Abstract
This article describes the synthesis of terpenic esters derived from geraniol and citronellol (geranyl and citronellyl alkanoates) through esterification reactions catalyzed by the immobilized lipases from Thermomyces lanuginosus (Lipozyme TL IM®) and Candida antarctica (Novozym 435®). Geraniol was esterified with oleic, lauric, and stearic acids; and citronellol was esterified with oleic and stearic acids. For all the synthesized flavor esters, the best conditions were 35 °C, and the molar ratio between acid and alcohol was 1:1. Geranyl and citronellyl alkanoates reached yields between 80-100% within 4 h of reaction. For the synthesis of the citronellyl and geranyl oleate, higher yields were obtained in the absence of organic solvents. For the esters from lauric and stearic acids, using solvent was indispensable to improve the miscibility between the substrates. The reuse of Novozym 435® and Lipozyme TL IM® was performed for two more cycles after the first use, with yields higher than 60%. The results demonstrated the efficiency of the reaction catalyzed by these two commercial enzymes and the feasibility of the methodology for the production of synthetic flavor esters through enzymatic catalysis. The flavor esters synthesized were not described in the literature up to the date, giving this research an innovative feature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Flavor esters are compounds widely used in several industrial formulations, mainly as perfumes and fragrances in cosmetics and personal care products [1, 2], as flavorings in food and beverage industry [3,4,5], or in pharmaceuticals [3, 6, 7]. The esters produced by terpenic alcohols and fatty acids have the highest variety of industrial applications [8,9,10] and are the most explored economically [11].
The economic interest for esters formed from geraniol is due to its presence in the vast majority of essential oils extracted from aromatic plants [12]. In addition to the industrial importance in aroma and fragrance, these have anti-infectious, immunostimulant, bactericidal, and pesticidal properties with low toxicity [13]. Esters formed from citronellol are widely studied for their active properties as natural repellents and pesticides [14, 15], besides flavorings.
The synthesis of aroma esters can be conducted by enzymatic reactions using lipases (triacylglycerol hydrolases, EC 3.1.1.3) [16,17,18]. Currently, several terpenic esters can already be produced through enzymatic reactions, such as eugenyl acetate esters, benzyl acetate, cinnamyl propionate [19], geranyl propionate [20], geranyl acetate [7], citronellyl laureate [4], and citronellyl acetate [21]. Different methodologies have been developed with the purpose of making the enzymatic synthesis of these esters feasible, such as the use of processes in continuous-flow [22], coupled reactors with membranes to improve the purification steps [23, 24], and the use of supercritical fluids aiming at eliminating the use of organic solvents [25, 26].
Traditionally, these compounds are isolated from plants by extraction or produced through chemical reactions involving strong acids. These methods have limitations: the high cost of extraction and demand for raw material, processing conditions with high temperature and pressure, hazardous chemical reagents and catalysts, by-product formation, and the necessity to additional purification steps [8, 27, 28]. In this way, enzymatic catalysis would overcome several of these limitations and be a greener alternative for the synthesis of the flavor esters.
The main objective of this work was to produce new terpenic esters derived from geraniol and citronellol. The geranyl laureate, oleic and stearate, and citronellyl oleic, and stearate were produced by enzymatic catalysis using the commercial lipases of Thermomyces lanuginosus (Lipozyme TL IM®) and Candida antarctica (Novozym 435®). All the terpenic esters studied were not reported in the literature up to the date, giving this research an innovative feature. Focusing on industrial applicability, the reagents were selected based on low toxicity and commercial interest, evaluating the need for organic solvents and aiming for higher purity of the products.
Materials and Methods
Materials
For the synthesis of the flavor esters, the following alcohols were used: geraniol (98%) and citronellol (95%) (Sigma-Aldrich); oleic (99%), lauric (99%), and stearic (95%) acids (Vetec); and the organic solvents hexane (99%) (Lafan) and isooctane (99.8%) (Sigma). The commercially immobilized lipases from Thermomyces lanuginosus (Lipozyme TL IM®) and Candida antarctica (Novozym 435®) were kindly donated by Novozymes (Araucaria-Brazil).
