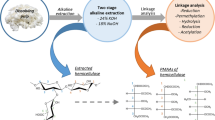
Abstract
Eucalyptus wood is the primary source of fibers to produce paper and cellulose in South American countries. The major by-product generated in the cellulose industry is sawdust derived from chip wood production, which is designated as Eucalyptus by-product (EB). The xylooligosaccharides (XOS) are xylose-based oligomers with proven effects over maintenance and stimulation of beneficial human gut bacteria. This study reported the EB extraction and characterization along with an assessment of hemicellulose hydrolysis using commercial xylanases to produce XOS. Hemicellulose derived from extracted and NaClO2 pretreated (HEEBPT) presented xylan content of 55%, which was similar to 58.5% found in commercial Birchwood hemicellulose (CBH). The enzymatic hydrolysis of HEEBPT and CBH presented 30% as maximum conversion of xylan into XOS without significant difference among the enzymatic extracts evaluated. The XOS production from EB was proven as a technically feasible alternative to recover a value-added product from hemicellulosic fraction generated in the cellulose industry. However, lignin removal with NaClO2 from EB affects the feasibility of an industrial process because they generate toxic compounds in the pretreatment step. Thus, further studies with alternative reagents, such as ionic liquids, are required to asses selectively lignin removal from EB.

Graphical Abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 2008, Brazil accounted for 31% of cellulose fiber production in the global market [1]. Later, in 2016, the Brazilian production of short cellulose fiber and paper was 16.4 and 10.3 million tons, respectively [2]. Recent data demonstrated that cellulose industry generates, approximately, 0.27 tons of solid waste to per ton of cellulose [3]. This high waste generation has been posing as a concern in the industry, which justified strategic studies regarding the utilization as feedstock to produce new value-added products [4].
The processing of Eucalyptus wood logs generates a large amount of Eucalyptus by-product (EB), which is oxidized for energy cogeneration. In this study, the EB presented more than 60% of cellulose and hemicellulose, thus resulting in low lignin content, which presents much higher energy recovery compared to the other fractions [5]. Therefore, the proper alternative to EB utilization would not be energy cogeneration, but the production of value-added products such as xylooligosaccharides (XOS). The hemicellulose from Eucalyptus has a backbone of β(-1,4)-D-xylopyranosyl in the main chain that may contain side-group consisting of 4-O-methyl-D-glucuronic acid, acetyl, and arabinosyl [6]. The hemicellulose is found in lignocellulosic materials cell wall linked to cellulose chains by hydrogen linkage and encapsulated by lignin [6, 7]. Thus, a physicochemical pretreatment is required for partial lignin removal and create access to the polysaccharide fraction (cellulose and hemicellulose) of lignocellulosic materials. The selective extraction of hemicellulose is essential to posterior utilization as a substrate for the biotechnological process involved in higher value-added product obtainment [8]. Several pretreatments can be used to perform delignification using bases, acids, organic solvents, or water (auto-hydrolysis) [9]. The oxidative pretreatment with sodium chlorite (NaClO2) and acetic acid (CH3COOH) in an aqueous phase is utilized to recover holocellulose by selective lignin removal [10, 11]. The NaClO2 in an acid medium (CH3COOH) forms chlorous acid, which is decomposed in chlorine dioxide (ClO2) and secondary products as chloride and chlorate ions, depending on temperature and pH [10]. In this pretreatment, the side-group in xylan molecules (4-O-methyl-D-glucuronic acid, acetyl, and arabinosyl) may also be partially removed by NaClO2 cleavage [10, 11].
Most of the hemicelluloses are extracted from the lignocellulosic cell wall using based on alkaline solutions, such as sodium hydroxide (NaOH) and potassium (KOH), which are often utilized [12]. The standard mechanism involved in hemicellulose extraction consists of dissolution followed by alkaline hydrolysis of ester type bounds (between lignin and hemicellulose) releasing hemicelluloses in aqueous medium [7, 13]. The addition of hydrogen peroxide (H2O2) to alkaline solutions is an alternative to improve hydrolysis because H2O2 is an efficient oxidant with low toxicity. Thus, the combination of alkaline with H2O2 increases lignin removal and hemicellulose extraction besides separating silica and greases [14]. In this combined treatment, the optimum pH reported in the literature is 11.6, which promotes H2O2 decomposition in peroxide anion (HOO-), the principal agent involved in degradation of chromophores groups (pka = 11.6 a 25 °C) [15,16,17]. Also, metals like iron, copper, and manganese accelerate H2O2 decomposition in alkaline conditions forming hydroxyl radicals (HO•) and superoxide anion (O2−•) that participate in delignification mechanism [15,16,17]. These radicals have positive effects in the delignification process; however, they also oxidize carbohydrates and negatively affect the process extraction. Thus, the metals found in the lignocellulosic material must be removed.
Extraction of hemicellulose from hardwoods and grasses has recently been reported in the literature. The extraction of hemicellulose from hardwoods in alkaline medium with addition of H2O2 and mild temperature was carried out with success; however, an oxidative pretreatment with NaClO2 was required to extract hemicellulose with high yield [18,19,20,21]. However, another study reports the extraction of hemicellulose from sugarcane bagasse. Unlike hardwoods, the bagasse allowed the extraction of the hemicellulose with high yields without any previous pretreatment of the material [21,22,23]. Thus, hardwoods are more resistant to the extraction process of hemicellulose, being this effect attributed to the high contents of lignin in wood when compared to bagasse [18].
Xylanases are enzymes produced by bacteria and fungi that perform hydrolysis of β(-1,4)-D-xylopyranosyl biding of xylan chain to release xylose monomers and XOS of different polymerization degrees [24, 25]. The main enzymes used in xylan chain depolymerization are endo-β-1,4-xylanases (EC 3.2.1.8) that break the main xylan chain into oligosaccharides units and the β-xylosidases (EC 3.2.1.37) cleaving the ends of non-reducing xylobiose and short-chain XOS into xylose monomers [26]. Generally, the endo-β-1,4-xylanases are utilized to hydrolyze internal glycosidic β-1,4 bonds of xylan resulting into XOS release, while the exo-β-xylanases act over the non-reducing terminals of XOS high molar mass to liberate xylobiose molecules; the release of xylose can also occur as a consequence of β-xylosidases acting over short-chain XOS or from xylobiose molecules [27, 28].
