Abstract
The two-component system “AfsQ1/Q2” plays a crucial role to activate the production of antibiotics ACT, RED, and CDA through directly binding the promoters of pathway-specific activator genes actII-ORF4, redZ, and cdaR respectively when grown under glutamate-supplemented minimal medium in Streptomyces coelicolor. In this report, we demonstrated that the RspA1/A2 (a homologous protein of two-component system AfsQ1/Q2) plays a regulatory role in salinomycin biosynthesis in Streptomyces albus. Gene deletion and complementation experiments showed that the RspA1/A2 promoted salinomycin production but inhibited cell growth when cultured in YMG medium supplemented with 3% soybean oil. More importantly, RspA1/A2 strengthens salinomycin biosynthesis by directly affecting the transcription of the pathway-specific activator gene slnR. Meanwhile, RspA1/A2 plays a negative role in the regulation of nitrogen assimilation and urea decarboxylation by interacting with the promoters of genes gdhA, glnA, amtB, and SLNWT_1828/1829. Gene sigW is located downstream of rspA1/A2 and encodes an extracytoplasmic function sigma factor. Moreover, it negatively regulates the salinomycin biosynthesis and promotes cell growth, which antagonizes the function of RspA1/A2. In short, these useful findings are proved helpful to enrich the understanding of the regulatory pathways of antibiotic biosynthesis by an ECF σ factor-TCS signal transduction system in Streptomyces.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptomyces albus, a gram-positive bacterium, is mainly isolated from the soil, which is a changing environment with limited nutrients [1]. This organism exhibits a wide range of adaptations to extreme nutrient limitation, including the formation of aerial mycelium and the production and secretion of antibiotics. Salinomycin, being one of the antibiotics biosynthesized by S. albus, has been widely utilized in agriculture as the treatment of coccidiosis for its ability to inhibit the growth of an array of Gram-positive bacteria [2].
The biosynthesis of antibiotics is regulated by various transcriptional regulators, mainly at two levels. At the lower level, the cluster-situated regulators (CSRs), which are located within the antibiotic biosynthetic clusters, could directly bind to the promoters of genes in clusters [3]. For salinomycin biosynthesis, SlnR is proved as a positive pathway-specific regulator and could modulate transcription of genes in the salinomycin biosynthetic cluster in S. albus [4]. At the upper level, pleiotropic regulators or global regulators have been shown to control the production of more than one antibiotic. In Streptomyces, the most abundant pleiotropic regulator is the two-component system (TCS), a significant fraction of which regulates antibiotic production and morphological differentiation. For example, TCS CutR/S was described as negatively regulating ACT production in S. coelicolor A3(2), although there was no reported information regarding the activator signal of the system [5]. Typically, TCS consists of a membrane-bound histidine kinase (HK), which reacts to specific environmental stimuli, and a response regulator (RR), which mediates the cellular signal transduction, mainly through regulating the transcription of target genes [6].
Under harsh environmental conditions, such as lack of amino acid or limited availability of carbon source, bacteria exerted stringent control of expression of many genes and enzymes, which involved a series of signal transduction systems. [7]. One of the predominant signal transduction mechanisms employed by microbes is the phosphotransfer pathway normally called as “two-component” signal transduction system (TCS) [8]. In Streptomyces coelicolor, the TCS PhoP/PhoR system was found to be associated with phosphate limitation response [9], in addition, the TCS VanR/VanS system was activated to protect the integrity of cell envelope when S. coelicolor A3(2) was exposed to vancomycin [10].
TCSs are widely found in bacteria, fungi, and plants. In particular, Streptomyces harbor a high number of TCSs as compared with other bacterial genera, which is probably due to the changing environment that these organisms must inhabit. The genome sequence of S. albus J1074 [11] had revealed the presence of 60 sensor kinase genes, 42 of which lie adjacent to genes encoding response regulators that form two-component systems. However, the function of most of them remains unknown, although some of them were proved to be involved in the regulation of cell growth, differentiation, and antibiotic production.
Previously, another TCS, the AfsQ1/Q2 was initially demonstrated for its ability to promote the biosynthesis of ACT and RED in Streptomyces lividans [12]. In 2009, Shu et al. found that afsQ1 mutant derived from S. coelicolor A3(2) exhibited significantly decreased ACT, RED, and calcium-dependent antibiotic (CDA) production and rapid growth of aerial mycelium when cultivated on defined minimal medium (MM) with 75 mM glutamate as the sole nitrogen source [13]; further research found that the protein AfsQ1 directly bound to the promoters of the CSR-genes actII-ORF4, cdaR, and redZ. AfsQ1 also activated a putative sigma factor gene sigQ, (deletion of sigQ leads to an increase in antibiotic production) which might play a role as an antagonist for the afsQ1/Q2 system. Moreover, the TCS AfsQ1/Q2 could also function as a repressor for nitrogen assimilation by directly interacting with the nitrogen metabolism-related genes glnA, amtB, glnII, gdhA, nirB, nasA, and ureA [14]. Chen et al. found that the deletion of afsQ1/Q2 resulted in markedly decreased expression of the whole coelimycin P2 biosynthetic genes cluster [15]. In 2014, the two-component system SmrAB, a homologous protein with the two-component system AfsQ1/Q2, inhibited avermectin production but promoted oligomycin production [16].