Synthesis of Geranyl and Citronellyl Alkanoates
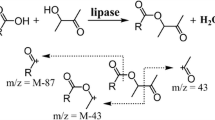
The terpenic esters derived from geraniol and citronellol were synthesized according to the reactions described in Fig. 1, obtaining as products the geranyl laureate, geranyl oleate, geranyl stearate, citronellyl laureate, citronellyl oleate, and citronellyl stearate. The experiments were conducted in an incubator chamber with 200 rpm orbital shaking. The reactions were studied at temperatures of 35, 45, and 55 °C; molar (alcohol:acid) ratios of 1:1, 1:2, and 1:3; and enzymatic loading of 3, 6, 10, and 14% (wimmobilized enzymes/wsubstrates). The esterification activity (U) was calculated based on the previously published data for the enzymes Lipozyme TL IM® and Novozym 435® by Sá et al. [19], comparing the activities to the amount of enzyme. One unit of activity (U) is the amount of enzyme necessary to consume 1 μmol of lauric acid per minute at the established experimental conditions.
The use of organic solvent was evaluated by adding 10 mL of hexane or isooctane. The reactions were conducted at 35 °C, the molar ratio (alcohol:acid) 1:3 and 10% (wbiocatalyst/wsubstrates) of each enzyme studied. The reactions were accompanied by quantification of the esters by gas chromatography; aliquots of 100 μL were withdrawn for analysis at each period.
Geranyl and Citronellyl Alkanoates Quantification
Geranyl and citronellyl alkanoates were determined by gas chromatography (Shimadzu GC-2010, São Paulo, Brazil) equipped with a data processor, using a capillary column of fused silica ZB-WAX (30 m × 250 μm × 0.25 μm) and flame ionization detector (FID), with programmed temperatures of 40 °C (8 min), 40–150 °C (10 °C min−1), 150–220 °C (10 °C min−1), and 220 °C (5 min), injector temperature at 250 °C, detector at 250 °C, injection in split mode, ratio of split 1:100, N2 (56 kPa and 2 mL min−1) as carrier gas, injected in volume 1 μL, and sample diluted in dichloromethane (2:10). Reaction yield was calculated measuring the reduced area of the limiting reagent based on the reaction stoichiometry [29,30,31].
Recovery and Reuse of the Biocatalyst in Consecutive Reactions
After each reaction, the biocatalysts were separated from the reaction system by simple filtration and washed with hexane to ensure the removal of product residues [32, 33]. After complete evaporation of the hexane at room temperature, the recovered biocatalysts were stored at 4 °C until the next use.
Results and Discussions
Synthesis of Geranyl and Citronellyl Alkanoates
Kinetic of the Esterification Reactions
For the kinetic study of the esterification, the reaction conditions were fixed at 35 °C and the molar ratio (alcohol:acid) of 1:1. The enzymatic reactions reached yields in esters between 82.7 and 97.6% (Table 1), while, in the absence of a catalyst, the maximum yield was 12% in 24 h of reaction, demonstrating the efficiency of lipases as biocatalysts in ester synthesis. In all cases, the use of Novozym 435® resulted in higher yields in shorter reactional times than the Lipozyme TL IM®, even when a smaller amount of enzyme was used. In the geranyl laurate synthesis, even when the total activity of the Novozym 435® (2.95 U) was lower than the Lipozyme TL IM® (3.42 U), the yield was higher for the first enzyme. The same result was observed in the citronellyl stearate synthesis, in which Novozym 435® (3.60 U) performed better than Lipozyme TL IM® (4.25 U). The results regarding the performance of the immobilized commercial enzymes are in agreement with the synthesis of citronellyl laurate by Habulin et al. [4] and also with the synthesis of benzyl propionate by Sá et al. [19]. Martins et al. [34] also reported better performance of the Novozym 435® compared with the Lipozyme TL IM® in the synthesis of several other flavor esters.
These differences in enzymatic activity and lipase performance in the reactions may be associated with different factors, such as the microorganism that produced the lipase, the type of immobilization and support, and a better fit of the substrate at the active site of the lipases [35]. Novozym 435® are immobilized on a support of macroporous resin of hydrophobic surface [36, 37]. The Lipozyme TL IM® is a lipase immobilized in silica gel with the hydrophilic surface [38]. For this reason, the water molecules produced as a by-product in the reactions may have been very close to the surface of the enzymes, which may have hindered the diffusivity of the substrates in the active site of the enzyme [19, 28].
According to the reaction stoichiometry, the maximum amount of water formed in the esterification does not exceed 4% (w/w). However, the presence of water on the reaction media did not shift the equilibrium towards the hydrolysis. The reactions reached their maximum yield in esters in the first hours, and they were continually monitored for more than 24 h without decreasing the ester amount.