The XOS consist of oligomer based in units of xylose and possess beneficial effects like reduction of cholesterol level, bifidobacteria growth stimulation, and caries prevention [29, 30]. The literature reports that XOS produced by enzymatic hydrolysis of hardwood xylan has a high potential for prebiotic effects on Bifidobacteria and Lactobacilli [19, 31].These XOS present easy application in the food industry because they are stable in a broad pH and temperature range and have organoleptic characteristics such being moderately sweet stable in a broad pH and temperature range [26]. Moreover, XOS also have been used as antioxidants, antiallergics, and preventive against anemia and arteriosclerosis [27, 29, 30]. Therefore, XOS has been presenting higher market value compared to biofuels, cellulose, and electricity [32]. In 2014, the market value of XOS was in the range of US$ 22-50 kg−1, varying according to purity [32]. In this context, this work aimed to extract and characterize the hemicellulose derived from EB and to evaluate enzymatic hydrolysis of hemicellulose using commercial xylanases to produce XOS.
Material and Methods
Lignocellulosic Source
The material denominated as EB was obtained as a by-product of wood logs processing by Fibria Cellulose Company. The feedstock utilized by the company is based on the mixture of Eucalyptus grandis and Eucalypytus urophylla in an average ratio of 1:1. After sampled, the EB was dried at 25 °C to remove excess of moisture and stocked in closed plastic bags at 25 °C before utilization.
Chemical Pretreatment of EB with Sodium Chlorite
The EB was milled and passed through a 0.84-mm sieve to obtain a standardized particle size. After, an ethanolic extraction (ethanol 95°) was performed in Soxhlet extractor for 6 h [33, 34]. The material free of extractives was denominated as extracted Eucalyptus by-product (EEB). The pretreatment of EEB (dry basis) demanded 0.3 g of NaClO2 (0.93%, w/v), 0.1 mL of anhydrous CH3COOH (0.31%, v/v), and 32 mL of deionized water [11, 19, 20]. In a 1-L polypropylene beaker, 20 g of EEB was added along of pretreatment with sodium chlorite/acetic acid in intervals between 1 and 4 h following the abovementioned ratio, with a controlled temperature of 70 °C. After 60, 120, 180, and 240 min of NaClO2 pretreatments, the materials were filtered in sintered glass filters number 3 (Schott, Germany) and washed with deionized water up to neutralization. This neutralized material (retained) was also washed with 200 mL of pure acetone and dried at 45 °C for 24 h. The fractions obtained at 60, 120, 180, and 240 min of pretreatment were denominated as EEBPT1, EEBPT2, EEBPT3, and EEBPT4, respectively. The assays were conducted in triplicate, and the results were expressed as average values with respective standard deviations. The pretreatment yield was calculated according to Eq. 1.
where PY, pretreatment yield using NaClO2 (%); MPi, EEB initial dry mass of untreated material (g); and MPf, final dry mass of EEBPT1, EEBPT2, EEBPT3, and EEBPT4 (g).
Hemicellulose Extraction
The EEB and EEBPT3 were subjected to a modified hemicellulose extraction process based on standard procedures reported in the literature [18,19,20,21]. Initially, 10 g of EEB and EEBPT3 were treated with 100 mL of ethylenediaminetetraacetic solution (EDTA) 0.2% (w/v), pH 5 throughout 1 h at 90 °C to remove metals, then materials were washed with deionized water, dried at 45 °C, and stored up to hemicelluloses extraction procedure [13]. After 5 g of materials previously treated with EDTA was added in a 600−mL polypropylene beaker along with 100 mL of H2O2 6% (w/v), with pH set to 11.6 using NaOH 2-5 M. Next, the mixture was placed in 80-rpm shaker for 4-12 h at 20 °C. At the end of reaction in the shaker, the material was filtered and pH set to 6 by the addition of HCl 6 M. The solution was concentrated threefold (related to volume) at 60 °C and precipitated using 95% (v/v) ethanol. After material sedimentation, the supernatant was removed and the amount precipitated was washed with 70% (v/v) ethanol. In this step, the material was kept static (12 h) to allow complete sedimentation and the supernatant was removed. The step involved in the washing with ethanol was repeated eightfold to obtain a clean supernatant [35]. After, the material was separated and dried at 45 °C for 24 h, being designated as hemicelluloses derived from extracted Eucalyptus by-product (HEEB) and hemicellulose derived from Eucalyptus by-product extracted and pretreated with NaClO2 for 180 min (HEEBPT). The assays were conducted in triplicate, and the results were expressed as average values with respective standard deviations. The yield in extractions EEB and EEBPT3 was calculated according to Eq. 2.
where RE, extraction yield of hemicellulose (%); MEi, EEB and EEBPT3 initial dry mass of the hemicellulose fraction (g); and MEf, final dry mass of HEEB and HEEBPT (g).
Enzymatic Hydrolysis of Solid Fractions
The EEB, EEBPT3, HEEB, HEEBPT, and commercial Birchwood hemicellulose (CBH, Sigma) were hydrolyzed with commercial xylanases: Celluclast from Trichoderma reesei (Novozymes-Brazil), Cellulase from T. reesei (Sigma-Brazil), and Xylanases from Bacillus subtilis expressed by Escherichia coli (Verdartis-Brazil). The enzymatic hydrolysis was performed in the ratio of 80 UI of xylanases/gram of substrate (dry basis) [36]. The reactions were carried out in 50-mL Falcon tubes containing 200 mg of sample, resulting in consistency (mass/water ratio) of 2% (w/v) in sodium acetate buffer 50 mM at pH 4.8. The assays were realized in triplicate under shaker agitation (120 rpm and 45 °C) for 72 h. During the enzymatic saccharification process, the reactions were monitored in the intervals of 3, 6, 12, 24, 48, and 72 h. After specified reaction times, 250 μL aliquots from the samples were treated at 100 °C for 5 min and centrifugated at 2600g while the supernatant was stored at −20 °C before utilization.
Characterization of Enzymatic Extracts
Total xylanases
The activity was assessed at 50 °C for 5 min with Birchwood xylan (Sigma) 1% (w/v) prepared in sodium acetate buffer 50 mM (pH 5.5). The readings were done in the spectrophotometer (Ultrospec™ 3100 UV/VIS-Amersham Biosciences) at 540 nm. The negative control consisted of the addition of DNS before the enzymatic extract [37].
β-xylosidase
This activity was measured at 50 °C for 30 min with a solution of p-nitrophenyl-β-D-xilopiranosídeo (pNPX) (Sigma) 0.1% (w/v), prepared in sodium acetate buffer 50 mM (pH 5.0). The absorbance readings at 410 nm were conducted in the spectrophotometer (Ultrospec™ 3100 UV/VIS-Amersham Biosciences) [38].
Proteins
Total protein quantification was made according to Lowry modified by Hartre [39]. All assays and readings were realized in triplicate.
Chemical Characterization of Solid Fractions
The EEB, EEBPTs, HEEB, HEEBPT, and a Birchwood xylan (HCM, Sigma) were chemically characterized to assess the amount of ashes, extractives, total lignin (insoluble and soluble), and carbohydrates. The assays were conducted in triplicate and the results presented as average percentages with respective standard deviation.