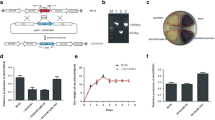
In this study, σw-rspA1/A2 (SLNWT_4830-SLNWT_4828/4829) in S. albus is homologous to the σQ-afsQ1/Q2 in S. coelicolor and σ25-smrAB in S. avermitilis as shown in Fig. 1b. Besides, they have a similar genetic organization (Fig. 1a). In our previous study, the transcription of TCS rspA1/A2 was found to be significantly upregulated in high-yield salinomycin production mutant according to the comparison of the mutant and the parental strain transcriptomes [17]. Herein, we elucidated the regulation of TCS RspA1/A2 in nitrogen metabolism and urea decarboxylation. In addition, we demonstrated the differential regulation role of TCS RspA1/A2 and σw in salinomycin biosynthesis and cell growth in S. albus.
Materials and Methods
Plasmids, Strains, and Growth Conditions
Plasmids, strains, and primers used in this study are shown in Table S1 and Table S2. S. albus DSM41398, A30 (gifted from Zhejiang Shenghua Biok Biology Co., Ltd.), and their derivatives were grown on ISP4 agar plates (BD, USA) for sporulation. For shaking flask fermentation, 150 μL of the A30 spore suspension (OD450 = 1) was inoculated into a 500-mL flask containing 100-mL fresh YMG medium (4.0% yeast extract, 10.0% malt extract, 4.0% glucose, 0.2% calcium carbonate) complemented with 3% soybean oil and further cultured at 34 °C for 7–8 days.
Construction of rspA1 Mutants
The separation between rspA1 and rspA2 is 36 bp, indicating rspA1 and rspA2 were co-transcribed, so we replaced a 586-bp fragment (located within the deletion region of rspA1 gene) with the kanamycin resistance gene to construct a rspA1/A2 gene deletion mutant in A30; a BamHI/HindIII fragment containing the kanamycin resistance gene neo was ligated with BamHI/HindIII-digested pJTU1278. Then, a 3.8-kb BamHI fragment of the left flanking region and a 4.0-kb HindIII fragment of the right flanking region were ligated with BamHI or HindIII-digested pJTU1278 to generate deletion plasmid pJTU-rspA1, respectively. After that, the resulted plasmid was transferred into A30 by conjugation from E. coli ET12567/pUZ8002, the selected colonies were further confirmed by DNA sequencing. Subsequently, we obtained rspA1 gene deletion mutant ΔrspA1, in which the rspA1 gene was completely deleted by double crossover recombination.
For complementation of gene rspA1, the whole CDS region of rspA1 was amplified by PCR with primers pair rspA1-F-NdeI and rspA1-R-EcoRI, and the product was cloned to pMD18-T and verified via sequencing. The NdeI/EcoRI fragment with rspA1 gene was ligated with NdeI-EcoRI-digested pIB139 to generate pIB-rspA1 [18]. Then, an EcoRI fragment containing the apramycin resistance gene aacIV was ligated with EcoRI-digested pIB-rspA1. The resulted plasmid was introduced into the wild-type competent cells through conjugation, and apramycin-resistant exoconjugants were selected and further confirmed by PCR with corresponding PCR primers. Constructions of sigW gene mutants in DSM41398 were also similarly performed.
RNA Preparation and Real-Rime RT-PCR
Samples were taken from S. albus cultures grown in YMG medium complemented with 3% soybean oil at 48, 72, and 96 h. RNA extractions were performed with an RNA extraction kit (Sangon Biotech Co., Ltd., Shanghai, P.R. China) by following the manufacturer’s instructions. To remove chromosomal DNA contamination, each RNA sample was treated with DNase I (Takara, Japan) for 5 min at 42 °C and subsequently confirmed by PCR using different primer pairs; then, the concentration of total RNA was determined by Nanodrop (Thermo USA). RNA samples (2 μg) from two biological replicates were then transcribed using a PrimeScriptTM RT Reagent Kit with gDNA Eraser (Takara, Shiga, Japan) for real-time RT-PCR. All these transcribed procedures mentioned above were performed by following the manufacturer’s instructions.