Influence of Organic Solvents in Esterification Reactions
The use of organic solvents in reactions plays a crucial role in enzymatic transformations and may affect the catalytic efficiency of the enzyme [7]. The nature and polarity of the organic solvents influence the rate of synthesis of the products catalyzed by lipases. The logarithm value of the partition ratio between the organic and the aqueous phases (log P) is used to describe the hydrophobicity of organic solvents [4]. Solvents with a log P > 2 value tend to provide better dissociation of weak organic acids and prevent the removal of the water layer essential for the maintenance of enzymatic activity. In contrast, hydrophilic solvents (log P < 2) with polar characteristics tend to remove essential water from the enzyme surface that leads to inactivation of the enzyme [39].
In the experiments carried out, the use of organic isooctane solvents (log P = 4.5) and hexane (log P = 3.5) was investigated. These solvents are widely used and present good results in enzymatic reactions, given their nonpolar characteristics [40]. As shown in Table 2, it was possible to obtain up to 100% yield to esters, with similar performance for both hexane and isooctane.
Esterification reactions of stearic and lauric acids were improved using solvents. Geranyl laurate was obtained with up to 10% yields with Lipozyme TL IM® and 43% with Novozym 435® in reactions without organic solvents. These values were increased to 89% and 99%, respectively, by adding hexane or isooctane, making the solvent indispensable for the reaction medium. Geranyl stearate was synthesized with 99% yield in the reactions with solvent and about 45% in the absence for both enzymes tested. Citronellyl stearate also showed a better yield in reactions performed with solvent (95%) than without (65%) for the two enzymes.
Both lauric and stearic acids have melting points at temperatures of 43.2 and 69.6 °C, respectively, while oleic acid has a melting point of 14 °C. As reactions were conducted at 35 °C, the use of the solvent was required to increase the solubility of the reagents in the reaction medium, facilitating the access of the substrates to the active site of the enzyme [39].
On the other hand, the use of solvents in the synthesis of geranyl oleate in a reaction catalyzed by Lypozyme TL IM® had an adverse effect: decreased the yield from 92.5 to 64.3 and 67.9% when adding hexane or isooctane in the reaction medium, respectively. This result demonstrates that the high yield can be achieved without the use of solvents when it is possible to have a reasonable degree of solubility between the reactants.
Evaluation of the Effect of Temperature in Esterification Reactions
The effect of the temperature, ranging from 35 to 55 °C, in the esterification reactions of geraniol and citronellol was studied. According to results, an increase in temperature did not influence the yield of the citronellyl (Fig. 2) and geranyl (Fig. 3) alkanoates. However, for the citronellyl oleate, the ester yield was reduced by about 17% when the temperature was rising from 35 to 55 °C.
Comparison of the synthesis of citronellyl alkanoates in the reactions at 35, 45, and 55 °C with Lipozyme TL IM® and Novozym 435® lipases. Reaction conditions: enzymatic loading of 3% (w/w about the reaction medium), hexane, RM 1:1 (acid:alcohol), the reaction time of 1 h and 200 rpm of orbital agitation
These values agree with previous studies of geranyl butanoate [9], geranyl acetate [41], and citronellyl laureate [42], which were synthesized in temperatures between 35 and 70 °C. The increase in temperature for syntheses involving enzymatic catalysis tends to be beneficial, since the probability of collision between the enzymes and substrate molecules increases proportionally [42]. However, an increase in temperature may result in conformational changes in the active site of the enzyme, decreasing its stability and even leading to its inactivation [41].
The possibility of conducting reactions at mild temperatures offers several advantages, such as high energy savings, reduction of possible problems related to the operational stability of the enzyme, and avoiding the volatilization of the reagents or organic solvents. According to the results, it is possible to perform the synthesis of flavor esters at 35 °C with excellent yields. Nonetheless, the presence of a solvent is required if the reactants have a melting point above 35 °C to improve the miscibility of the reaction medium.
Reuse of the Biocatalyst in Consecutive Reactions
The use of immobilized enzymes, such as Lipozyme TL IM® and Novozym 435®, allows the recovery and reuse of the biocatalyst in new operating cycles, impacting on a cost reduction [21]. The study of the reuse of the biocatalysts in the esterification reactions was performed in two additional cycles (R1 and R2) after the first use (R0).