Ashes determination
The amount was measured according to specific literature in the field [40,41,42].The calculations were made using Eq. 3.
where AA, ashes amount (%); Mi, solids initial dry mass (g); and Mf, solids final dry mass after oxidation (g).
Lignin quantification
The samples provided 300 mg (dry basis) that were treated with 3 mL sulfuric acid 72% (w/w) at 30 °C for 60 min in glass vials. After 60 min, the contents were transferred to 250 mL Erlenmeyer and complemented with 79 mL of deionized water (reaching 82 mL). The suspensions were autoclaved (121 °C) for 60 min. After cooling, the suspensions were filtered in sintered glass filters number 3 (Schott, Germany), which were previously dried at 105 °C for 1 h. The retained material was washed in two stages of 5 mL with deionized water and dried until reached constant mass. The dried residue corresponded to insoluble lignin (Klason lignin) [33, 34]. The quantification of Klason lignin was done according to Eq. 4.
where LI, Klason lignin content (g); Mfilt+res, mass dry sinterized filter containing Klason lignin (g); and mfilt, mass dry sintererized filter empty (g).
The filtrate was added at 100 mL, and the amount of soluble lignin was determined by absorbance measured in a spectrophotometer UV-VIS at 205 nm (Ultrospec™ 3100 UV/VIS-Amersham Biosciences). The measurements of soluble lignin concentration were made considering the molar extinction coefficient of 105 L g−1 cm−1, which is the average of absorptivities presented by lignin models [33, 34]. The calculation of soluble and total lignin mass was based in Eqs. 5 and 6.
where Ls, soluble lignin content (g); Ahid, acid hydrolyzates absorbance (205 nm); and f, acid hydrolyzate dilution factor.
where LT, total lignin content (%); and Mi, fractions initial dry mass (g).
Carbohydrates and organic acids
The acid hydrolyzates were filtered by cartridges SEP-PACK C18 and injected in liquid chromatography (HPLC, Shimadzu model NEXERA XR). The chromatographic analysis was performed according to following conditions: BIO-RAD Aminex HPX-87H column (300 × 7.8 mm); oven temperature at 60 °C; H2SO4 5 mM as eluent (0.6 mL min−1); 20 μL sample volume; refractive index detector at 60 °C (Shimadzu, model RID- 20A). The amount of cellulose, xylan, and arabinosyl side-groups were calculated according to Eq. 7. All assays were conducted in triplicate and the results presented as average percentages with respective standard deviation.
where C, cellulose, xylan, arabinosyl or acetyl groups content (%); Mf, glucose, xylose, arabinose, or acetic acid mass (g); f, hydrolysis factor for cellulose (0.9), xylan (0.88), arabinosyl groups (0.88), and acetyl groups (0.72); and Mi, initial fractions dry mass (g).
Determination of XOS
The XOS content in the enzymatic hydrolyzates was assessed by liquid chromatography (HPLC, Shimadzu model NEXERA XR) according to methodology adapted [36]. The analysis parameters were BIO-RAD Aminex HPX-87C (300 × 7.8 mm) column; 80 °C oven temperature; ultrapure water (Milli Q) at 0.6 mL min−1 as eluent; 20 μL sample volume; refractive index detector at 60 °C (Shimadzu, model RID- 20A); and total analysis time of 15 min. Before injection, the pH of the samples was set to 6.5 using NaOH 1 M. Xylose (Sigma), xylobiose (Megazyme), xylotriose (Megazyme), xylotriose (Megazyme), xylopentaose + xylohexaose (Megazyme), and oligosaccharides with more than 6 xylose units were quantified. The conversion of xylan to XOS was calculated by Eq. 8.
where ς, xylan conversion to xylose, xylobiose, xylotriose, xylotetraose or oligosaccharides with more than 6 xylose units (> X6) (%); XOS, concentration of xylose, xylotriose, xylotetraose, or oligosaccharides with more than 6 xylose units (g L-−1); XOS factor, hydrolysis index of xylan into xylose (0.88), xylobiose (0.936), xylotriose (0.956), xylotetraose (0.967), and oligosaccharides with more than 6 xylose unit (0.978); and m, initial xylan dry mass (g), for EEB (0.0282 g), EEBPT3 (0.032 g), HEEB (0.041 g), HEEBPT (0.11 g), and HCM (0.117 g). All assays were conducted in triplicate and the results presented as average percentages with respective standard deviation.
Results and Discussion
Pretreatment of Eucalyptus By-product with NaClO2 and Chemical Characterization
The EB presented an ethanol-extractable amount of 3.4% (w/w) and generated an insoluble fraction denominated EEB (extracted Eucalyptus by-product). The content of cellulose, xylan, acetyl groups, lignin, ashes, and extractives in EB were 43.9%, 14.1%, 3.3%, 27.2%, 0.7%, and 3.4% (w/w), respectively, according to Table 1. The sum of all EB components was 92.6% (w/w). The chemical composition of EB was similar to other studies reporting the analysis of Eucalyptus wood [5, 43, 44]. However, the arabinose quantification methodology was not responsive enough to detect this compound in EB. Thus, the arabynosyl side-groups (commonly found in Eucalyptus wood) were considered undetectable. Moreover, 4-O-methyl-D-glucuronic, often found in hemicellulose chain of hardwood was not quantified in the samples of this study [40]. Besides, oxidized sugars formed in acid hydrolysis and arabinosyl and 4-O-methyl-D-glucuronic side-groups were part of the non-assessed content [40]. A comparison of the macromolecular chemical composition of EB and hardwood with sugarcane bagasse pointed to similar cellulose content, despite higher lignin (+ 29%) and lower (− 32%) hemicellulose in the composition [18,19,20].
The NaClO2 pretreatment over EEB was done to reduce lignin content while preserving hemicellulose fraction (xylan + acetyl side-groups) and cellulose, which generated an insoluble fraction denominated EEBPT (Extracted Eucalyptus by-product pretreated with NaClO2). In the extracted material (EEBPT3), it was noticed bleaching when compared to EEB, which is common due to the removal of lignin and some derivatives [11, 28, 29]. The primary goal of NaClO2 pretreatment was to obtain a model (EEBPT) to be compared with EEB in the subsequent procedures of hemicellulose extraction.