SYBR premix Ex TaqTMGC Kit Perfect Real Time (Takara, Shiga, Japan) was used and about 1 μL cDNA was added in 25 μL volume of PCR reaction for real-time RT-qPCR. The PCR was conducted using CFX96 Real-Time System (Bio-Rad, USA) and the conditions were 95 °C for 5 min; then 40 cycles of 95 °C for 5 s and 58 °C for 30 s; and an extension at 72 °C for 10 min. For all the RT-PCR assays, 16S rRNA was used as an internal control. The relative fold changes of gene transcription were calculated using the 2−ΔΔCt method [19]. RT-qPCR experiments were conducted with three independent biological replicates and error bars indicate the standard deviations (SD).
Overproduction and Purification of RspA1
The open reading frame of the rspA1 gene was amplified by PCR from the genomic DNA of S. albus. After digesting by restriction enzymes, the gene encoding protein RspA1 was cloned into plasmid pET28a. E. coli BL21 (DE3) strain was used as a host to express RspA1 protein. A single colony was selected to 5 mL LB contained kanamycin resistance at 37 °C for overnight culture. Then, cells were transferred to 100 mL LB supplemented with 50 mg/L kanamycin. A total of 0.5 mM isopropyl-b-d-thiogalactoside (IPTG) was added when cells were grown to OD600 = 0.4–0.8; then, the temperature was lowered to 20 °C for 12 h.
Protein His-RspA1 was purified as described previously [20], the fractions were analyzed by SDS-PAGE, and the protein concentration was determined by the BCA method using bovine serum albumin as a standard.
Electrophoretic Mobility Shift Assay
The binding sites were supposed to be located on the upstream region (− 300 to + 50 bp relative to the transcription start site of target genes), probes were designed and attached with a universal primer (5′-AGCCAGTGGCGATAAG-3′), and PCR products were biotin-labeled by PCR with the 5′-biotin-modified universal primer (Table S2). Concentrations of PCR products were determined by Nanodrop2000 (Thermo Scientific, Germany). EMSAs were carried out according to the protocol provided by the Chemiluminescent EMSA Kit (Beyotime Biotechnology, China). The binding reaction contained 10 mM Tris-HCl pH 8.0, 25 mM MgCl2, 50 mM NaCl, 1 mM DTT, 1 mM EDTA, 0.01% Nonidet P40, 50 mg/L poly[d(I-C)], and 10% glycerol. After binding, the samples were separated on a non-denatured PAGE gel in an ice bath at 100 V for 90 min and transferred to the N+ nylon membrane (GE Amersham, USA); then, bands were detected by BeyoECL Plus after dyeing (Beyotime Biotechnology, China).
Determination of Salinomycin Production
Determination of salinomycin production was conducted by following the method described in previous studies [21, 22]. The fermentation cultures of the wild-type strain and mutants were extracted with 9 volumes of methanol with the help of ultrasonic extraction for 30 min. Samples were then applied to liquid chromatography after passing through 0.2-μm filters. The liquid chromatography was operated at a flow rate of 1 mL/min with an Agilent Eclipse TC-C18 column (4.6 by 250 mm; particle size, 5 μm) with UV detection at 210 nm. The column temperature was set at 25 °C, and the mobile phase was Merck HPLC grade acetonitrile-2% aqueous acetic acid (92:8 [v/v]), and the injection volume was 20 μL.
Results
The RspA1/A2 System Regulates Antibiotic Production and Cell Growth in S. albus
In order to investigate the function of RspA1/A2, we constructed a rspA1 deletion mutant using a kanamycin-resistant gene (neo) replacement cassette; the rspA1 deletion mutant (named ΔrspA1) was confirmed by PCR. Compared with the parental strain, the ΔrspA1 mutant has shown no obvious phenotype difference when cultured on ISP4 solid medium (Fig. S1). However, salinomycin bioassays using Bacillus subtilis as an indicator strain formed a smaller inhibition zone in the ΔrspA1 strain in contrast to the parental type A30 and the complemented strain ΔrspA1a (Fig. S1). Therefore, this finding suggested that gene rspA1 defect may result in less yield of salinomycin production.
For a more detailed study of the difference in their ability to synthesize salinomycin, a shaking flask fermentation in the YMG medium complemented with 3% soybean oil was conducted. Samples were taken for after every 24 h, and we found that, throughout the 7-day fermentation, mutant ΔrspA1 achieved higher biomass than that of the original strain A30. Meanwhile, salinomycin synthesis was decreased by the deletion of rspA1 (Fig. 2a, b). Hence, these results demonstrated that the TCS RspA1/A2 played a positive role in the regulation of salinomycin biosynthesis.
