Abstract
d-amino acid oxidase (DAAO) is widely used in the industrial preparation of l-amino acids, and cultivating Escherichia coli (E. coli) expressing DAAO for the biosynthesis of l-phosphinothricin (l-PPT) is very attractive. At present, the biomass production of DAAO by fermentation is still limited in large-scale industrial applications because the expression of DAAO during the fermentation process inhibits the growth of host cells, which limits higher cell density. In this study, the factors that inhibit the growth of bacterial cells during a 5-L fed-batch fermentation process were explored, and the fermentation process was optimized by co-expressing catalase (CAT), by balancing the biomass and the enzyme activity, and by adding exogenous d-alanine (d-Ala) to relieve the limitation of DAAO on the cells and optimize fermentation. Under optimal conditions, the DO-STAT feeding mode with DO controlled at 30% ± 5% and the addition of 27.5 g/L lactose mixed with 2 g/L d-Ala during induction at 28 °C resulted in the production of 26.03 g dry cell weight (DCW)/L biomass and 390.0 U/g DCW specific activity of DAAO; an increase of 78% and 84%, respectively, compared with the initial fermentation conditions. The fermentation strategy was successfully scale-up to a 5000-L fermenter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
d-amino acid oxidase (DAAO, EC 1.4.3.3) is a flavoenzyme-containing flavin adenine dinucleotide as the prosthetic group, and it catalyzes oxygen-dependent oxidative deamination of amino acid d-isomers with absolute stereospecificity, producing α-keto acids, ammonia, and hydrogen peroxide (H2O2) [1,2,3,4,5]. Because of its strict stereoselectivity, DAAO has been used to produce unnatural l-amino acids [6, 7]. l-amino acids are important intermediate compounds in the synthesis of pharmaceuticals [8, 9]. Moreover, DAAO has been widely used in industrial catalysis. For example, DAAO is a catalyst for the enzymatic conversion of cephalosporin C to 7-aminocephalosporanic acid (7-ACA), and it is a key compound for the synthesis of many β-lactam drugs [10,11,12]. Commercial phosphinothricin (PPT), generally a racemic mixture, is an organophosphorus herbicide with the advantages of low toxicity, high safety, and broad spectrum. In addition to its herbicidal activity, PPT also has fungicidal and insecticidal activities, and the use of PPT as a pesticide can prevent and control crop pests and diseases. Only the l-PPT isomer has herbicidal activity, not the d-isomer (d-PPT). DAAO can be used as a specific and efficient reaction catalyst for the preparation of chiral amino acids as intermediate products in the biosynthesis of the herbicidal l-PPT [13,14,15]. The market for glyphosate and paraquat is shrinking, and phosphinothrin-resistant crops are being promoted; therefore, the market demand for l-PPT is enormous and the prospects are broad.
With the rapid and high-quality development of biotechnology in recent years, methods for efficient DAAO production have been continuously discovered and molecularly modified to improve enzyme activity, allowing related biological enzymatic catalysis to be used for efficient production of DAAO, and laying the foundation for large-scale application. Therefore, large-scale production of DAAO is a hot-spot of current research, and the efficient production of DAAO by genetically engineered bacterial strains by optimizing the fermentation conditions is the most effective method to improve the industrial production of DAAO. Unfortunately, host cell growth is negatively affected by the expressed DAAO, resulting in a significant limitation of cell growth [16, 17]. Various studies have improved biomass production by different methods. Zheng et al. attempted to balance enzyme activity and biomass production using N-terminal modification; however, while the mutant had increased enzyme activity, the biomass decreased by 40% to 22 g dry cell weight (DCW)/L during fed-batch fermentation in a 7.5-L fermenter [18]. Sae-Jin Kim et al. proposed to increase the biomass production by delaying the induction time and the optimal induction time occurred when the optical density at 600 nm (OD600) reached 40, and achieved a typical cell growth pattern with a OD600 of 80 after 48 h in a 30-L fermenter. However, this fermentation period was too long for industrial application [17]. The interference of expressed DAAO on the biosynthesis of the cell wall is generally assumed to be responsible for the toxic effect of DAAO on cell growth [16]. Cell growth is controlled by the peptidoglycan (PG) cell wall, a rigid and essential structure composed of glycan strands cross-linked by d-amino acid-containing short peptides [19]. When d-amino acids are depleted by DAAO, cell growth is naturally inhibited. In addition, H2O2, which is toxic to E. coli cells, is produced during the action of the DAAO catalyst on d-amino acids [20,21,22].