The EEBPT yields after 60 and 120 min of pretreated with NaClO2 were 90%, while in 180 and 240 min were lower (85%) (Table 1). Thus, the NaClO2 pretreatments resulted in efficient and high yield process presenting 13.1% lignin content removal without significant losses of other components. Literature also reports NaClO2 pretreatments, but applied to sugarcane bagasse with the following yields 91.4% (60 min), 89% (120 min), 82% (180 min), and 80.2% (240 min) (w/w) [11]. Comparatively, the sugarcane bagasse yields (NaClO2 pretreatments) in 60 and 120 min were similar to those found with EEBPTs (90%; 60 and 120 min), while higher reaction times (180 and 240 min) provided lower yields (81%) compared to those evaluated in EEBPTs (approximately 85%; reaction times of 180 and 240 min) (Table 1).
The chemical characterization of macromolecular components (cellulose, xylan, acetyl side-group, and lignin) and other low molar mass compounds (ashes and extractives) of EB and EEBPTs submitted to mass balances is presented in Table 1. The analysis of EEBPTs pretreated with NaClO2 for 60, 120, 180, and 240 min presented cellulose content (42.5%, 43.9%, 42.1%, and 42.1%, (w/w)), xylan (13.9%, 14.2%, 13.3%, and 13.4% (w/w)), acetyl side-group (2.7%, 2.8%, 2.8%, and 2.9% (w/w)), total lignin (23.5%, 21.5%, 14.2%, and 14.1% (w/w), and ashes (0.3%, 0.2%, 0.7%, and 0.9% (w/w)), respectively. Cellulose and xylan content have not presented significant modification regarding EEBPTs compared to EB. However, lignin content in EEBPTs for 60, 120, 180, and 240 min was reduced 14.5%, 21.0%, 48.4%, and 48.2% (w/w), respectively. In this sense, the NaClO2 pretreatment was selective towards lignin removal (48.4% in 180 min) and kept cellulose and xylan (EB) intact. Thus, the Eucalyptus by-product pretreated with NaClO2 for 180 min (EEBPT3) was selected as model for hemicellulose extraction and XOS production.
Hemicellulose Extraction from EEB and EEBT3
The hemicellulose derived from EEB was obtained through NaOH and KOH solutions in different concentrations (2 M and 5 M) and extraction times (4 h and 8 h). However, the concentration of H2O2 was maintained in 6% (w/w). In these conditions, the xylan content of extracted hemicelluloses varied in the range of 6.4-11.2% (w/w) (data not shown). An additional assay was performed with NaOH 2 M and 12 h reaction time aiming to increase xylan content from extracted hemicelluloses. Table 2 presents the yields and chemical composition of hemicelluloses derived from EEB and EEBPT3 in the abovementioned conditions. The EEBPT3 presented higher hemicellulose extraction yield (62.6%, w/w) compared to EEB (22.8%, w/w). In a recent study on Mahogany (Swietenia sp.) and mango wood (Mangifera sp.) pretreated with NaClO2, the hemicellulose extraction yields were 77.2% and 62.6% (w/w), respectively, which support the yield obtained in EEBPT3 (this study) [19]. The contents of anhydroglucose, xylan, arabinosyl side-group, lignin, and ashes in HEEB, HEEBPT, and CBH were ranked as follows: 1-5.5%, 20.5-58.5%, 2.1-3.2%, 4-4.8%, and 12.6-28.2% (w/w), respectively (Table 2). The chemical composition of HEEB and HEEBPT was also compared to CBH, because of the similarity between EB and Birchwood content using vegetal cell tissue distribution and chemical composition (Table 2) [45]. The sum of HEEB, HEEBPT, and CBH quantified components were 62.2%, 79.5%, and 76.7% (w/w), respectively. Thus, HEEBPT and CBH showed the highest content of xylan (55% and 58.5%, w/w) while HEEB presented xylan and ashes content of 23.7% and 28.2% (w/w), respectively. The differences between hemicelluloses chemical composition are likely related to the delignification process, in which NaClO2 promotes surface, area and porosity increase allowing enhanced access to the hemicellulosic fraction. Nevertheless, hemicellulose extraction from non-chemically pretreated sugarcane bagasse in similar conditions (NaOH 2 M, 4 h extraction length) yielded approximately 86% (w/w) [18, 46]. Even though, HEEB extraction yield was low (22.8% (w/w), it is still comparable to the study which obtained extraction degree of 26.6% (w/w) from wheat straw using H2O2 2% (w/w) and setting solution pH for 11.6 with NaOH for 16-h reaction time [13]. However, hemicellulose extraction yield from HEEB was low (22.8%, w/w), compared to 76.9% (w/w) achieved in the wheat straw extraction, which suggests that EEB extraction process was limited by intrinsic elevated lignin content because of increased extraction resistance. Thus, a delignification process is strongly recommended to decrease hemicellulose extraction resistance [47].
The extraction difficulty (EEB) was assessed by the ratio of lignin to hemicellulose content, as demonstrated in Table 1. As a result, EEBPT3 presented a ratio (lignin/hemicellulose) of 0.9, while EB showed 1.6, which was 1.8-fold higher when compared to EEBPT3. This ratio was directly related to the content of lignin in EB and EEBPT3 (27.2% and 14.2%, (w/w), respectively) because hemicellulose content in EB and EEBPT3 was similar (17.4% and 16.1%, (w/w), respectively) (Table 1). Consequently, lignin content in EB and EEBPT3 confirmed interference in hemicellulose extraction, which implies in need of pretreatment to remove this compound from Eucalyptus biomass.
Figure 1a and b report the aspect of extracted hemicelluloses considering that Fig. 1a presents HEEB, while Fig. 1b refers to HEEBPT. It is noticeable higher bleaching aspect of HEEBPT (Fig. 1b) compared to HEEB (Fig. 1a). The different hemicelluloses correlation occurred because of the higher lignin content in EB compared to EEBPT3.
Evaluation of Commercial Enzymatic Extracts and HPLC Utilization for XOS Analysis
Table 3 shows proteins content and specific activity of total xylanases and β-xylosidases in commercial enzymatic extracts. The extracts Celluclast, Cellulases, and Xylanases presented protein content of 25.4, 40.0, and 4.5 mg mL−1; total xylanases specific activity (27.3, 17.5, and 28.3 UI mg−1), and β-xylosidases (0.2, 0.1, and 0.2 UI mg−1), respectively (Table 3). The total xylanases specific activity were similar to those found in Celluclast and Xylanases, but Cellulases enzymatic extract demonstrated 38% less activity. However, β-xylosidases specific activity exhibited values below 0.2 UI mg−1 in both evaluated extracts in this study, indicating that are suitable for XOS production because the high activity of β-xylosidases would drastically increase xylose generation rate [48].