RspA1/A2 system regulates salinomycin production and cell growth in S. albus. a Growth curves in YMG liquid medium complemented with 3% soybean oil. b Time course of salinomycin production in A30, rspA1 deletion strain (ΔrspA1), and complemented strain (ΔrspA1a). c Transcript levels of genes rspA1 and slnR in A30 and rspA1 deletion strain (ΔrspA1). d EMSAs of His-RspA1 protein with the upstream promoter region of slnR. The DNA probe (10 ng) was incubated with a protein concentration gradient (0, 0.5, and 4.0 μg). An excess of poly (d[I-C]) was included in every lane as an internal control to avoid nonspecific binding of the protein to the DNA
Furthermore, the transcription levels of gene rspA1 and positive pathway-specific regulator of salinomycin biosynthesis gene slnR were detected in 48, 72, and 96 h by RT-qPCR in the ΔrspA1 mutant and the original strain A30. Based on the results, no rspA1 was detected and transcription of slnR was drastically reduced as compared with the original strain A30 (Fig. 2c). These results indicated that RspA1 regulated salinomycin biosynthesis by effecting the transcription level of pathway-specific regulator gene slnR. Moreover, in order to determine whether RspA1 directly activates slnR transcription or not, EMSA analysis was conducted. The results suggested that RspA1 positively regulated the transcription of slnR by directly binding to the promoter region of slnR (Fig. 2d).
sigW Is Directly Activated by RspA1
Gene sigW, which encodes a sigma factor σw, locates upstream of the gene rspA1. The gap between sigW and rspA1 is 184 bps (Fig. 1a). To investigate whether the expression of sigW is controlled by RspA1, the transcript level of sigW was determined in the rspA1 deletion mutant ΔrspA1 and the original strain A30. Real-time RT-PCR analysis showed a marked reduction of the sigW transcription in ΔrspA1 mutant in 48 h, 72 h, and 96 h (Fig. 3a). These findings indicate that RspA1 positively controls the expression of sigW.
Transcriptional analysis of gene sigW and EMSAs of the interaction between RspA1 protein and the promoter regions of rspA1 and sigW genes. a Real-time RT-PCR analysis of gene sigW transcription levels at 48, 72, and 96 h in the ΔrspA1 mutant and A30 grown in the YMG medium. The relative transcription level of each gene was obtained after normalization to the internal reference 16S rRNA at corresponding time points. Error bars show standard deviation from triple independent experiments. b EMSAs of His-RspA1 protein with the upstream promoter region of sigW. The DNA probe (10 ng) was incubated with a protein concentration gradient (0, 0.5, and 4.0 μg). An excess of poly[d(I-C)] was included in every lane as an internal control to avoid nonspecific binding of the protein to the DNA
To assess the direct or indirect regulation of sigW by RspA1, EMSA was conducted with a probe of the promoter region between sigW and rspA1. EMSA result show that the protein RspA1 could specifically interact with the probe, and the motif website MEME predicted a potential RspA1-binding site with the direct repeat GTGAC-n6-GTGTC sequence from positions − 101 to − 86 relative to the translational start site of sigW based on the consensus motif of protein AfsQ1 (http://meme-suite.org/) (Fig. 3b), illustrating that sigW was directly activated by RspA1.
Deletion of sigW Inhibits Cell Growth and Promotes Salinomycin Biosynthesis
To further identify the sigW’s functions in S. albus, gene sigW was deleted in wild-type strain S. albus DSM41398. The complemented strain and overexpressed strain were also generated using pIB139-based vector pIB139-sigW containing the sigW coding region. As shown in Fig. 4a, deletion of sigW inhibited cell growth and the complementation of sigW restored the growth defect. Besides, the overexpressed strain ΔsigWb also generated by expressing the sigW gene under the strong promoter PermE* and it accumulated more cell biomass, indicating that σw plays a positive role in cell growth. However, gene sigW exert opposite impacts on salinomycin biosynthesis, deletion of sigW leads to increased production of salinomycin. In short, these findings indicate that sigW has a positive effect on cell growth and a negative effect on salinomycin production.
Role of σw on salinomycin production and cell growth in Streptomyces albus. a Effect of σw on salinomycin production and cell growth in Streptomyces albus DSM41398. The wildtype containing the plasmid pIB139 was designated WT/pIB139, the sigW deletion mutant was named ΔsigW, the complemented strain and overexpressed strain named ΔsigWa and ΔsigWb, respectively. b Transcription level of gene sigW, rspA1, and slnR in sigW mutants and the parental strain DSM41398 in 48 and 96 h cultured in YMG medium complemented with 3% soybean oil. Error bars show standard deviation from three independent experiments
RT-qPCR experiments were performed to determine the effect of sigW on the transcription of genes rspA1 and slnR (Fig. 4b). Transcription of sigW was not detected in sigW-deleted mutant ΔsigW, which was restored in the complemented strain ΔsigWa. Either in the wild-type strain or a sigW deletion mutant ΔsigW, transcription of rspA1 shows no obvious change, indicating that the transcription of rspA1 was not controlled by SigW. Meanwhile, the fact that the deletion of sigW could enhance the transcription of slnR was observed. These findings are consistent with the result of salinomycin production in sigW deletion mutant ΔsigW, which demonstrates that SigW has a negative impact on salinomycin production likely by repressing the transcription of slnR. Additionally, RspA1 and SigW have opposite effects on salinomycin production and cell growth in Streptomyces albus. Moreover, these results are in good agreement with the previous report on sigQ-afsQ1/Q2 in S. coelicolor [14].