In this work, a fed-batch fermentation of genetically engineered E. coli BL21 (DE3) expressing DAAO was studied in a 5-L fermenter containing a 2-L fermentation medium. Optimization resulted in high biomass and high enzyme activity of DAAO expressed by E. coli, which could be adapted for industrial applications. Because the optimization of the feeding mode cannot eliminate the restriction of DAAO on cell growth, reduction of H2O2 concentrations, the by-product of the fermentation process, by co-expression of CAT in the recombinant strain was also studied. In addition, the effect of d-alanine (d-Ala; the key d-amino acid involved in the synthesis of E. coli cell walls) on biomass production during the fed-batch fermentation was studied because of the known activity of DAAO on d-Ala. Figure 1 shows the optimization process in this work. In addition, after the optimization of the 5-L fermentation scale, scale-up fermentation was carried out in 50-L and 5000-L fermenters, respectively.
Materials and Methods
Bacterial Strain and Plasmid
The gene daao, encoding DAAO from Rhodotorula gracilis (RgDAAO), was synthesized into the first open reading frame of pCDFduet-1 vector in vitro [23]. The gene cat, encoding catalase (CAT) from Geobacillus sp., was inserted into the second multiple cloning site of pCDFDuet-DAAO using the One Step Cloning Kit (Vazyme, Nanjing). The constructed pCDFDuet-DAAO-CAT was transformed into E. coli BL21 (DE3) [24].
Chemicals
d-Ala was purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). dl-Glufosinate substrate (purity > 85%) was provided by Dezhou Lvba Fine Chemical Co., Ltd. (Dezhou, China). A glycerin assay kit was purchased from NanJing JianCheng Biological Engineering Research Institute Co., Ltd. (Nanjing, China). SDS-PAGE and Bis-Tris buffer were purchased from GenScript (Nanjing, China). A hydrogen peroxide assay kit was purchased from Sigma-Aldrich (USA). Unless otherwise stated, all other chemicals were of analytical grade and commercially available.
Media
Luria–Bertani (LB) medium was the primary seed medium, and terrific broth (TB) medium was secondary seed medium. The basal fed-batch fermentation medium comprised 15 g/L peptone, 12 g/L yeast extract, 12 g/L glycerin, 10 g/L NaCl, 5 g/L (NH4)2SO4, 0.375 g/L MgSO4·7H2O, 1.36 g/L KH2PO4, 2.28 g/L K2HPO4·3H2O [23]. Compound feeding medium comprised 75 g/L peptone, 50 g/L yeast extract, 500 g/L glycerin, 5 g/L NaCl, 5 g/L MgSO4·7H2O, 20 g/L (NH4)2SO4, 3 g/L KH2PO4, 3 g/L K2HPO4·3H2O. The above mediums were all supplemented with 50 μg/mL streptomycin.
Fed-batch Fermentation in a 5-L Fermenter
For the primary seed preparation, the recombinant strain was cultured in a 250-mL shake flask with a 50-mL primary seed medium at 37 °C and 180 rpm for 12 h in an incubator shaker (DHZ-052DR, Shanghai Bocai Biological Technology Co., Ltd.). The primary seed culture was inoculated into a 500-mL shake flask with a 100-mL secondary seed medium and shaken at 37 °C and 180 rpm for 12 h in an incubator shaker. The cultivated seed (200 mL) was inoculated into a 5-L fermenter (Biotech-5BJ, Shanghai BaoXing Bio-Engineering Equipment Co.) containing 2 L of fed-batch fermentation medium.
Before inoculation, 50% (v/v) aqueous H3PO4 and 50% (v/v) aqueous NH3·H2O was used to adjust the pH to 7.0. The recombinant strains were cultured under the following conditions: 37 °C, 600 rpm agitation, 2.0 vvm aeration, pH 7.0. When the OD600 reached 18–20, lactose was added to the fermentation broth, and the culture was induced at 28 °C for approximately 18 h. When the carbon source in the basal fermentation medium was exhausted, a feeding medium was added into the fermenter.
