numerous experimental and clinical studies prove that the oxidative stress plays a role in the development of the arterial hypertension. Many cells in blood vessels may be generators of ROS, for example: fibroblasts, endothelial, and smooth muscles cells. The main source of ROS is the NADPH oxidase activation. The other sources are the biosynthesis of uric acid by xanthine oxidase, disturbed production of nitrogen oxide (NO) by nitrogen oxide synthase (NOS; particularly in case of deficiency of tetrahydrobiopteridine) and the generation of peroxynitrite (ONOO–) [14].
There have been no clinical studies conducted which would determine the pathomechanism of the increase of arterial blood pressure in persons exposed to lead compounds for a long time. Great majority of investigation devoted to the toxicity of lead are experimental animal studies. In that investigation, acute poisoning is caused by high doses of lead administered in a short period of time. In clinical studies, the exposure to that metal is significantly lower. Lead enters the body mainly via the respiratory tract contrary to experimental studies that this element is administrated intraperitoneally or with drinking water. Moreover, the exposure time is shorter than several years. It makes the effect on human body different than that in acute poisoning. There are many people exposed to lead compounds due to their professions. Many of them are not monitored (employees of companies carrying out overhauls in foundries, employees of small plants producing batteries, of scrap-metal collecting centers, producers of crystals, etc.). It seems that these people require specific preventive examinations, with emphasis on diseases of circulatory system, particularly arterial hypertension.
Our earlier studies demonstrated that among people substantially exposed to lead (PbB exceeding 40 μg/dl), arterial hypertension is much more frequent (OR 4.4 95%CI 1.4–14.5). It is accompanied by the increased lipid peroxidation [7]. It seems probable that the enhanced synthesis of ROS influenced by lead increases arterial blood pressure as a result of changing activity of antioxidant enzymes responsible for the removal of ROS.
Materials and Methods
The examined population included 92 employees of metal works exposed to lead in the southern region of Poland. In order to determine the degree of exposure to lead compounds, the concentration of lead (PbB) and zinc protoporphirin (ZPP) in blood sample has been marked. Workers (aged 28–55) have been exposed to lead for about 10 to 30 years, and the values of PbB and ZPP were higher than normal levels (PbB > 35 μg/dl or ZPP > 5 μg/dl (normal values of non-occupational exposure to lead should not exceed the PbB of 10 μg/dl and ZPP of 2.5 μg/dl). Workers with malignant tumors, diabetes, serious liver, kidney, or heart insufficiency have been excluded. Blood pressure was measured every month over the period of 6 months. The examined population exposed to Pb has been divided into four groups:
-
normal blood pressure—Pb-normotensive group (systolic pressure <129 and diastolic pressure <85)
-
high-normal blood pressure—Pb-high-normotensive group (systolic pressure 130–139 or diastolic pressure 85–89)
-
first degree of hypertension—Pb-hypertensive-1 degree (Pb-HT-1) group (systolic pressure 140–159 or diastolic pressure 90–99)
-
second degree of hypertension—Pb-hypertensive-2 degree (Pb-HT-2) group (systolic pressure ≥160 or diastolic pressure ≥100)
The control group consisted of 30 office workers with normal blood pressure and no history of occupational exposure to lead. They all had normal PbB and ZPP levels. None of the controlled individuals had a history of abnormalities regarding the above parameters. Only environmental exposure to lead occurred in the group controlled.
Blood (10 ml) was collected by venipuncture into 10-ml sterile tubes containing ethylenediamine tetra acetic acid solution as anticoagulant to obtain erythrocytes.
In the whole blood, PbB and ZPP were determined. Analysis of PbB was done by graphite furnace atomic absorption spectrophotometry using Unicam 929 and 939OZ Atomic Absorption Spectrometers with GF90 and GF90Z Graphite Furnaces. Data are provided in microgram per deciliter. Concentration of ZPP was assayed directly using Aviv Biomedical hematofluorometer model 206 which measured the ratio of fluorescence of ZPP to absorption of the light by sample (by hemoglobin) and is presented as μg ZPP/g of hemoglobin (μg/g Hb).