According to the results (Table 1), the reuse of the enzymes proved to be effective in most of the studied reactions, being possible to reach yields over 90% for all the flavor esters synthesized. However, the esters formed from lauric and stearic acid showed a reduction in its yield after the second use. According to Lerin et al. [33], enzymatic activity and the yield of the products are directly related to the substrates and solvents used in the reaction. In this way, the product was not completely removed after washing the biocatalyst with solvent, or even the contact with the solvent can lead to changes in the lipase microenvironment (pH, amount of water around the enzyme), affecting the enzymatic activity. Considering that lauric and stearic acids form esters have a greasy aspect, the residues removed from the enzyme surface with hexane may have been inefficient and diminished the performance in the following cycles.
In all cases, Novozym 435® maintained good yields in both recycles, suggesting that it is less sensitive to acid exposure and that Lipozyme TL IM® is more sensitive to such contact [34]. Kim and Park [43] studied the enzymatic reuse of Novozym 435® in esterification reactions to produce flavor esters with yields above 95% after 20 cycles. Martins et al. [34] also achieved yields above 80% in 10 cycles, with the same enzyme.
Conclusions
All the esters studied were synthesized with an excellent experimental yield, with yields between 82 and 98%, in a maximum of 4 h of reaction. Immobilized enzymes Novozym 435® and Lipozyme TL IM® proved to be efficient biocatalysts in the synthesis of geranyl and citronellyl alkanoates from lauric, oleic, and stearic acids. The main characteristics of this method were the simplicity of operation and excellent yields in short reaction times. The high conversion of the oleate esters without organic solvent in short reaction time was very satisfactory, showing that the elimination of solvents in the enzymatic synthesis of flavor esters is feasible. Data of this study contribute to the better knowledge of enzymatic production of terpene esters in the presence of organic solvents.
References
Varma, M. N., & Madras, G. (2010). Kinetics of enzymatic synthesis of geranyl butyrate by transesterification in various supercritical fluids. Biochemical Engineering Journal, 49(2), 250–255.
Ben Akacha, N., & Gargouri, M. (2015). Microbial and enzymatic technologies used for the production of natural aroma compounds: Synthesis, recovery modeling, and bioprocesses. Food and Bioproducts Processing, 94, 675–706.
Serri, N. A., Kamaruddin, A. H., & Len, K. Y. T. (2010). Studies of reaction parameters on synthesis of Citronellyl laurate ester via immobilized Candida rugosa lipase in organic media. Food and Bioproducts Processing, 88(2), 327–332.
Habulin, M., Šabeder, S., Sampedro, M. A., Knez, Z. (2008) Enzymatic synthesis of citronellol laurate in organic media and in supercritical carbon dioxide. Biochemical Engineering Journal, 42 (1), 6-12
Liu, D., Chen, J., & Shi, Y. (2018). Advances on methods and easy separated support materials for enzymes immobilization. TrACTrends in Analytical Chemistry., 102, 332–342.
Dhake, K. P., Deshmukh, K. M., Patil, Y. P., Singhal, R. S., & Bhanage, B. M. (2011). Improved activity and stability of Rhizopus oryzae lipase via immobilization for citronellol ester synthesis in supercritical carbon dioxide. Journal of Biotechnology, 156(1), 46–51.
Badgujar, K. C., & Bhanage, B. M. (2014). Synthesis of geranyl acetate in non-aqueous media using immobilized Pseudomonas cepacia lipase on biodegradable polymer film: Kinetic modelling and chain length effect study. Process Biochemistry, 49(8), 1304–1313.
You, P., Su, E., Yang, X., Mao, D., & Wei, D. (2011). Carica papaya lipase-catalyzed synthesis of terpene esters. Journal of Molecular Catalysis B: Enzymatic, 71(3–4), 152–158.
Damnjanovic, J. J., Zuza, M. G., Savanovic, J. K., Bezbradica, D. I., Mijin, D. Z., Boskovic-Vragolovic, N., & Knezevic-Jugovic, Z. D. (2012). Covalently immobilized lipase catalyzing high-yielding optimized geranyl butyrate synthesis in a batch and fluidized bed reactor. Journal of Molecular Catalysis B: Enzymatic, 75, 50–59.
Da Silva, J. M. R., & Nascimento, M. D. G. (2012). Chemoenzymatic epoxidation of citronellol catalyzed by lipases. Process Biochemistry, 47(3), 517–522.