Scale-up Culture Conditions
Scale-up cultivation was performed in a 50-L fermenter (Biotech-30BS-100JS, Shanghai bxbio Biological Equipment Engineering Co.) containing a 30-L fermentation medium and a 5000-L fermenter (Biotech-J5000, Shanghai BaoXing Bio-Engineering Equipment Co.) containing a 3000-L fermentation medium. The primary seed preparation for fermentation in a 50-L fermenter was cultured in a 500-mL shake flask with a 100-mL shake flask and shaken at 37 °C and 180 rpm for 12 h in an incubator shaker. The primary seed culture (100 mL) was inoculated into a 2-L shake flask with a 900-mL secondary seed medium and shaken at 37 °C and 180 rpm for 10 h in an incubator shaker and the cultivated seed (2 L) was inoculated into the 50-L fermenter. Except that the agitation was changed to 250 rpm, the other culture conditions were the same as a 5-L fermentation. For the 5000-L fermentation, the mature seed (30 L, OD600 4–6) in the 50-L fermenter was pneumatically injected into a 500-L fermenter as the primary seed through a pipeline connecting the two fermenters. The mature seed (150 L, OD600 4–6) in the 500-L fermenter was injected into a 5000-L fermenter as the secondary seed in the same way. The recombinant strains were cultured under the following conditions: 37 °C, 180 rpm agitation, 270 m3/h aeration, pH 7.0. When the OD600 reached 10–12, lactose was added to the fermentation broth, and the culture was induced at 28 °C for approximately 13 h. When the carbon source in the basal fermentation medium was exhausted, a feeding medium was added into the fermenter. Taking into account the cost problem and the difficulty of preparation after scale-up, the composition of the feed medium is changed from a complex compound feed medium to pure glycerol.
Analytical Methods
The detection method for dl-PPT: the concentration and enantiomeric excess (e.e.) of d-PPT and l-PPT were analyzed with a fluorescence detector (UltiMate FLD-3100; Ex 340 nm and Em 450 nm) during HPLC analysis (ThermoFisher UltiMate 3000) with a C18 column (Unitary C18; 4.6 mm × 250 mm, 5 μm) using a previously reported derivatization method [25, 26]. The detection method for d-Ala was the same as dl-PPT, with the ratio of methanol and ammonium acetate aqueous solution in the mobile phase changed to 30:70.
The residual glycerin was detected using a glycerin assay kit. The working solutions R1 and R2 were mixed in a 4:1 ratio, and the supernatant of the fermentation broth was diluted to 1 mL with deionized water. A 50-μL aliquot of the diluted mixture was moved to a new e-tube, to which 150 μL the working solution mixture was added, then the mixture was incubated at 37 °C for 20 min. The absorbance of the sample at 550 nm was tested using a Microplate reader (SpectraMax M5, Molecular Devices, USA). The concentration of the residual glycerin was determined with a standard plot prepared under the same conditions.
Biomass was measured based on the OD600 measured with an BioPhotometer (Eppendorf AG, Hamburg, Germany) and converted into DCW using the fitting and calibrated calculation formula (DCW = 0.313OD600 + 0.9).
Definition of DAAO enzyme activity (U): under the conditions of 30 °C and pH 8.0, the amount of enzyme required to consume 1 μM d-PPT per minute is equal to one unit of enzyme activity. Specific enzyme activity (U/g DCW) is defined as the enzyme activity per DCW per gram.
Results and Discussion
Optimization of Feeding Mode of the Recombinant Strain Expressing DAAO
Although DAAO has been successfully applied to produce of 7-ACA [12, 27], the technology of using RgDAAO to transform d-PPT is still immature, and production by fermentation is always at a disadvantage in industrial applications. Therefore, in this study, the feed fermentation mode was optimized for the recombinant strain expressing DAAO.
Many factors affected fed-batch fermentation, such as dissolved oxygen (DO), temperature, pH, and culture methods. Exploring the effects of various feed fermentation processes on the production of DAAO is necessary to improve both biomass and enzyme activity.
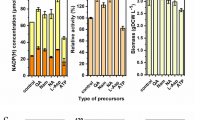
In the optimization process, the final concentration of lactose was 20 g/L. Initially, the feeding mode was kept constant at a rate of 15 mL/h (Fig. 2a); the resulting biomass was 14.6 g DCW/L, and the specific activity was approximately 210 U/g DCW. As shown in Fig. 2b and Fig. 2c, changing the feeding medium flow rate has little effect on the fermentation yield. The comparison of biomass and enzyme activity is shown in Fig. 2d.
When the feeding rate is too slow, the nutrients in the fermentation broth are insufficient, resulting in low bacterial biomass. A fast feeding rate will result in excessive growth of the bacteria, leading to the accumulation of fermentation by-products such as acetic acid, which will affect the growth of the bacteria and the expression of enzymes. Therefore, choosing an appropriate feeding rate will have a positive effect on fermentation. In this work, the optimal feeding rate was determined to be 20 mL/h. Because the constant-speed feeding caused the unfavorable accumulation of glycerin during the late fermentation stage, the feeding mode was changed to variable-speed feeding, which was determined by the concentration of residual glycerin. The concentration of the residual glycerol was controlled at 1 g/L, 3 g/L, and 5 g/L respectively. The results indicated that there was an advantage of maintaining the concentration of the remaining glycerol at 1 g/L through variable-speed feeding in the fermentation broth (Fig. 3) When the concentration of residual glycerol in the fermentation broth was maintained at a low level (1 g/L), the biomass was further increased based on the best constant-speed feeding mode (20 mL/h). This feeding mode is more adapted to the growth rate of the bacteria, which improves the biomass and enzyme activity.