The remaining blood was centrifuged. The sediment of erythrocytes was rinsed three times, using 0.9% NaCl. Then erythrocytes were hemolysed with deionized water. In 10% hemolysate, the activity of study enzymes and concentration of hemoglobin were indicated by means of Drabkin reagent and MDA.
The activity of SOD was indicated by the Oyanagui [15] method. Enzymatic activity is expressed in nitric unit (NU) in each milligram of hemoglobin (Hb); 1 NU means 50% of inhibition by SOD of nitric ion production in this method.
The catalase was indicated by the Aebi [16] kinetic method; 2.5 ml of substrate was mixed with substance consisting of 50 mM TRIS/HCl buffer with pH = 7.4 and perhydrol with 50 μl of haemolysate. Enzymatic activity is expressed in IU/mg Hb.
GPx activity in erythrocytes was assayed by the kinetic method [17]. Briefly, reduced GSH is oxidized by H2O2 to GSSG then glutathione reductase is recovered back to GSH using NADPH+H+. Decrease in absorbance is measured at 340 nm. The activity of GPx is determined as the quantity of μmol of NADPH+H+ used to recover GSH in 1 min, converted to 1 g of hemoglobin (IU/g Hb).
Glutathione reductase (GR) activity was also assayed by the kinetic method. The decrease of the concentration of NADPH+H+ after reduction of GSSG back to GSH was measured. Activity of GR was determined as the quantity of μmol of NADPH+H+ used to recover GSH in 1 min converted to 1 g of hemoglobin (IU/g Hb) [18].
Lipid peroxidation in erythrocytes was determined fluorometrically according to Ohkawa [19]. Data are shown as nmol of MDA converted to 1 g of hemoglobin (nmol/g Hb).
The statistical analysis was performed using Statistic 7.1 PL software. Statistical methods included mean, standard deviation, and standard error of mean (SEM). Shapiro–Wilk’s test was used to verify normality and Levene’s test to verify homogeneity of variances. An analysis of variance or Kruskal–Wallis ANOVA test was used for multiple comparisons of data. Additional statistical comparisons were made by t test, t test with separate variance estimates or Mann–Whitney U test. Spearman nonparametric correlation was calculated. A value of p < 0.05 was considered to be significant.
Results
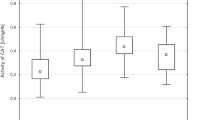
There were no differences in age, body mass index (BMI), smoking habits, arterial systolic blood pressure (SBP) and arterial diastolic blood pressure (DBP) between the Pb-normotensive group, when compared to the control normotensive non Pb-exposed group (Table 1), while the PbB, ZPP, MDA concentration, GPx, and SOD activity were significantly higher in Pb-normotensive group (Table 2, Figs. 1 and 2).
Body mass index, age, and years of exposure to lead increased significantly in both Pb-HT groups when compared with Pb-normotensive group (Table 1 and Fig. 3). There were no differences in years of non-exposure to lead and number of cigarettes smoked per day in the study population exposed to lead. In both Pb-HT groups, the mean lead exposure indicators PbB and ZPP were higher than in Pb-normotensive or Pb-high-normotensive groups, but only PbB reached the level of statistical significance (Table 2 and Fig. 4).
The concentration of MDA in erythrocytes increased in Pb-HT-1 group at 48% and in Pb-HT-2 at 72% in comparison to the Pb-normotensive group (Fig. 1). The activity of GPx decreased significantly in Pb-HT-1 group at 30% and in Pb-HT-2 at 43%, in comparison to Pb-normotensive group (Fig. 2), and there were no changes when these groups were compared to the control normotensive non-Pb-exposed group. No significant differences were observed among the other studied activities of enzymes in erythrocytes such as SOD, catalase, and GR in the study population exposed to lead (Table 2).