In addition, further experimentation will be necessary to clearly identify the SigW promoter signature. Unfortunately, our attempts to overproduce the protein SigW in order to study its interaction with the target genes rspA1 and slnR were not successful (data not shown).
RspA1 Functions as a Transcriptional Repressor of Nitrogen Assimilation
In S. coelicolor, expressions of glnA, glnII, amtB, gdhA, nirB, nasA, and ureA were controlled by AfsQ1 [14]. In order to assess the influence of protein RspA1 on those genes in Streptomyces albus, the promoter region of glnA, amtB, gdhA, nirB, and nasA were amplified and labeled by biotin; genes glnII and ureA were not found in the genome of Streptomyces albus DSM41398 by KEGG database (https://www.kegg.jp/), which indicated that other homologous proteins might perform similar functions. As shown in Fig. 5a, EMSA results show promoter regions of genes glnA, amtB, and gdhA gave retarded signals; however, no retarded signals occurred with the promoter regions of nirB and nasA (data not shown), indicating that RspA1 specially bound to the promoters of genes glnA, amtB, and gdhA. Based on the consensus motif of RspA1 in S. coelicolor, we recognized the RspA1-binding sites in promoter regions of genes glnA, amtB, gdhA, slnR, and sigW in Streptomyces albus DSM41398 via the website MEME (http://meme-suite.org/). A putative RspA1 motif bound (“GTGAC-6bps-GTGTC”) was identified by weblogo (Fig. 5b), which shows a little difference with the motif of AfsQ1 (GT/AnAC-n6 –GtnAC) in S. coelicolor [14].
RspA1 functions as a transcriptional repressor of nitrogen assimilation. a EMSA of His-RspA1 protein with upstream promoter regions of gdhA, amtB, and glnA. The DNA probe (10 ng) was incubated with a protein concentration gradient (0, 0.5, and 4.0 μg). An excess of poly(d[I-C]) was included in every lane as an internal control to avoid nonspecific binding of the protein to the DNA. b The consensus motif of RspA1 in Streptomyces albus. c The transcription levels of genes rspA1, gdhA, amtB, glnA, and slnR at 72 h in A30 and rspA1 deletion mutant ΔrspA1. Each experiment was performed in triplicates
In order to check the impact of protein RspA1 on the nitrogen metabolism, we performed quantitative RT-PCR (qRT-PCR) analysis. Interestingly, the results have shown that gene transcript levels of genes gdhA, amtB, and glnA are upregulated in mutant ΔrspA1 (Fig. 5c), which indicated the upregulation of nitrogen metabolism when rspA1 is deleted and RspA1 functions as a transcriptional repressor of nitrogen assimilation.
RspA1 Directly Regulates Urea Amidolyase
Degradation of a wide range of nitrogen-containing biomolecules generates urea, and many plants, fungi, and bacteria can utilize this urea as a nitrogen source [23, 24]. The first step of urea utilization is the conversion of urea to ammonium, which is catalyzed by two distinct enzymes; urease or urea amidolyase (UA) [25]. In S. coelicolor, urease is used to break down the urea into NH4+ and CO2. However, urease is not found in the genome sequence of Streptomyces albus DSM41398. Instead, we found that urease is substituted by urea amidolyase (UA) to take the charge of degrading urea. In Streptomyces albus, urea amidolyase (UA) is separately encoded by two genes: gene SLNWT_1828 encodes a urea carboxylase (UC) and SLNWT_1829 encodes an allophanate hydrolase (AH). As shown in Fig. 6a, the gap between SLNWT_1828 and SLNWT_1829 is − 4 bp (Fig. 6a), suggesting that these two genes are co-transcribed and the products of those two genes work together to catalyze the conversion of urea to ammonium in the urea cycle (Fig. 6b).