Because dissolved oxygen (DO) in aerobic fermentation will significantly affect cell growth, product synthesis, and normal cell metabolism, fermentation using the DO-STAT feedback feeding mode was used. DO-STAT is a fermentation regulation method that uses the increase in dissolved oxygen caused by the lack of nutrients as a feed signal. Because of the delay of dissolved oxygen monitoring process, the floating range for the DO in the fermenter was set at 10% ± 5%, 30% ± 5%, and 40% ± 5%. Biomass (18.3 g DCW/L) and specific activity (251 U/g DCW) are highest when DO is approximately 30% ± 5% (Table 1). The biomass of the recombinant stain expressing RgDAAO increased 1.25 times as a result of the optimization of the feeding mode. The results demonstrate that the DO-STAT feeding mode is the best for the recombinant strain expressing RgDAAO when DO is controlled at 30% ± 5%.
The By-product H2O2 in the Fermentation Process of DAAO
The recombinant strain expressing DAAO usually cannot grow to high biomass even during fed-batch fermentation. In order to determine the mechanism for the inhibitory effect of DAAO on host cell growth, the by-products in the fermentation broth were analyzed. During the fermentation process, H2O2 was observed to accumulate rapidly after induction. Studies have shown that H2O2 is harmful to cell metabolism even at concentrations of a few micromoles [20, 28].
During the process described above for optimizing the feeding mode of the recombinant strain expressing DAAO, the concentration of H2O2 in the fermentation broth was analyzed. In DO-STAT feeding mode, when DO was controlled at 30% ± 5%, 20 g/L lactose was used to induce the expression of DAAO. The concentration of H2O2 produced during the fermentation process is shown in Fig. 4a. H2O2 accumulated rapidly after induction, continued to increase, and accumulated slowly in the late fermentation period. After induction, the growth of the strain gradually entered the stable phase, and DAAO began to be expressed, which lead to the accumulation of H2O2.
Studies have shown that DAAO catalyzes d-Ala, which is an amino acid necessary for the synthesis of E. coli cell walls, and also produces H2O2. In order to prove that d-Ala can act as a substrate of RgDAAO catalysis, 5 g/L of the recombinant strain expressing DAAO was mixed with an initial concentration of 20 mM/L d-Ala and incubated at 30 °C and pH 8.0 in a total volume was 30 mL. After reacting for 5 h, the residual concentration of d-Ala was 50% of the initial concentration, and the accumulation of H2O2 was detected during the reaction as shown in Fig. 4b. This indirectly shows that the rapid accumulation of H2O2 during the fermentation process after induction is a result of the conversion of d-Ala by DAAO. Therefore, removal of H2O2 from the fermentation broth is important for optimal cell growth and enzyme expression.
Fed-batch Fermentation Expressing DAAO-CAT to Relieve H2O2 Toxicity
In order to reduce the effects of H2O2 toxicity on cell metabolism, Ju et al. proposed replacing the critical methionine residues in DAAO with leucine [29]. In this work, CAT was added to the fermentation system in order to remove H2O2. A recombinant strain that co-expressed DAAO and CAT was constructed by our laboratory. The purpose of DAAO and CAT co-expression is to remove H2O2 from the fermentation broth during the fermentation process. In this study, fed-batch fermentation for the expression of DAAO-CAT was first studied and optimized. According to the fermentation characteristics of the recombinant strain expressing DAAO, lower glycerol concentrations and proper DO which is at 30% ± 5% are optimal for cell growth and enzyme expression, which has also been confirmed for the fermentation of the recombinant strain expressing DAAO-CAT (Fig. 5a). The biomass of the recombinant strain expressing DAAO-CAT was increased by 1.5 times (21.6 g DCW/L) and the specific activity was increased by 1.4 times (292 U/g DCW) during the fed-batch fermentation during DO-STAT feeding where DO was controlled at 30% ± 5%.