Correlations between the studied parameters in lead-exposed population have been shown in Table 3. Arterial blood pressure (both SBP and DBP) positively correlated with BMI (R = 0.47–0.48), age (R = 0.22–0.35), years of lead exposure (R = 0.20–0.30), and PbB (R = 0.20–0.25) and negatively correlated with GPx (R = –0.23 and –0.30). Additionally, SBP positively correlated with MDA (R = 0.28). There were no significant correlations between BMI and MDA, BMI and GPx, age and MDA, and age and GPx. Also, no significant relations between studied parameters and other antioxidant enzymes (SOD, CAT, GR) were noticed.
Discussion
The study proved that in normotensive individuals exposed to lead compounds increased erythrocyte, MDA concentration was found compared to normotensive control non-exposed to Pb. It testifies to the intensified peroxidation of lipids due to the action of ROS. Besides, the increased activity of antioxidant enzymes, such as GPx and SOD, was observed. It seems that the increased activity of the above enzymes can be an adaptive mechanism as consequence of oxidative stress induced by lead in erythrocytes. Some clinical studies confirm the activation of antioxidants as a result of lead action [13, 20]. Also, in vitro administration of lead acetate (500 μmol/l) to cell cultures showed an increase of both MDA concentration and antioxidant enzymes activity (GPx, CAT) [21]. On the other hand, the incubation of erythrocytes in the presence of lead (5 μmol/l) inhibited the activity of GPx, SOD, and catalase [22]. It is possible that Pb2+ induces expression of antioxidant enzymes and can simultaneously inhibit enzyme activity by binding to amino acids in protein, mainly to sulfhydryl groups [22]. Lead can activate the expression of different factors as heme oxygenase-1, NF-kappaB, and Cyp1a1 mRNA [23].
In the present study, lead-exposed populations were divided on the basis of values of arterial blood pressure into normotensive, high-normotensive, and hypertensive groups. The concentration of erythrocyte MDA in hypertensive subjects was higher by about 48% and 72%, depending on the degree of hypertension (Fig. 1). Many studies confirm that ROS play an important role in the arterial hypertension. The most significant ROS is superoxide radical O2 •– and, as the consequence of transformations with the participation of SOD, hydrogen peroxide. Besides, O2 •– react with nitrogen oxide (NO) and formed one of the most toxic ROS—peroxynitrite (ONOO–). Those ROS are responsible for the non-enzymatic peroxidation of arachidonic acid. In consequence, it leads to the formation of isoprostanes. They cause vessel contraction and stimulate the production of endothelin and the proliferation of smooth muscles of blood vessels as well as the aggregation of platelets. Moreover, peroxynitrite reacts with proteins. It leads to the formation of nitrosothiols and nitrotyrosine as a result of inhibition of the activity of prostacycline synthase. As a consequence of the above processes, blood vessels contract and arterial blood pressure increases [24]. In this study, the analysis of correlation has shown that arterial blood pressure (both SBP and DBP) positively correlated with the time of exposure to lead and PbB and systolic blood pressure positively correlated with MDA. It seems that lead can at least partially increase blood pressure by generating ROS.
Clinical studies in individuals with hypertension indicated changes within the enzymatic antioxidative system in erythrocytes. The results are not unequivocal. Most often, decreased activity of enzymes is described; in some studies, their activity appears to remain unchanged. In some other publications, the researchers state they found increase in the activity. Very often, the increase in the activity of one enzyme is accompanied by decrease or lack of change concerning other enzymes. Practically, all studies reported intensified oxidative stress.