RspA1 directly regulates urea carboxylase in Streptomyces albus. a Organization of SLNWT_1828 and SLNWT_1829 in Streptomyces albus. b The catalysis route of urea to ammonium. c The transcription level of gene SLNWT_1828 in 72 and 96 h in A30 and rspA1 mutant. d EMSA of His-RspA1 protein with upstream promoter regions of SLNWT_1828. The DNA probe (10 ng) was incubated with a protein concentration gradient (0, 0.5, and 4.0 μg). An excess of poly[d(I-C)] was included in every lane as an internal control to avoid nonspecific binding of the protein to the DNA
In S. coelicolor, the ureABC operon is directly controlled by AfsQ1/Q2. Therefore, we speculate that RspA1/A2 might also be involved in the regulation of urea amidolyase (UA) in S. albus. To address this hypothesis, as an initial approach, RT-qPCR assays are performed and results show that transcript level of SLNWT_1828 is upregulated in rspA1 deletion mutant (named ΔrspA1) as compared with the parental strain A30 at 72 and 96 h (Fig. 6c), inferring that RspA1 negatively regulates the decarboxylation of urea. To assess whether RspA1/A2 directly regulates those two genes, EMSA assays are performed. A 222-bp probe (from − 203 to 20 bp relative to the translational start site of SLNWT_1828) covering the intergenic region (140 bp) between SLNWT_1826 and SLNWT_1828 is designed. As shown in Fig. 6d, RspA1 interacts specifically with the designed probe. Moreover, it was found that at lower protein concentrations (0.5–4 μg), two shifted bands are observed in the gels, and by increasing the RspA1 concentration to 4 μg, an increase in the abundance of the upper band is clearly observed. The results demonstrate that RspA1 restrains SLNWT_1828 and SLNWT_1829 transcription possibly cooperated via direct interaction with the intergenic region between SLNWT_1826 and SLNWT_1828.
Discussion
Almost all TCSs studied in Streptomyces come from S. coelicolor [26], such as PhoP/PhoR, CutR/S, AbsA1/A2, and RapA1/A2 [5, 27]; majority of them are pleiotropic but conditionally required signal transduction systems for both secondary metabolism and morphological development in Streptomyces coelicolor. Similarly, like TCS DraR-K, the S. coelicolor mutants with deletion of either one or both of draR and draK exhibited significantly reduced actinorhodin (ACT) production but increased undecylprodigiosin (RED) production, and the further involvement in primary metabolism on minimal medium (MM) was separately supplemented with a high concentration of different nitrogen sources [28].
The AfsQ1/Q2 system was initially proved for its ability to promote the production of ACT and RED in Streptomyces lividans when cultured in MM medium complemented with 75 mM glutamate. However, disruption of either afsQ1 or afsQ2 in S. coelicolor led to no detectable change in secondary metabolism or morphogenesis when the mutants were grown under rich medium compared with the parental strain. Shu et al. also found a similar phenomenon in the afsQ1 deletion mutant in S. coelicolor when cultured in rich medium [13], which may be, due to the abundant nutrition, restored the deletion of afsQ1. In contrast, Luo et al. proved that deletion of smrAB stimulated avermectins production but inhibited oligomycin production in Streptomyces avermitilis when cultured under FM-I-rich medium [16]. However, in this study, we demonstrated that RspA1/A2 system (homologous proteins of AfsQ1/Q2) positively regulates antibiotic production in S. albus when cultured in YMG medium complemented with 3% soybean oil (Fig. 3), either FM-I or YMG medium contained abundant nitrogen source along with MM medium complemented with 75 mM glutamate, which may account for the difference in secondary metabolism or morphogenesis. Furthermore, transcript levels of genes glnA, amtB, gdhA, and SLNWT_1828 were upregulated which mean that the nitrogen metabolism was more active in the rspA1 deletion mutant ΔrspA1 as compared with the parental strain A30. Particularly, increased expression of genes gdhA and SLNWT_1828 enhanced the flux of nitrogen source for the production of other intermediate metabolites, thus keeping the C/N/P ratio in an equilibrium state. Conclusively, this may explain that rspA1 deletion mutant ΔrspA1 accumulates more biomass as compared with the parental strain A30 (Fig. 2a).
RspA1/A2 can strengthen salinomycin production by directly interacting with the pathway-specific activator gene slnR (Fig. 3d). SlnR activates the transcription of most of the genes belonging to the salinomycin gene cluster [4]. The gene sigW is located downstream of RspA1/A2 and encodes an extracytoplasmic function sigma factor. Besides, sigma factor SigW was found that it could regulate the salinomycin biosynthesis and promote cell growth (Fig. 4a), which antagonize the function of RspA1/A2. The effect of Sigma-TCS system on secondary metabolite differs among Streptomyces species; for example, SigE-CseB/CseC work in coordination to respond to specific signals by the cell envelope [29]; SigQ antagonizes the function of AfsQ1-Q2 in ACT, RED, and CDA production, similar with SigW-RspA1/A2 in Streptomyces albus; Sig25-SmrAB works together to regulate the avermectin and oligomycin production in Streptomyces avermitilis [16]. These findings helpfully enrich the understanding of the regulation of antibiotic biosynthesis by the Sigma-TCS signal transduction system in Streptomyces.