A chromatographic comparison between standards and products derived from enzymatic hydrolysis of xylan is depicted in Fig. 2a b. Figure 2a presents the typical chromatogram obtained with a BIO-RAD Aminex HPX-87C column in the elution of the following analytical standards of XOS with respective retention time: xylopentaose + xylohexaose (X6 + X5) (peak 1; 7.2 min), xylotetraose (X4) (peak 2; 7.5 min), xylotriose (X3) (peak 3; 8.2 min), xylobiose (X2)), and xylose (X1) (peak 5; 11.4 min). The chromatographic methodology could not perform complete separation of xylopentaose and xylohexaose (Fig. 2a, peak 1); thus xylopentaose and xylohexaose were considered as a mixture and prepared together in the analytical curve. Figure 2b presents the chromatographic profile of products obtained by enzymatic hydrolysis of HEEBPT fraction using Celluclast enzymatic extract for 72 h. According to the retention times depicted in Fig. 2a, it was possible to confirm the presence of X4 (peak 2; 7.5 min), X2 (peak 4; 9.2 min), and X1 (peak 5; 11.4 min). Moreover, there was an indication of XOS containing more than 6 repeat units of xylose (> X6 = XOS, retention time = 6.7 min), which were quantified by the analytical curve of xylopentaose + xylohexaose since there were not commercially available standards for this XOS.
Chromatographic profile of XOS in column BIO-RAD Aminex HPX-87C. a Chromatogram with XOS analytical standards. b Chromatogram of the enzymatic hydrolysis of HEEBPT with Celluclast enzymatic extract after 72 h. (X1) = Xylose, (X2) = Xylobiose, (X3) = Xylotriose, (X4) = Xylotetraose, (X5/X6) = Xylopentaose + Xylohexaose and (> X6) = oligosaccharides with more of six repetitive units. XOS = (>X6) + (X4) + (X3) + (X2) + (X1)
Enzymatic Hydrolysis of By-product Fractions
The enzymatic hydrolysis assays of EEB, EEBPT3, HEEB, HEEBPT, and CBH were realized with 3 different enzymatic extracts, as shown in Table 3. The direct enzymatic hydrolysis over EEB and EEBPT3 presented maximum conversion of xylan into XOS (> X6 + X4 + X2) of 8% and 16% (w/w), respectively (data not shown). In this context, direct enzymatic hydrolysis of EEB and EEBPT3 did not result in satisfactory yields for XOS production because the materials (EEB and EEBPT3) were recalcitrant towards enzymatic hydrolysis of xylan fraction.
Table 1S and Fig. 3abc report the enzymatic hydrolysis results of CBH. The conversion of xylan into > X6 were ranked according to commercial extracts employed during reaction time (3–72 h), as follows: 5.1-15.4% (Celluclast), 3.1-8.2% (Cellulase), and 5.1-12.9% (Xylanases) (w/w). The conversion of xylan into X4 and X2 were also ranked, as follows: 4.9-7.7% and 5.3-14.1% (Celluclast), 6-8.4% and 4.9-13.7% (Cellulase), and 4.16.6% and 5.5-13.5% (Xilanases) (w/w), respectively. The conversions of xylan into XOS were also ranked as follows: 18.3-30% (Celluclast), 16.827.6% (Cellulase), and 16.4-26.1% (Xylanases) (w/w). However, X6/X5 and X3 were not detected along enzymatic hydrolysis (4-72 h).
Conversion xylan to (X2) = Xylobiose, (X3) = Xylotriose, (X4) = Xylotetraose, and (> X6) = oligosaccharides with more of six repetitive units over time by direct hydrolysis enzymatic of commercial hemicellulose. Birchwood (HCM); (a) Celluclast. (b) Cellulase. (c) Xylanases. Eucalyptus by-product extracted and pretreated with sodium chlorite for 180 min (HEEBPT); (d) Celluclast. (e) Cellulase. (f) Xylanases
The concentrations of X1 and XOS obtained as a result of CBH enzymatic hydrolysis are presented in Table 1S. The amount of X1 and XOS were ranked according to commercial extracts along enzymatic hydrolysis (3-72 h), as follows: 0.8-3.4 g L−1 and 2.1-3.5 g L−1 (Celluclast); 0.8-3.3 g L−1, and 2.0-3.2 g L−1 (Cellulase); 0.4-3.4 g L−1 and 1.9-3.1 g L−1 (Xylanases), respectively. The results did not show any significant difference among the 3 enzymatic extracts regarding XOS conversions and concentrations resultant from CBH enzymatic hydrolysis.
A recent study on CBH hydrolyzed with commercial xylanases of Thermomyces lanuginosus was reported [20]. The authors mentioned above demonstrated that enzymatic hydrolysis of CBH (60 h) produced approximately 1.8 mg mL−1 of XOS. The main XOS obtained were xylotriose (0.8 g L−1), xylotetraose (0.4 g L−1), xylopentaose (0.35 g L−1), and xylobiose (0.25 g L−1). The total concentration of XOS derived from CBH culminated in 12.8%, (w/w) of xylan conversion into XOS, supporting our results of average conversions (17%) achieved in the enzymatic hydrolysis of CBH (72 h) using extracts of Celluclast, Xylanases, and Cellulases.
Figure 4 presents the kinetic profiles of CBH enzymatic hydrolysis. In general, the profiles (Fig. 4a–c) depicted an increase in XOS production up to 12 h (approximately 30%, w/w) persisting in this level until 24 h. After this interval, there was a decrease in XOS production. Nevertheless, Fig. 4a also exhibited a continuous increase in xylose generation along 3 to 72 h of catalysis, achieving the highest conversion of 29% (w/w) in both enzymatic extracts evaluated. The conversion of xylan into XOS and xylose has become similar in 48 h of catalysis in the enzymatic extracts assayed. A kinetic study of CBH enzymatic hydrolysis using xylanases from Talaromyces amestolkiae CIB supports our results concerning the hydrolysis of CBH by xylanases derived from commercial extracts Celluclast, Xylanases, and Cellulases [49].
Conversion xylan to XOS (filled triangle) and xylose (empty square) over time by direct hydrolysis enzymatic of commercial hemicellulose from Birchwood (HCM). a Celluclast. b Cellulase. c Xylanases. XOS = (> X6) + (X4) + (X2). (X1) = Xylose, (X2) = Xylobiose, (X3) = Xylotriose, (X4) = Xylotetraose, and (> X6) = oligosaccharides with more of six repetitive units. Average coefficient of variation, calculated from triplicates of XOS for Celluclast, Cellulase, and Xylanases, were 1.2, 1.7, and 1.2%, respectively. Average coefficient of variation, calculated from triplicates of xylose for Celluclast, Cellulase, and Xylanases, were 1.7, 1.6, and 1.4%, respectively
Table 2S presents the results of HEEB enzymatic hydrolysis. In this fraction, the xylan conversions into > X6 were ranked according to the commercial extracts along the length of enzymatic hydrolysis (3-72 h), as follows: 3.7-6.8% (Celluclast), 3.6-5.8% (Cellulase), and 2.2-5.9% (Xylanases) (w/w). The conversions of xylan into X4 and X2 were ranked as follows: 1.8-3.3% and 1.2-4.7% (Celluclast), 1.9-2.9% and 1.2-4.3% (Cellulase), and 1.8-3.5% and 1.1-7.1% (Xylanases) (w/w), respectively. The conversions of xylan into XOS were ranked as 10.1-13.3% (Celluclast), 8.9-11.5% (Cellulase), and 8.8-13.7% (Xylanases) (w/w). However, X6/X5 and X3 were not detected along 3-72 h enzymatic hydrolysis, as observed in Table 2S. The exception occurred for conversion of 0.8 ± 0.1 and 0.4 ± 0.02 (w/w) xylan into X3 in reaction time of 3 and 6 h, respectively, when Celluclast extract was employed.