The Italian study concerning people with arterial hypertension in middle age (aged 46–48 years) revealed the decreased activity of SOD by 16% and increased activity of GPx by 13% in erythrocytes, with accompanying increase of MDA in blood serum [25]. The study carried out in Taiwan indicated that in the group of people with arterial hypertension, almost twice as high activity of erythrocyte SOD was noted in comparison to normotensive [26]. No connection has been found between polymorphism of the catalase and occurrence of arterial hypertension [27]. On the other hand, no changes in the activity of GPx and the concentration of selenium, with simultaneous increased concentration of the products of lipid peroxidation have been observed in the groups of children with arterial hypertension from Poland and Turkey [28, 29]. A decrease of SOD by 8% and catalase by 12% has been observed in erythrocytes in the group of Polish elderly people (average age 76 years) with arterial hypertension, with accompanying increase of erythrocyte MDA by 43% and over 1.5 times increase of serum carbonyl groups [30]. Population studies in Portugal revealed that among people with arterial hypertension the activity of GPx in whole blood was decreased by 30%, in comparison with control, while the activity of SOD in erythrocytes remained unchanged [31]. In Spain, in the group of people with recently diagnosed arterial hypertension, a drop in the activity of SOD by 13%, reduced GPx by 18% in erythrocytes [32], reduction of CuZn-SOD, GR, and GPx mRNA concentrations in mononuclear cells has been observed [33]. Similar results have been obtained by Redon among middle-aged people (average age 43–50 years)—decreased activity of all antioxidant enzymes (SOD, GPx, and catalase), both in erythrocytes and in leukocytes, with associated increase of MDA [34].
The above results are discordant. Most probably, this is due to the differences in age, nationality, diet used, pollution, environment, etc. No change in the activity of antioxidant enzymes has been found among children, whereas in the population of middle-aged and elderly people, the activities of enzymes were usually lower. Disturbances of the antioxidative system eventually lead to the aggravation of oxidative stress, which has been described practically by a decisive majority of researchers.
In our study, we have observed a drop in the activity of GPx in erythrocytes by 30–43%, depending on the degree of hypertension. It seems possible that prolonged exposure to lead reduces the activity of that enzyme and, as a result, disturbs the distribution of hydrogen peroxide. It causes an increase of MDA concentration, which may be confirmed by the negative correlations between GPx and PbB. One of the reasons of reduced activity of GPx may be the deficiency of selenium. Interactions between selenium and lead have been described. Lead may react with selenium, making up an insoluble complex (lead selenide); it may also impair the uptake of selenium [35]. The experimental studies revealed that administration of selenium before exposure to lead prevents the occurrence of disturbances in the enzymatic antioxidant system [36]. Changes in erythrocyte SOD activity may be explained by differences in the concentrations of copper (Cu) and zinc (Zn), which are components of this enzyme. Both elements play a role in the development of arterial hypertension [37, 38]. Deficiency of those metals may disturb the function of SOD and other enzymes, particularly in those that take part in the transport of electrolytes, e.g., the sodium–potassium ATP-ase in erythrocytes [38]. In our study, we did not find statistically significant changes in the activity of SOD in the population exposed to lead, although in the groups with arterial hypertension, the activity of SOD was 10% lower in comparison to Pb-normotensive.
In Pb-HT groups, significantly higher body mass index (in Pb-HT1 group 29.0 kg/m2 and in Pb-HT2 group 31.6 kg/m2) has been found. Clinical studies show that among obese individuals, SOD and GPx activity appears to be significantly lower, as well as concentration of zinc or selenium in blood, than those in controls [39–41]. There are many factors that induce hypertension. Studies of the relations between arterial systolic and diastolic blood pressure in lead-exposed population have shown that blood pressure depends on several factors, such as age, BMI, years of lead exposure, and blood lead level (Table 3). When analyzing the relation between the above factors and oxidative/antioxidative balance, only PbB positively correlated with MDA. Age and BMI are very strong factors that induce hypertension, but they seem to be independent of oxidative stress. Generation of ROS, induced by lead, can be one (but not the only one) of the factors that influence the development of hypertension in lead exposed subjects. The reasons for increase of blood pressure in lead exposure remain unrecognized.