Conclusion
Collectively, we can conclude that RspA1/A2 plays a positive regulatory role in salinomycin biosynthesis and functions as a transcriptional repressor of nitrogen assimilation and urea decarboxylation in Streptomyces albus. Moreover, SigW-RspA1/A2 functions together in the regulation of salinomycin biosynthesis and morphological differentiation, and SigW antagonizes the function of RspA1/A2. Many studies suggest that the Sigma-TCS system has pleiotropic roles on the regulation of carbon, nitrogen, and phosphate metabolism in Streptomyces; however, further studies are required to explore the roles of RspA1/A2 in regulating carbon metabolism and phosphate metabolism.
References
Worthen, D. B. (2008). Streptomyces in Nature and Medicine: The Antibiotic Makers. Journal of the History of Medicine and Allied Sciences, 63(272), 273–274.
Gumila, C., Ancelin, M. L., Delort, A. M., Jeminet, G., & Vial, H. J. (1997). Characterization of the potent in vitro and in vivo antimalarial activities of ionophore compounds. Antimicrobial Agents and Chemotherapy, 41(3), 523–529.
Jianqiang, H., Jing, S., Virginie, M., BjRn, S., David, W., Bibb, M. J., Nitsara, K., Chih-Jian, L., Kao, C. M., & Buttner, M. J. (2010). Cross-regulation among disparate antibiotic biosynthetic pathways of Streptomyces coelicolor. Molecular Microbiology, 58, 1276–1287.
Zhu, Z., Li, H., Yu, P., Guo, Y., Luo, S., Chen, Z., Mao, X., Guan, W., & Li, Y. (2017). SlnR is a positive pathway-specific regulator for salinomycin biosynthesis in Streptomyces albus. Applied Microbiology and Biotechnology.
Chang, H. M., Chen, M. Y., Shieh, Y. T., Bibb, M. J., & Chen, C. W. (1996). The CutR/S signal transduction system of Streptomyces lividans represses the biosynthesis of the polyketide antibiotic actinorhodin. Molecular Microbiology, 21, 1075–1085.
Mascher, T., Helmann, J. D., & Unden, G. (2006). Stimulus perception in bacterial signal-transducing histidine kinases. Microbiology and Molecular Biology Reviews, 70(4), 910–938.
Krell, T., Lacal, J., Busch, A., Silvajiménez, H., Guazzaroni, M. E., & Ramos, J. L. (2010). Bacterial sensor kinases: Diversity in the recognition of environmental signals. Annual Review of Microbiology, 64(1), 539–559.
Bourret, R. B., & Silversmith, R. E. (2010). Two-component signal transduction. Annual Review of Biochemistry, 13, 113–115.
Alberto, S. L., Antonio, R. G., Alexander Kristian, A., & Martín, J. F. (2008). Target genes and structure of the direct repeats in the DNA-binding sequences of the response regulator PhoP in Streptomyces coelicolor. Nucleic Acids Research, 36, 1358–1368.
Hutchings, M., Hong, H., & Buttner, M. (2010). The vancomycin resistance VanR/S two-component signal transduction system of Streptomyces coelicolor. Molecular Microbiology, 59, 923–935.
Zaburannyi, N., Rabyk, M., Ostash, B., Fedorenko, V., & Luzhetskyy, A. (2014). Insights into naturally minimised Streptomyces albus J1074 genome. BMC Genomics, 15, 97. https://doi.org/10.1186/1471-2164-15-97.
Ishizuka, H., Horinouchi, S., Kieser, H. M., Hopwood, D. A. and Beppu, T.(1992). A putative two-component regulatory system involved in secondary metabolism in Streptomyces spp. Journal of Bacteriology, 174, 7585-7594, 23.
Shu, D., Chen, L., Wang, W., Yu, Z., Ren, C., Zhang, W., & Yang, S. (2009). afsQ1-Q2-sigQ is a pleiotropic but conditionally required signal transduction system for both secondary metabolism and morphological development in Streptomyces coelicolor. Applied Microbiology and Biotechnology, 81(6), 1149–1160.
Rui, W., Yvonne, M., Jin, W., Weiwen, Z., Guoping, Z., Wolfgang, W., Yinhua, L., & Weihong, J. (2013). Identification of two-component system AfsQ1/Q2 regulon and its cross-regulation with GlnR in Streptomyces coelicolor. Molecular Microbiology, 87, 30–48.
Chen, S., Zheng, G., Zhu, H., He, H., Chen, L., Zhang, W., Jiang, W., & Lu, Y. (2016). Roles of two-component system AfsQ1/Q2 in regulating biosynthesis of the yellow-pigmented coelimycin P2 in Streptomyces coelicolor. FEMS Microbiology Letters, 363, fnw160.