Badgujar, K. C., & Bhanage, B. M. (2015). Immobilization of lipase on biocompatible co-polymer of polyvinyl alcohol and chitosan for synthesis of laurate compounds in supercritical carbon dioxide using response surface methodology. Process Biochemistry, 50(8), 1224–1236.
Murcia, M. D., Gómez, M., Gómez, E., Gómez, J. L., Hidalgo, A. M., Sánchez, A., & Vergara, P. (2018). Kinetic modelling and kinetic parameters calculation in the lipase-catalysed synthesis of geranyl acetate. Chemical Engineering Research and Design, 138, 135–143.
Adenubi, O. T., Ahmed, A. S., Fasina, F. O., McGaw, L. J., Eloff, J. N., & Naidoo, V. (2018). Pesticidal plants as a possible alternative to synthetic acaricides in tick control: A systematic review and meta-analysis. Industrial Crops and Products, 123, 779–806.
Tavares, M., da Silva, M. R. M., de Oliveira de Siqueira, L. B., Rodrigues, R. A. S., Bodjolle-d’Almeira, L., dos Santos, E. P., & Ricci-Júnior, E. (2018). Trends in insect repellent formulations: A review. International Journal of Pharmaceutics, 539(1–2), 190–209.
Hussein, H. S., Salem, M. Z. M., & Soliman, A. M. (2017). Repellent, attractive, and insecticidal effects of essential oils from Schinus terebinthifolius fruits and Corymbia citriodora leaves on 62 two whitefly species, Bemisia tabaci, and Trialeurodes ricini. Scientia Horticulturae , 216, 111–119.
Silva, N. C. A., Miranda, J. S., Bolina, I. C. A., Silva, W. C., Hirata, D. B., de Castro, H. F., & Mendes, A. A. (2014). Biochemical Engineering Journal, 82, 1139–1149.
Carvalho, C. C. C. R., & Fonseca, M. M. R. (2006). Biotechnology Advances, 24(2), 134–142.
Sharma, R., Chisti, Y., & Banerjee, U. C. (2001). Biotechnology Advances, 19(8), 627–662.
Sá, A. G. A., Meneses, A. C., Lerin, L. A., Hermes, P. H. A., Sayer, C., & De Oliveira, D. (2018). Bioprocess Biosyst. Eng., 41(5), 585–591.
Ferraz, L. I. R., Possebom, G., Alvez, E. V., Cansian, R. L., Paroul, N., de Oliveira, D., & Treichel, H. (2015). Biocatalysis and Agricultural Biotechnology, 4(1), 44–48.
Badgujar, K. C., & Bhanage, B. M. (2014). Enzyme and Microbial Technology, 57, 16–25.
Adarme, C. A. A., Leão, R. A. C., de Souza, S. P., Itabaiana, I., de Souza, R. O. M. A., & Rezende, C. M. (2018). Molecular Catalysis, 453(March), 39–46.
Nigiz, F. U. (2018). Chemical Engineering and Processing: Process Intensification, 128(December 2017), 173–179.
Jyoti, G., Keshav, A., & Anandkumar, J. (2015). Journal of Engineering (United States), 2015.
Couto, R., Vidinha, P., Peres, C., Ribeiro, A. S., Ferreira, O., Oliveira, M. V., Macedo, E. A., Loureiro, J. M., & Barreiros, S. (2011). Geranyl Acetate Synthesis in a Packed-Bed Reactor Catalyzed by Novozym in Supercritical Carbon Dioxide and in Supercritical Ethane. Industrial and Engineering Chemistry Research, 50(4), 1938–1946.
Bourkaib, M. C., Randriamalala, H., Dettori, L., Humeau, C., Delaunay, S., Chevalot, I., & Guiavarc’h, Y. (2018). Enzymatic synthesis of geranyl acetate in packed bed reactor in supercritical carbon dioxide under various pressure-temperature conditions and reactor configurations. Process Biochemistry, 71, 118–126.
Trusek-holownia, A., & Noworyta, A. (2007). An integrated process: Ester synthesis in an enzymatic membrane reactor and water sorption. Journal of Biotechnology, 130(1), 47–56.
Sá, A. G. A., de Meneses, A. C., de Araújo, P. H. H., & de Oliveira, D. (2017). A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends in Food Science and Technology, 69, 95–105.
Machado, J. R., Pereira, G. N., dos Santos de Oliveira, P., Zenevicz, M. C., Lerin, L., dos Reis Barreto de Oliveira, R., Cabral de Holanda Cavalcanti, S., Ninow, J. L., & de Oliveira, D. (2017). Process Biochemistry, 58, 114–119.