The free-radical activity of lead may substantially contribute to search for medicines. The antioxidants as vitamins (vitamin C, E, beta-caroten), increasing the level of glutathione in cell (N-acetylcysteine, S-adenosyl-l-methionine), or hipotensive drugs containing free SH groups (captopril), would prevent the toxic activity of that metal [11]. Perhaps the reduction of oxidative stress would prevent the increase of arterial blood pressure in the population exposed to lead compounds. So far, no effective methods have been developed for prevention of unfavorable activities of lead in human body, in case of chronic intoxications with that element.
To sum up, in the group of individuals exposed to compounds containing lead, among whom, arterial hypertension has been diagnosed, higher body mass index, age, values of blood lead level, and a prolonged exposure to lead have been noticed, accompanied by an intensified oxidative stress and the decrease in the activity of glutathione peroxidase in erythrocytes.
References
H. C. Gonick, Y. Ding, S. C. Bondy, Z. Ni and N. D. Vaziri, Lead-induced hypertension: interplay of nitric oxide and reactive oxygen species, Hypertension. 30, 1487–1492 (1997).
S. A. Korrick, D. J. Hunter, A. Rotnitzky, H. Hu and F. E. Speizer, Lead and hypertension in a sample of middle-aged women, Am. J. Public Health. 89, 330–335 (1999).
Z. Ni, S. Hou, C. H. Barton and N. D. Vaziri, Lead exposure raises superoxide and hydrogen peroxide in human endothelial and vascular smooth muscle cells, Kidney Int. 66, 2329–2336, (2004).
N. D. Vaziri and Y. Ding, Effect of lead on nitric oxide synthase expression in coronary endothelial cells: role of superoxide, Hypertension 37, 223–226 (2001).
M. Carmignani, A. R. Volpe, P. Boscolo, N. Qiao, M. Di-Gioacchino, A. Grilli and M. Felaco, Catcholamine and nitric oxide systems as targets of chronic lead exposure in inducing selective functional impairment, Life Sci. 68, 401–415 (2000).
F. Dalloz, V. Maupoil, S. Lecour, F. Briot and L. Rochette, In vitro studies of interactions of NO. donor drugs with superoxide and hydroxyl radicals, Mol. Cell. Biochem. 177, 193–200 (1997).
S. Kasperczyk, E. Birkner, A. Kasperczyk and J. Kasperczyk, Lipids, lipid peroxidation and 7-ketocholesterol in workers exposed to lead, Hum. Exp. Toxicol. 24, 287–295 (2005).
S. Kasperczyk, E. Birkner, A. Kasperczyk and J. Zalejska-Fiolka, The activity of superoxide dismutase and catalase in people protractedly exposed to lead compounds, Ann. Agric. Environ. Med. 11, 291–296 (2004).
S. Kasperczyk, A. Kasperczyk, A. Ostałowska, M. Dziwisz and E. Birkner, Activity of glutathione peroxidase, glutathione reductase and lipid peroxidation in erytrocytes in workers exposed to lead, Biol. Trace Elem. Res. 102, 79–84 (2004).
A. Skoczyńska, Lipid peroxidation as a toxic mode of action for lead and cadmium, Med. Pr. 48, 197–203 (1997).
H. Gurer and N. Ercal, Can antioxidants be beneficial in the treatment of lead poisoning?, Free. Radic. Biol. Med. 29, 927–945. (2000).
W. Wąsowicz, J. Gromadzińska and K. Rydzyński, Blood concentration of essential trace elements and heavy metals in workers exposed to lead and cadmium, Int. J. Occup. Med. Environ. Health. 14, 223–229 (2001).
X. B. Ye, H. Fu, J. L. Zhu, W. M. Ni, Y. W. Lu, X. Y. Kuang, S. L. Yang, and B. X. Shu, A study on oxidative stress in lead-exposed workers, J. Toxicol. Environ. Health A. 57, 161–172 (1999).