Shuai, L., Di, S., Jianya, Z., Zhi, C., Ying, W., & Jilun, L. (2014). An extracytoplasmic function sigma factor, σ(25), differentially regulates avermectin and oligomycin biosynthesis in Streptomyces avermitilis. Applied Microbiology and Biotechnology, 98, 7097–7112.
Zhang, K., Mohsin, A., Dai, Y., Chen, Z., Zhuang, Y., Chu, J. and Guo, M. (2019).Combinatorial effect of ARTP mutagenesis and ribosome engineering on an industrial strain of Streptomyces albus S12 for enhanced biosynthesis of salinomycin. Frontiers in Bioengieering and Biotechnology, pp. 212.
Bierman, M., Logan, R., O’Brien, K., Seno, E. T., Rao, R. N., & Schoner, B. E. (1992). Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene, 116(1), 43–49.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods, 25(4), 402–408.
You, D., Wang, M. M., & Ye, B. C. (2017). Acetyl-CoA synthetases of Saccharopolyspora erythraea are regulated by the nitrogen response regulator GlnR at both transcriptional and post-translational levels. Molecular Microbiology, 103(5), 845–859.
Lu, C., Zhang, X., Jiang, M., & Bai, L. (2016). Enhanced salinomycin production by adjusting the supply of polyketide extender units in Streptomyces albus. Metabolic Engineering, 35, 129–137.
Zhang, X., Lu, C., & Bai, L. (2017). Mechanism of salinomycin overproduction in Streptomyces albus as revealed by comparative functional genomics. Applied Microbiology and Biotechnology, 101(11), 4635–4644.
Mobley, H. L., Island, M. D., & Hausinger, R. P. (1995). Molecular biology of microbial ureases. Microbiology and Molecular Biology Reviews, 59, 451–480.
Mobley, H. L., & Hausinger, R. P. (1989). Microbial ureases: significance, regulation, and molecular characterization. Microbiological Reviews, 53(1), 85–108.
Zhao, J., Zhu, L., Fan, C., Wu, Y., & Xiang, S. (2017). Structure and function of urea amidolyase. Bioscience Reports, 38, BSR20171617.
Rodríguez, H., Rico, S., Díaz, M., & Santamaría, R. I. (2013). Two-component systems in Streptomyces: Key regulators of antibiotic complex pathways. Microbial Cell Factories, 12(1), 127.
Apel, A. K., Alberto, S. L., Antonio, R. G., & Martín, J. F. (2007). Phosphate control of phoA, phoC and phoD gene expression in Streptomyces coelicolor reveals significant differences in binding of PhoP to their promoter regions. Microbiology, 153(10), 3527–3537.
Zhenyu, Y., Hong, Z., Fujun, D., Weiwen, Z., Zhongjun, Q., Sheng, Y., Huarong, T., Yinhua, L., & Weihong, J. (2012). Differential regulation of antibiotic biosynthesis by DraR-K, a novel two-component system in Streptomyces coelicolor. Molecular Microbiology, 85, 535–556.
Hee-Jeon, H., Paget, M. S. B., & Buttner, M. J. (2008). A signal transduction system in Streptomyces coelicolor that activates the expression of a putative cell wall glycan operon in response to vancomycin and other cell wall-specific antibiotics. Molecular Microbiology, 69, 1199–1211.
Acknowledgments
We thank Zhejiang Biok Biology Co., Ltd. for providing strains and other experimental help.
Funding
This research was supported by the National Natural Science Foundation of China (81373286) and Fundamental Research Funds for the China Central Universities (No.22221818014 and No.22221817014) and 111 Project (B18022) to Meijin Guo.
Author information
Authors and Affiliations
Contributions
All authors saw and approved the manuscript. All authors contributed significantly to the work. Meijin Guo and Kuipu Zhang conceived the project. Meijin Guo, Kuipu Zhang designed experiments and analyzed results. Kuipu Zhang wrote the manuscript with the help of Ali Mohsin, Muhammad Fahad Ali, Meijin Guo, Yingping Zhuang, and Ju Chu. Kuipu Zhang performed experiments, supported by Yichen Dai, Zhongbing Chen.
Corresponding author
Ethics declarations
Conflict of Interest
Author Zhongbing Chen was employed by the company Zhejiang Biok Biology Co., Ltd. All other authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The presenting author of this manuscript in ACB2019 is Kuipu Zhang.
Electronic Supplementary Materials
ESM 1
(DOCX 40 kb)
Rights and permissions
About this article
Cite this article
Zhang, K., Mohsin, A., Dai, Y. et al. Role of a Two-Component Signal Transduction System RspA1/A2 in Regulating the Biosynthesis of Salinomycin in Streptomyces albus. Appl Biochem Biotechnol 193, 1296–1310 (2021). https://doi.org/10.1007/s12010-020-03357-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03357-z