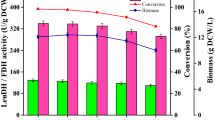
Primary fermentation optimization conditions (a and d) and advantages of the recombinant strain co-expressing DAAO-CAT (b and c). Constant-speed feeding: (A) 15 mL/h, (B) 20 mL/h, (C) 25 mL/h; Constant-glycerin feeding: (D) 5 g/L, (E) 3 g/L, (F) 1 g/L; DO-STAT feeding: (G) 10% ± 5%, (H) 30% ± 5%, (I) 40% ± 5%
The growth curves of the two recombinant strains are shown in Fig. 5b, and the concentration of H2O2 in the fermentation broth of DAAO and DAAO-CAT are shown in Fig. 5c. The biomass is significantly different after induction. Compared with the fermentation time for DAAO to reach the maximum biomass, the fermentation time for DAAO-CAT to reach the same biomass only takes 17 h, which was shortened by approximately 4 h. When DAAO and CAT were co-expressed, cell growth was significantly accelerated, and after induction, the biomass of the DAAO-CAT strain was higher than that of the DAAO recombinant strain. Although CAT was co-expressed, part of the H2O2 in the fermentation broth was removed, reducing the accumulation and allowing the strain to be more resistant to H2O2, which is reflected in an accelerated cell growth. However, the reduction of H2O2 also promotes the forward reaction rate of DAAO, which produces additional H2O2. Studies indicated that catalase forms an intermediate compound I (Cpd I) when decomposing H2O2. When the H2O2 concentration is low, Cpd I may react with external one-electron/hydrogen donors resulting in the formation of Compound II (Cpd II, an oxoferryl derivative without radical site [porFeIV = O]) [30]. In contrast to Cpd II of heme peroxidases, Cpd II of catalase is not effectively reduced to the native enzyme; therefore, Cpd II accumulation leads to catalase inactivation [31]. Co-expression of CAT can only remove part of the growth restriction during the fermentation process and cannot fundamentally prevent the inhibitory effect of DAAO on the growth of host cells during the fermentation process.
Based on research on H2O2 in the fermentation process, the expression of DAAO will lead to the production of H2O2, so it is necessary to balance the specific activity and the biomass. Because the high concentration of lactose will make the fermentation broth sticky and not conducive to cell growth, the lactose was added in two doses if the total concentration was greater than 20 g/L. As shown in Fig. 5d, the specific activity decreased with lactose concentration. In contrast, the biomass increased with decreasing lactose concentrations, especially for the 12.5 g/L and 15 g/L doses, which indirectly indicates that the expression of DAAO inhibited the growth of host cells. Based on the results from this study, the optimal lactose concentration is 27.5 g/L, resulting in a biomass of 20.2 g DCW/L and a specific activity of 395 U/g DCW, which was 1.85 times higher than the specific activity before optimization.
d-Ala Relieves the Inhibitory Effect of DAAO
Based on the fed-batch fermentation of the recombinant strain expressing DAAO-CAT, the growth curve (Fig. 5b) indicates that cell growth is still inhibited during the late fermentation stage (biomass only reaching 21.6 g DCW/L), although reaching the same fermentation level compared with that before shortening the fermentation time. During the decomposition of d-Ala by DAAO, H2O2 was produced as a by-product while the synthesis of the E. coli cell wall was weakened. In this work, based on the previously optimized fermentation conditions, the influence of d-Ala on fermentation was explored.
Study showed that d-Ala can be transported into cells [32]. Because the expression of DAAO inhibits the growth of host cells, and is generated after induction with lactose, d-Ala was added under the following culture conditions: fed-batch fermentation with DO-STAT feeding mode (DO was controlled at 30% ± 5%), cells were cultured at 37 °C until the OD600 reached approximately 20, and a total of 27.5 g/L lactose mixed with d-Ala was added in two additions to induce the cells at 28 °C. Because the addition of d-Ala promotes the forward conversion reaction of DAAO, excess d-Ala is detrimental to biomass production and enzyme activity. The optimal concentration of d-Ala resulted in a large increase in biomass, reaching 26.03 g DCW/L, and the specific activity was minimally negatively affected, maintaining 390 U/g DCW (Fig. 6a). d-Ala relieves the inhibition of DAAO on the growth of host cells, and results in a similar biomass to that resulting from induction with a low concentration of lactose.