T. M. Paravicini and R. M. Touyz, NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities, Diabetes Care. 31Suppl 2, S170–S180 2008, Feb.
Y. Oyanagui, Reevaluation of assay methods and establishment of kit for superoxide dismutase activity, Anal. Biochem. 142, 290–296 (1984).
H. Aebi, Catalase in vitro. Methods Enzymol. 105, 121–126 (1984).
D. E. Paglia and W. N. Valentine, Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase, J. Lab. Clin. Med. 70, 158–169 (1967).
R. Richterich, Chemia kliniczna [Clinical chemistry], Wydawnictwo Lekarskie PZWL [Medical Publishing PZWL], Warszawa [Warsaw] (1971).
H. Ohkawa, N. Ohishi, and K. Yagi, Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction, Anal. Biochem. 95, 351–358 (1979).
I. Ergurhan-Ilhan, B. Cadir, M. Koyuncu-Arslan, C. Arslan, F.M. Gultepe and G. Ozkan, Level of oxidative stress and damage in erythrocytes in apprentices indirectly exposed to lead, Pediatr. Int. 50, 45–50 (2008)
N. Aykin-Burns and N. Ercal, Effects of selenocystine on lead-exposed Chinese hamster ovary (CHO) and PC-12 cells, Toxicol. Appl. Pharmacol. 214, 136–143, (2006)
E. Sugawara, K. Nakamura, T. Miyake, A. Fukumura and Y. Seki, Lipid peroxidation and concentration of glutathione in erythrocytes from workers exposed to lead, Br. J. Ind. Med. 48, 239–342 (1991).
H.M. Korashy and A.O. El-Kadi, The role of redox-sensitive transcription factors NF-kappaB and AP-1 in the modulation of the Cyp1a1 gene by mercury, lead, and copper, Free Radic. Biol. Med. 44, 795–806 (2008)
G. Wójcicka, J. Bełtowski and A. Jamroz, Oxidative stress in hypertension, Postepy Hig. Med. Dosw. 58, 183–193 (2004).
C. Russo, O. Olivieri, D. Girelli, G. Faccini, M. L. Zenari, S. Lombardi and R. Corrocher, Anti-oxidant status and lipid peroxidation in patients with essential hypertension, J. Hypertens. 16, 1267–1271 (1998).
Yang H. Y., P. F. Kao, T. H. Chen, B. Tomlinson, W. C. Ko and P. Chan, Effects of the angiotensin II type 1 receptor antagonist valsartan on the expression of superoxide dismutase in hypertensive patients, J. Clin. Pharmacol. 47, 397–403 (2007).
Y. M. Hsueh, P. Lin, H. W. Chen, H. S. Shiue, C. J. Chung, C. T. Tsai, Y. K. Huang, H. Y. Chiou and C. J. Chen, Genetic polymorphisms of oxidative and antioxidant enzymes and arsenic-related hypertension, J. Toxicol. Environ. Health A. 68, 1471–1484 (2005).
J. Śladowska, A. Wierzbicka, M. Litwin, J. Antoniewicz, A. Niemirska, Z. Wawer, P. Socha, E. Skorupa and G. Grenda, Oxidative stress and hypertensive arteriopathy in children with primary hypertension-preliminary results, Przegl. Lek. 63, 107–110 (2006).
S. Oztezcan, S. Doğru-Abbasoğlu, U. Mutlu-Türkoğlu, S. Cetiner, R. Eker-Omeroğlu, G. Aykaç-Toker and M. Uysal, The investigation of prooxidant-antioxidant balance in serum of children with family histories of essential hypertension, Res. Commun. Mol. Pathol. Pharmacol. 111, 167–174 (2002).