Table 2S also exhibits the concentrations of X1 and XOS derived from HEEB enzymatic hydrolysis. The concentrations of X1 and XOS were ranked according to the performance of commercial extracts along the enzymatic hydrolysis (3-72 h), as follows: 0.2-0.8 g L−1 and 0.4-0.5 g L−1 (Celluclast); 0.1-0.8 g L−1 and 0.4-0.5 g L−1 (Cellulase); 0.2-0.8 g L−1 and 0.4-0.6 g L−1 (Xylanases), respectively. The highest concentration of XOS was 0.6 g L−1 (t = 48 h), which was obtained using Xylanases enzymatic extract. It is worth mentioning that the highest concentration of X1 was 0.8 g L−1 (72 h) which was found for both extracts (Celluclast, Cellulase, and Xylanases) (Table 2S). The results pointed to the absence of significant differences among the conversions and concentrations of XOS performed by the enzymatic extracts evaluated for HEEB hydrolysis.
Figure 1S depicts the kinetic profile of HEEB enzymatic hydrolysis. According to Figures 1Sabc, there was an increase in XOS production from 3 to 48 h of catalysis, yielding approximately 10% conversion (w/w). However, it was reported a decrease in XOS production after 48 h while xylose concentration was increasing up to 20% (w/w) at 72 h. The conversions of xylan into XOS and xylose became similar at 48 h catalysis for the commercial extracts Celluclast (Figure 1Sa) and Xylanases (Figure 1Sc). The exception occurred to Cellulases because conversions became equivalent in 24 h of catalysis (Figure 1Sb).
According to Table 2S and Figure 1S, the production of XOS by hydrolysis of HEEB was highly limited because, the maximum conversion of xylan into XOS was 13.7% (w/w) using Xylanases extract for 48 h. In contrast, the conversion of CBH into XOS resulted in 29% as the maximum for both enzymatic extracts evaluated (Table 1S, Figs. 3 and 4).
Recently, specialized literature has reported enzymatic hydrolysis of 6 hemicelluloses obtained from sugarcane bagasse, not-chemically pretreated, extracted in different concentrations of KOH (24, 10, and 5% (w/v), reaction times (0.5 and 3 h), and temperatures (35, 70. and 121 °C). In the abovementioned study, the hemicelluloses presented 70% (w/w) as average content of xylan. These hemicelluloses were hydrolyzed with Xylanases from Trichoderma reesei and Aspergillus fumigatus [48]. The authors reported conversions of xylan into XOS after 12 h of enzymatic hydrolysis yields of 16% and 24% (w/w), respectively. The results of our study are supported by those obtained because we achieved 10% of xylan conversion into XOS (20.5% xylan content) after 12 h enzymatic hydrolysis for both extracts evaluated (Table 2S and Figure 1S). The lower conversion obtained in our study is more likely related to xylan content in sugarcane bagasse (70%, w/w) compared to that found in HEEB (20.5%, w/w), which is threefold lower [48].
Table 3S and Fig. 3d e f present the results of HEEBPT enzymatic hydrolysis. In this set, the conversions of xylan into > X6 were ranked according to commercial extracts, hydrolysis time (3-72 h), as follows: 6.1-17.4% (Celluclast), 2.7-9.2% (Cellulase), and 5.8-15.4% (Xylanases) (w/w). The conversions of xylan into X4 and X2 were ranked as follows: 5.5-7.8% and 4.8-14.7% (Celluclast); 6.8-9.2% and 6-15.1% (Cellulase); 5.9-7.3% and 6-14% (Xylanases) (w/w), respectively. The conversions of xylan into XOS were also ranked, as follows: 19-30.8% (Celluclast), 18.8-30.1% (Cellulase), and 19.3-29.6% (Xylanases) (w/w). However, in this assay, X6/X5 and X3 were not detected along the enzymatic hydrolysis (4-72 h).
The amount of X1 and XOS derived from HEEBPT hydrolysis are shown in Table 3S. The concentrations of X1 and XOS were ranked according to the commercial extracts and reaction time (3-72 h), as follows: 0.9-3.3 g L−1 and 2.1-3.4 g L−1 (Celluclast); 0.4-3.7 g L−1 and 2.1-3.3 g L−1 (Cellulase); 0.4-3.4 g L−1 and 2.1-3.3 g L−1 (Xylanases), respectively. The results demonstrated that conversion degree and XOS concentration were similar among the three enzymatic extracts, which resulted in negligible difference for HEEBPT hydrolysis. Moreover, the enzymatic hydrolysis of CBH presented similar results to those obtained for HEEBPT (Tables 1S and 3S).
Similarly, a study was developed aiming at XOS production using hardwood. In the work by Meranti (Shorea) sawdust was pretreated with NaClO2 and submitted to enzymatic hydrolysis (60 h catalysis) by immobilized commercial xylanases from Thermomyces lanuginosus [20]. This enzymatic hydrolysis of xylan extracted from Meranti wood yielded approximately 0.3 mg mL−1 of XOS. The main XOS produced from extracted xylan were xylobiose (0.1 mg mL−1) and xylotriose (0.2 mg mL−1). According to the authors, the total concentration of XOS derived from extracted xylan resulted in 2.1% of XOS conversion. Thus, HEEBPT of our study was 93% more efficient in the production of XOS when compared to XOS generated from xylan of Meranti wood. Another study on xylan enzymatic hydrolysis (Clostridium sp. BOH3 xylanases) using sawdust from Mahogany (Swietenia sp.) and mango woods (Mangifera sp.), pretreated with NaClO2 was recently reported in specialized literature [19]. The authors demonstrated that enzymatic hydrolysis (24 h catalysis) of the abovementioned woods resulted in xylan to XOS conversions of 57.2% and 50.4% (w/w), respectively. The main XOS produced from Mahogany and Mango woods were xylobiose (41.2% and 51.2%), xylotriose (35.5% and 38.7%), xylotetraose (16.2% and 6.9%), and xylopentaose (5.7% and 2%) (w/w) respectively. Given these yields, xylan hydrolysis was more efficient for XOS production compared to that reported in HEEBPT, which is more likely related to the composition of the feedstock.