The effect of d-Ala (a) and optimized condition (b) on fermentation. (A): original fermentation expressing DAAO; (B): induction of low concentration of lactose (12.5 g/L) co-expressing DAAO and CAT; (C): induction of high concentration of lactose (27.5 g/L) co-expressing DAAO and CAT; (D): induction of high concentration of lactose (27.5 g/L) mixed d-Ala (2 g/L) co-expressing DAAO and CAT
In this work, the co-expression of CAT was used to remove H2O2, the concentration of lactose was optimized to adjust the relationship between biomass and specific activity, and lactose mixed with d-Ala was added for induction, which relieved the inhibitory effect of DAAO on host cell growth during fed-batch fermentation (Fig. 6b). Compared with previous studies, the biomass of the recombinant strain co-expressing DAAO and CAT in fed-batch fermentation under the optimal fermentation conditions in this study reached a high level. Table 2 summarizes the results of some of the higher biomass production obtained in the previous literature in comparison with the results obtained in this study. Although the biomass obtained in some studies is similar, the fermentation time in this study is the shortest, which is a great advantage in industrial applications.
Scale-up Cultivation
To adapt the production of DAAO by fermentation to industrial applications, scale-up cultivation was performed in a 50-L fermenter containing a 30-L medium and a 5000-L fermenter containing a 3000-L medium based on the optimal condition in a 5-L fermenter. Fed-batch fermentation in the 50-L fermenter containing a 30-L fermenter medium resulted in a DAAO specific activity of 353.1 U/g DCW and a biomass production of 19.68 g DCW/L. Furthermore, a DAAO specific activity of 341.7 U/g DCW and a biomass production of 18.43 g DCW/L was obtained in the fed-batch 5000-L fermentation, (fermentation process is shown in Fig. 7). These fermentation results suggested that the process of scale-up did not have a major impact on the specific activity of DAAO, but the biomass has been reduced by the difference in parameter control and the change in the composition of the feeding medium, the inorganic salt and nitrogen source in the compound feeding medium contribute to the increase of cell density.
Conclusions
In this work, the fed-batch fermentation conditions were optimized and scale-up fermentation was carried out. Under optimal conditions, the fermentation of E. coli expressing DAAO-CAT relieved the inhibition on cell density and resulted in high specific activity. The biomass was 26.03 g DCW/L and the specific activity was 390 U/g DCW, an increase of 78% and 84%, respectively, compared with the original conditions, which indicate that the detoxification of DAAO was beneficial. In the scale-up experiments, 50-L and 5000-L fermentation processes resulted 75.6% and 70.8% of the biomass, and 90.5% and 87.6% of the specific activity in comparison with a 5-L fed-batch fermentation.
Data Availability
The authors declare that the data and materials are transparent.
Code Availability
All data, models, and code generated or used during the study appear in the submitted article.
References
Han, H. J., Zhu, B., Fu, X. Y., You, S. H., Wang, B., Li, Z. J., Zhao, W., Peng, R. H., & Yao, Q. H. (2015). Overexpression of d-amino acid oxidase from Bradyrhizobium japonicum, enhances resistance to glyphosate in Arabidopsis thaliana. Plant Cell Reports, 34(12), 2043–2051. https://doi.org/10.1007/s00299-015-1850-5.
Han, L., Zhao, Y. K., Cui, S., & Liang, B. (2018). Redesigning of microbial cell surface and its application to whole-cell biocatalysis and biosensors. Applied Biochemistry and Biotechnology, 185(2), 396–418. https://doi.org/10.1007/s12010-017-2662-6.
Pollegioni, L., & Molla, G. (2011). New biotech applications from evolved d-amino acid oxidases. Trends in Biotechnology, 29(6), 276–283. https://doi.org/10.1016/j.tibtech.2011.01.010.
Takahashi, S., Abe, K., & Kera, Y. (2015). Bacterial d-amino acid oxidases: Recent findings and future perspectives. Bioengineered, 6(4), 237–241. https://doi.org/10.1080/21655979.2015.1052917.
Xue, Y. P., Cao, C. H., & Zheng, Y. G. (2018). Enzymatic asymmetric synthesis of chiral amino acids. Chemical Society Reviews, 47(4), 1516–1561. https://doi.org/10.1039/C7CS00253J.
Fotheringham, I., Archer, I., Carr, R., Speight, R., & Turner, N. J. (2006). Preparative deracemization of unnatural amino acids. Biochemical Society Transactions, 34(2), 287–290. https://doi.org/10.1042/BST0340287.
Pilone, M. S., & Pollegioni, L. (2009). Enzymes, d-amino acid oxidases. In Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology (pp. 1–13).
Chen, Y., Goldberg, S. L., Hanson, R. L., Parker, W. L., Gill, I., Tully, T. P., Montana, M. A., Goswami, A., & Patel, R. N. (2011). Enzymatic preparation of an (S)-amino acid from a racemic amino acid. Organic Process Research & Development, 15(1), 241–248. https://doi.org/10.1021/op1001534.