K. Kędziora-Kornatowska, J. Czuczejko, K. Szewczyk-Golec, J. Motyl, L. Szadujkis-Szadurski, T. Kornatowski, H. Pawluk and J. Kędziora, Effects of perindopril and hydrochlorothiazide on selected indices of oxidative stress in the blood of elderly patients with essential hypertension, Clin. Exp. Pharmacol. Physiol. 33, 751–756 (2006).
M. L. Pavão, T. Figueiredo, V. Santos, P. A. Lopes, R. Ferin, M. C. Santos, J. Nève and A. M. Viegas-Crespo, Whole blood glutathione peroxidase and erythrocyte superoxide dismutase activities, serum trace elements (Se, Cu, Zn) and cardiovascular risk factors in subjects from the city of Ponta Delgada, Island of San Miguel, The Azores Archipelago, Portugal, Biomarkers. 11, 460–471 (2006).
J. Pedro-Botet, M. I. Covas, S. Martín and J. Rubiés-Prat, Decreased endogenous antioxidant enzymatic status in essential hypertension, J. Hum. Hypertens. 14, 343–345 (2000).
F. J. Chaves, M. L. Mansego, S. Blesa, V. Gonzalez-Albert, T. Jiménez, M. C. Tormos, O. Espinosa, V. Giner, A. Iradi, G. Saez and J. Redon, Inadequate cytoplasmic antioxidant enzymes response contributes to the oxidative stress in human hypertension, Am. J. Hypertens. 20, 62–69 (2007).
J. Redón, M. R. Oliva, C. Tormos, V. Giner, J. Chaves, A. Iradia and G. T. Sáez, Antioxidant activities and oxidative stress byproducts in human hypertension, Hypertension 41, 1096–1101 (2003).
P. D. Whanger, Selenium in the treatment of heavy metal poisoning and chemical carcinogenesis, J. Trace Elem. Electrolytes Health Dis. 6, 209–221 (1992).
A. I. Othman and M. A. El Missiry, Role of selenium against lead toxicity in male rats, J. Biochem. Mol. Toxicol. 12, 345–349 (1998).
S. Tubek, Role of zinc in regulation of arterial blood pressure and in the etiopathogenesis of arterial hypertension, Biol. Trace Elem. Res. 117, 39–51 (2007).
K. Kędzierska, J. Bober, K. Ciechanowski, E. Gołembiewska, E. Kwiatkowska, I. Noceń, B. Dołęgowska, G. Dutkiewicz and D. Chlubek, Copper modifies the activity of sodium-transporting systems in erythrocyte membrane in patients with essential hypertension, Biol. Trace Elem. Res. 107, 21–32 (2005).
M. Ghayour-Mobarhan, A. Taylor, S. Lanham-New, D. J. Lamb, M. A. Nezhad, S. M. R. Kazemi-Bajestani, F. Ghafouri, C. Livingstone, T. Wang and G. A. A. Ferns, Serum selenium and glutathione peroxidase in patients with obesity and metabolic syndrome, Pakistan J. Nutr. 7, 112–117 (2008)
M. Ozata, M. Mergen, C. Oktenli, A. Aydin, S. Y. Sanisoglu, E. Bolu, M. I. Yilmaz, A. Sayal, A. Isimer, I. C. Ozdemir, Increased oxidative stress and hypozincemia in male obesity, Clin. Biochem. 35, 627–631 (2002)
I. Gjørup, T. Gjørup and B. Andersen, Serum selenium and zinc concentrations in morbid obesity. Comparison of controls and patients with jejunoileal bypass, Scand. J. Gastroenterol. 23, 1250–1252 (1988)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasperczyk, S., Kasperczyk, J., Ostałowska, A. et al. The Role of the Antioxidant Enzymes in Erythrocytes in the Development of Arterial Hypertension among Humans Exposed to Lead. Biol Trace Elem Res 130, 95–106 (2009). https://doi.org/10.1007/s12011-009-8323-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-009-8323-z