Figure 5 presents the kinetic profile of HEEBPT enzymatic hydrolysis. According to Fig. 5a–c, there was a steady increase on XOS production up to 12 h catalysis (approximately 30% conversion (w/w)) maintaining this plateau up to 24 h, which resembled the profile of CBH enzymatic hydrolysis (Table 1S; Fig. 4). Further support to the results of HEEB and HEEBPT can be found in the kinetic profiles shown in the study hydrolyzing xylan from corncob powder [50].
Conversion xylan to XOS (Triangle filled) and xylose (Square empty) over time by direct hydrolysis enzymatic of hemicellulose from Eucalyptus by-product extracted and pretreated with sodium chlorite for 180 min (HEEBPT). a Celluclast. b Cellulase. c Xylanases. XOS = (> X6) + (X4) + (X2). (X1) = Xylose, (X2) = Xylobiose, (X3) = Xylotriose, (X4) = Xylotetraose, and (> X6) = oligosaccharides with more of six repetitive units. Average coefficient of variation, calculated from triplicates of XOS for Celluclast, Cellulase, and Xylanases, were 2.1, 2.7, and 3.9%, respectively. Average coefficient of variation, calculated from triplicates of xylose for Celluclast, Cellulase, and Xylanases, were 2.9, 0.7, and 5.2%, respectively
According to Tables 1S, 2S, and 3S and Fig. 3, the production of XOS was more efficient in HEEBPT and CBH compared to HEEB. The XOS produced by CBH was similar to HEEBPT. For instance, xylan conversion in XOS was 30.8% and 29.7% for HEEBPT and CBH (Celluclast; 12 h catalysis), respectively. However, maximum conversions of xylan in XOS in HEEBPT and HEEB were 30.8% and 10.9% (w/w), respectively (Celluclast; 12 h catalysis), as shown in Tables 2S and 3S. Thus, HEEBPT conversion was almost threefold (2.8) higher than HEEB. Moreover, this contrast still increases when comparing HEEB XOS concentration (0.45 g L−1) with HEEBPT (3.39 g L−1), pointing to a value sevenfold (7.5) higher. These various conversion (2.8) and concentration (7.5) ratios of XOS for HEEB and HEEBPT mostly occurred due to xylan content in both substrates. This affirmation relied on a chemical analysis that demonstrated 20.5% (w/w) of xylan in HEEB, while HEEBPT presented 55% (w/w) (Table 2). The xylan content almost threefold (2.7) higher compared to HEEB explained higher XOS amount produced with HEEBPT (Tables 1S, 2S and 3S).
The substrates to product dynamics, along with the enzymatic hydrolysis (Celluclast; Cellulases; Xylanases), pointed to a decrease in xylan to XOS conversion in CBH, HEEB, and HEEBPT in hydrolysis time course regardless of the enzymatic extract evaluated. On the other hand, the conversions of xylan in xylose increased, as observed in Figure 1S and Figs. 4 and 5 (Tables 1S, 2S and 3S). This rise in xylan to xylose conversion during enzymatic hydrolysis is unfavorable to the XOS production process. However, these results are corroborated by literature because similar effects using xylan extracted from sugarcane bagasse, corncob, and hardwood were already reported [19, 20, 36, 50].
Conclusion
The hemicellulose extraction from EEBPT3 was the most efficient (62.6%) when compared to EEB (22.8%). Moreover, HEEBPT and CBH presented higher xylan content (55% and 58.5%) respectively, compared to HEEB (20.5%). The XOS production from HEEBPT and CBH was approximately 2.8 times higher, in terms of conversion than EEB. However, the need for lignin removal from EEB (prior or concomitant to hemicellulose extraction procedures) affects the feasibility of an industrial process because the pretreatment with NaClO2 can generate toxic compounds. In this sense, further studies with alternative reagents, such as ionic liquids, are required to asses selectively lignin removal from EEB in the change of NaClO2.
The XOS production from HEEB can be a biorefining strategy to use the hemicellulosic fraction of EB generated in the cellulosic sector to produce a high value-added product. Also, the insoluble fraction generated in the hemicellulose extraction stage (rich in cellulose) could be used for the glucooligosaccharides and/or nanocellulose production. The sum of these bioproducts (XOS, glucooligosaccharides, and nanocellulose) has a high retail price in the international market. Moreover, the final “residue” of the glucooligosaccharides and/or nanocellulose (lignin-rich-fraction) production could be harnessed for energy in cogeneration.
Abbreviations
- EB:
-
Eucalyptus by-product
- XOS:
-
xylooligosaccharides
- H2O2 :
-
hydrogen peroxide
- EEB:
-
extracted Eucalyptus by-product
- EEBPT:
-
extracted Eucalyptus by-product pretreated
- EEBPT1 :
-
extracted Eucalyptus by-product pretreated for 1 h
- EEBPT2 :
-
extracted Eucalyptus by-product pretreated for 2 h
- EEBPT3 :
-
extracted Eucalyptus by-product pretreated for 3 h
- EEBPT4 :
-
extracted Eucalyptus by-product pretreated for 4 h
- EDTA:
-
tetracyclic ethylenediamine acid
- NaOH:
-
sodium hydroxide
- KOH:
-
potassium hydroxide
- HCl:
-
hydrochloric acid
- NaClO2 :
-
sodium chlorite
- HEEB:
-
hemicellulose from extracted Eucalyptus by-product
- HEEBPT:
-
hemicellulose from extracted Eucalyptus by-product pretreated with NaClO2 for 180 min
- CBH:
-
commercial birchwood hemicellulose
- DNS:
-
3,5-dinitrosalicylic acid
- pNPX:
-
p-nitrophenyl-β-D-xylopyranoside
- HPLC:
-
high-performance liquid chromatograph
- H2SO4 :
-
sulfuric acid
- X1 :
-
xylose
- X2 :
-
xylobiose
- X3 :
-
xylotriose
- X4 :
-
xylotetraose
- X6 + X5 :
-
xylopentaose + xylohexaose
- > X6 :
-
xylooligosaccharides with more than 6 xylose repeated units
References
Magaton, A. S., Veloso, D. P., & Colodette, J. L. (2008). Characterization of O-acetyl-(4-O-methylglucuronic) xylans from Eucalyptus urograndis. Química Nova, 31(5), 1085–1088.
Farinha e Silva, C. A., Bueno, J. M., Neves, M. R. (2017). A INDÚSTRIA DE CELULOSE E PAPEL NO BRASIL. Guia ABTCP (Techical brazilian Association of Celullose and Paper) - Fornecedores e Fabr. 16-28.