Trost, E. M., & Fischer, L. (2002). Minimization of by-product formation during d-amino acid oxidase catalyzed racemate resolution of d/l-amino acids. Journal of Molecular Catalysis B: Enzymatic, 19, 189–195. https://doi.org/10.1016/S1381-1177(02)00166-2.
Bianchi, D., Bortolo, R., Golini, P., & Cesti, P. (1998). Enzymatic transformation of cephalosporin c to 7-aca by Simultaneous Action of Immobilized d-amino acid oxidase and glutaryl-7-ACA acylase. Applied Biochemistry and Biotechnology, 73(2-3), 257–268. https://doi.org/10.1007/BF02785660.
Hardianto, D., Royani, J. I., & Safarrida, A. (2016). Cephalosporin C acylase from microbes for one-step enzymatic transformation of cephalosporin C to 7-aminocephalosporanic acid. Journal of Pure and Applied Microbiology, 10(4), 2495–2499. https://doi.org/10.22207/jpam.10.4.03.
Ma, X. Q., Deng, S. W., Su, E. Z., & Wei, D. Z. (2015). One-pot enzymatic production of deacetyl-7-aminocephalosporanic acid from cephalosporin C via immobilized cephalosporin C acylase and deacetylase. Biochemical Engineering Journal, 95, 1–8. https://doi.org/10.1016/j.bej.2014.11.015.
Duke, S. O. (2018). The history and current status of glyphosate. Pest Management Science, 74(5), 1027–1034. https://doi.org/10.1002/ps.4652.
Freudl, R. (2018). Signal peptides for recombinant protein secretion in bacterial expression systems. Microbial Cell Factories, 17(1), 52. https://doi.org/10.1186/s12934-018-0901-3.
Kang, X. M., Cai, X., Liu, Z. Q., & Zheng, Y. G. (2019). Identification and characterization of an amidase from Leclercia adecarboxylata for efficient biosynthesis of l-phosphinothricin. Bioresource Technology, 289, 121658. https://doi.org/10.1016/j.biortech.2019.121658.
Chien, L. J., Wu, J. M., Kuan, I. C., & Lee, C. K. (2004). Coexpression of Vitreoscilla hemoglobin reduces the toxic effect of expression of d-amino acid oxidase in E. coli. Biotechnology Progress, 20(5), 1359–1365. https://doi.org/10.1021/bp0498589.
Kim, S. J., Kim, N. J., Shin, C. H., & Kim, C. W. (2008). Optimization of culture condition for the production of d-amino acid oxidase in a recombinant Escherichia coli. Biotechnology and Bioprocess Engineering, 13(2), 144–149. https://doi.org/10.1007/s12257-008-0005-8.
Zheng, J. X., Yang, T. W., Zhou, J. P., Xu, M. J., Zhang, X., Rao, Z. M., & Yang, S. (2017). Efficient production of d-amino acid oxidase in Escherichia coli by a trade-off between its expression and biomass using N-terminal modification. Bioresource Technology, 243, 716–723. https://doi.org/10.1016/j.biortech.2017.07.007.
Kuru, E., Hughes, H. V., Brown, P. J., Hall, E., Tekkam, S., Cava, F., de Pedro, M. A., Brun, Y. V., & VanNieuwenhze, M. S. (2012). In situ probing of newly synthesized peptidoglycan in live bacteria with fluorescent d-amino acids. Angewandte Chemie, 51(50), 12519–12523. https://doi.org/10.1002/ange.201206749.
Jang, S., & Imlay, J. A. (2007). Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. Journal of Biological Chemistry, 282(2), 929–937. https://doi.org/10.1002/ange.201206749.
Kumar, S. R., & Imlay, J. A. (2013). How Escherichia coli tolerates profuse hydrogen peroxide formation by a catabolic pathway. Journal of Bacteriology, 195(20), 4569–4579. https://doi.org/10.1128/JB.00737-13.
Park, S., You, X., & Imlay, J. A. (2005). Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx- mutants of Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America, 102(26), 9317–9322. https://doi.org/10.1074/jbc.M607646200.
Xu, J. M., Zhang, K., Cao, H. T., Li, H., Cheng, F., Cao, C. H., Xue, Y. P., & Zheng, Y. G. (2020). Development of a biocatalytic cascade for synthesis of 2-oxo-4-(hydroxymethylphosphinyl) butyric acid in one pot. Biocatalysis and Biotransformation, 1–8. https://doi.org/10.1080/10242422.2020.1797697.