Chiaradia, V., Paroul, N., Cansian, R. L., Detofol, M. R., Lerin, L. A., Oliveira, J. V., & Oliveira, D. (2012). Applied Biochemistry and Biotechnology, 168(4), 742–751.
Paroul, N., Grzegozeski, L. P., Chiaradia, V., Treichel, H., Cansian, R. L., Oliveira, J. V., & de Oliveira, D. (2010). Production of geranyl propionate by enzymatic esterification of geraniol and propionic acid in solvent-free system. Journal of Chemical Biology, 85(12), 1636–1641.
Castro, H. F., & Anderson, W. A. (1995). Fine Chemicals by Biotransformation Using Lipases. Quimica Nova, 18(6), 544–554.
Lerin, L., Ceni, G., Richetti, A., Kubiak, G., Vladimir Oliveira, J., Toniazzo, G., Treichel, H., Oestreicher, E. G., & Oliveira, D. (2011). Successive cycles of utilization of Novozym 435 in three different reaction systems. Brazilian Journal of Chemical Engineering, 28(2), 181–188.
Martins, A. B., da Silva, A. M., Schein, M. F., Garcia-Galan, C., Záchia Ayub, M. A., Fernandez-Lafuente, R., & Rodrigues, R. C. (2014). Comparison of the performance of commercial immobilized lipases in the synthesis of different flavor esters. Journal of Molecular Catalysis B: Enzymatic, 105, 18–25.
Yadav, G. D., & Dhoot, S. B. (2009). Synthesis of cinnamyl laurate in non-aqueous media. Journal of Molecular Catalysis B: Enzymatic, 57(1–4), 34–39.
Verdasco-Martín, C. M., Villalba, M., dos Santos, J. C. S., Tobajas, M., Fernandez-Lafuente, R., & Otero, C. (2016). Effect of chemical modification of Novozym 435 on its performance in the alcoholysis of camelina oil. Biochemical Engineering Journal, 111, 75–86.
José, C., Austic, G. B., Bonetto, R. D., Burton, R. M., & Briand, L. E. (2013). Investigation of the stability of Novozym® 435 in the production of biodiesel. Catalysis Today, 213, 73–80.
Yang, H., Mu, Y., Chen, H., Su, C., Yang, T., & Xiu, Z. (2014). Sn-1,3-specific interesterification of soybean oil with medium-chain triacylglycerol catalyzed by Lipozyme TL IM. Chinese Journal of Chemical Engineering, 22, 1016–1020.
Mohamad, N., Aziah, N., Mahat, N. A., Jamalis, J., Huyop, F., Aboul-enein, H. Y., & Abdul, R. (2015). Simple adsorption of Candida rugosa lipase onto multi-walled carbon nanotubes for sustainable production of the flavor ester geranyl propionate. Journal of Industrial and Engineering Chemistry, 32, 99–108.
Trusek Holownia, A., & Noworyta, A. (2007). An integrated process: Ester synthesis in an enzymatic membrane reactor and water sorption. Journal of Biotechnology, 130, 47–56.
Gupta, A., Dhakate, S. R., Pahwa, M., Sinha, S., Chand, S., & Mathur, R. B. (2013). Geranyl acetate synthesis catalyzed by Thermomyces lanuginosus lipase immobilized on electrospun polyacrylonitrile nanofiber membrane. Process Biochemistry, 48(1), 124–132.
Abdullah, A. Z., Sulaiman, N. S., & Kamaruddin, A. H. (2009). Biocatalytic esterification of citronellol with lauric acid by immobilized lipase on aminopropyl-grafted mesoporous SBA-15. Biochemical Engineering Journal, 44(2-3), 263–270.
Kim, H., & Park, C. (2017). Enzymatic synthesis of phenethyl ester from phenethyl alcohol with acyl donors. Enzyme and Microbial Technology, 100, 37–44.
Acknowledgments
The authors also thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the financial support and Laboratório Integrado de Engenharia Bioquímica (Lieb) at Federal University of Santa Catarina for supporting this research work.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva Corrêa, L., Henriques, R.O., Rios, J.V. et al. Lipase-Catalyzed Esterification of Geraniol and Citronellol for the Synthesis of Terpenic Esters. Appl Biochem Biotechnol 190, 574–583 (2020). https://doi.org/10.1007/s12010-019-03102-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03102-1