Cao, C. H., Gong, H., Dong, Y., Li, J. M., Cheng, F., Xue, Y. P., & Zheng, Y. G. (2020). Enzyme cascade for biocatalytic deracemization of d, l-phosphinothricin. Journal of Biotechnology, 325, 372–379. https://doi.org/10.1016/j.jbiotec.2020.09.024.
Cao, C. H., Cheng, F., Xue, Y. P., & Zheng, Y. G. (2020). Efficient synthesis of l-phosphinothricin using a novel aminoacylase mined from Stenotrophomonas maltophilia. Enzyme and Microbial Technology, 135, 109493. https://doi.org/10.1016/j.enzmictec.2019.109493.
Lv, S. Z., Guo, Y. X., Xue, Y. P., Xu, J. M., & Zheng, Y. G. (2019). Efficient separation of l-phosphinothricin from enzymatic reaction solution using cation-exchange resin. Separation Science and Technology, 55(4), 779–787. https://doi.org/10.1016/j.enzmictec.2019.109493.
Luo, H., Li, Q., Yu, H., & Shen, Z. (2004). Construction and application of fusion proteins of d-amino acid oxidase and glutaryl-7-aminocephalosporanic acid acylase for direct bioconversion of cephalosporin C to 7-aminocephalosporanic acid. Biotechnology Letters, 26(11), 939–945. https://doi.org/10.1023/B:bile.0000025907.33332.be.
Zhang, C. C., Zhang, S. S., Liu, W., Guo, T. T., Gu, R. X., & Kong, J. (2019). Potential application and bactericidal mechanism of lactic acid-hydrogen peroxide consortium. Applied Biochemistry and Biotechnology, 189(3), 822–833. https://doi.org/10.1007/s12010-019-03031-z.
Ju, S. S., Lin, L. L., Chien, H. R., & Hsu, W. H. (2000). Substitution of the critical methionine residues in Trigonopsis variabilis d-amino acid oxidase with leucine enhances its resistance to hydrogen peroxide. FEMS Microbiology Letters, 186(2), 215–219. https://doi.org/10.1111/j.1574-6968.2000.tb09107.x.
Alfonso-Prieto, M., Vidossich, P., & Rovira, C. (2012). The reaction mechanisms of heme catalases: an atomistic view by ab initio molecular dynamics. Archives of Biochemistry and Biophysics, 525(2), 121–130. https://doi.org/10.1016/j.abb.2012.04.004.
Gebicka, L., & Krych-Madej, J. (2019). The role of catalases in the prevention/promotion of oxidative stress. Journal of Inorganic Biochemistry, 197, 110699. https://doi.org/10.1016/j.jinorgbio.2019.110699.
Young, G. B., Jack, D. L., Smith, D. W., & Saier Jr., M. (1999). The amino acid/auxin: proton symport permease family. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1415(2), 306–322. https://doi.org/10.1016/S0005-2736(98)00196-5.
Romano, D., Molla, G., Pollegioni, L., & Marinelli, F. (2009). Optimization of human d-amino acid oxidase expression in Escherichia coli. Protein Expression and Purification, 68(1), 72–78. https://doi.org/10.1016/j.pep.2009.05.013.
Deng, S. W., Su, E. Z., Ma, X. Q., Yang, S., & Wei, D. (2014). High-level soluble and functional expression of Trigonopsis variabilis d-amino acid oxidase in Escherichia coli. Bioprocess and Biosystems Engineering, 37(8), 1517–1526. https://doi.org/10.1007/s00449-013-1123-z.
Kim, S. J., Park, H. W., Shin, C. H., & Kim, C. W. (2014). Establishment of a cryopreservation method for the industrial Use of d-amino acid oxidase-overexpressing Escherichia coli. Bioscience, Biotechnology, and Biochemistry, 73(2), 299–303. https://doi.org/10.1271/bbb.80507.
Funding
This work was financially supported by the National Key R & D Program of China (2018YFA0901400).
Author information
Authors and Affiliations
Contributions
Jian-Miao Xu, Hui-Ting Cao, and Kai Zhang made substantial contributions to the design of the work, performed the most analysis, experiments, and wrote the manuscript. Ming Wang, Bao-Jian Ma, and Liu-Yu Wang participate in the experiment. Jian-Miao Xu, Feng Cheng, Ya-Ping Xue, and Yu-Guo Zheng revised it critically for important intellectual content and approved the version to be published.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, JM., Cao, HT., Wang, M. et al. Development of a Combination Fermentation Strategy to Simultaneously Increase Biomass and Enzyme Activity of d-amino Acid Oxidase Expressed in Escherichia coli. Appl Biochem Biotechnol 193, 2029–2042 (2021). https://doi.org/10.1007/s12010-021-03519-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03519-7