Marques, M. L., Silva, E. J., Velasco, F. M., & Junior, C. C. M. F. (2014). Potencialidades do uso de resíduos de celulose (DREGS/GRITS) como agregado em argamassas. Revista Brasileira de Produtos Agroindustriais, 16(4), 423–431.
Cano, A., & Cristina, P. (2007). Xylooligosaccharide recovery from agricultural biomass waste treatment with enzymatic polymeric membranes and characterization of products with MALDI-TOF-MS. Journal of Membrane Science, 291(1-2), 96–105.
Neto, F. S. P. P., Lucca, E. A., & Masarin, F. (2016). Particle size influence on the chemical composition from waste Eucalyptus in the processing for industrial production of cellulose pulp. Chemical Engineering Transactions, 50, 337–342.
Ek, M., Gellerstedt, G,. & Henriksson, G. (2009). Pulp and paper chemistry and technology, vol. 1: Wood Chemistry and Biotechnology (1st ed.). Berlin: Walter de Gruyter GmbH & Co. KG.
Balakshin, M. Y., Capanema, E. A., & Chang, H. (2007). MWL fraction with a high concentration of lignin-carbohydrate linkages: isolation and 2D NMR spectroscopic analysis. Holzforschung., 61(1), 1–7.
Morgante, C. M., Bonturin, N., & Miranda, E. A. (2015). Extração da fração hemicelulósica de cavacos de madeira visando sua aplicação em biorrefinarias. Blucher Chemical Engineering Proceedings, 1, 1009–1014.
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y Y., Holtzapple, M., & Ladish, M. (2005). Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresource Technology, 96, 673–686.
Browning, B. L. (1967). Methods of wood chemistry (1st ed., Vol. 2). New York: Wiley.
Siqueira, G., Várnai, A., Ferraz, A., & Milagres, A. M. F. (2013). Enhancement of cellulose hydrolysis in sugarcane bagasse by the selective removal of lignin with sodium chlorite. Applied Energy, 102, 399–402.
Seok, J., Lee, Y. Y., & Hyun, T. (2016). A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresource Technology, 199, 42–48.
Fang, J. M., Sun, R. C., Salisbury, Fowler, P., & Tomkinson, J. (1999). Comparative study of hemicelluloses from wheat straw by alkali and hydrogen peroxide extractions. Polymer Degradation and Stability, 66, 423–432.
Curreli, N., Fadda, M. B., Rescigno, A., Rinaldi, A. C., Soddu, G., Sollai, F., Vaccarrgiu, S., Sanjust, E., & Rinaldi, A. (1997). Mild alkaline/oxidative pretreatment of wheat straw. Process Biochemistry, 32(8), 665–670.
Sun, H. J., Yoshida, S., Park, N. H., & Kusakabe, I. (2002). Preparation of (1→4)-β-D-xylooligosaccharides from an acid hydrolysate of cotton-seed xylan: suitability of cotton-seed xylan as a starting material for the preparation of (1→4)-β-D-xylooligosaccharides. Carbohydrate Research, 337(7), 657–661.
Yang, B., Boussaid, A., Mansfield, S. D., Gregg, D. J., & Saddler, J. N. (2002). Fast and efficient alkaline peroxide treatment to enhance the enzymatic digestibility of steam-exploded softwood substrates. Biotechnology and Bioengineering, 77(6), 678–684.
Kadla, JF., Chang, H. (2001). The Reactions of Peroxides with Lignin and Lignin Model Compounds, vol. 785: Oxidative delignification Chemistry (American Chemical Society, ed). pp.108–129.
Brienzo, M., Siqueira, A. F., & Milagres, A. M. F. (2009). Search for optimum conditions of sugarcane bagasse hemicellulose extraction. Biochemical Engineering Journal, 46, 194–204.
Rajagopalan, G., Kavitha, S., & Kun-Lin, Y. (2017). Production of prebiotic-xylooligosaccharides from alkali pretreated mahogany and mango wood sawdust by using purified xylanase of Clostridium strain BOH3. Carbohydrate Polymers, 167, 158–166.
Sukri, S. S. M., & Sakinah, A. M. (2018). Production of high commercial value xylooligosaccharides from meranti wood sawdust using immobilised xylanase. Applied Biochemistry and Biotechnology, 184(1), 278–290.
Akpinar, O., Erdogan, K., & Bostanci, S. (2010). Enzymatic production of xylooligosaccharide from selected agricultural wastes. Food and Bioproducts Processing, 87, 45–51.
Figueiredo, F. C., Carvalho, A. F. A., Brienzo, M., Campioni, T. S., & Oliva-Neto, P. (2017). Chemical input reduction in the arabinoxylan and lignocellulose alkaline extraction an xylooligosaccaharides production. Bioresource Technology, 228, 164–170.
Sporck, D., Reinoso, F. A. M., Rencoret, J., Gutiérrez, A., Del Rio, J. C., Ferraz, A., & Milagres, A. M. F. (2017). Xylan extraction from sugarcane bagasse pretreated using alkaline and enzymatic approaches. Biotechnology for Biofuels, 10(1), 296.
Wong, K. K., Tan, L. U., & Saddler, J. N. (1988). Multiplicity of beta-1,4-xylanase in microorganisms: functions and applications. Microbiological Reviews, 52(3), 305–317.
Knob, A., & Carmona, E. C. (2010). Purification and characterization of two extracellular xylanases from Penicillium sclerotiorum: a novel acidophilic xylanase. Applied Biochemistry and Biotechnology, 162(2), 429–443.
Santos, L. F., Freire, L., & Ishii, P. L. (2011). Xilanases: principais metodologias e parâmetros cinéticos. Journal of Biotechnology and Biodiversity, 2, 7–15.
Gibson, G. R., & Roberfroid, M. B. (1995). Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. The Journal of Nutrition, 125(6), 1401–1412.
Juturu, V., & Wu, J. C. (2014). Microbial exo-xylanases: a mini review. Applied Biochemistry and Biotechnology, 174(1), 81–92.
Vázquez, M. J., Alonso, J. L., Domínguez, H., & Parajó, J. C. (2001). Xylooligosaccharides: manufacture and applications. Trends in Food Science and Technology, 11, 387–393.
Álvarez, C., González, A., Negro, M. J., Ballesteros, I., Oliva, J. M., & Sáez, F. (2017). Optimized use of hemicellulose within a biorefinery for processing high value-added xylooligosaccharides. Industrial Crops and Products, 99, 41–48.
Freitas, C., Carmona, E., Brienzo, M. (2019). Xylooligosaccharides production process lignocellulose biomass and bioactives effect. Bioactive Carbohydrates and Dietary Fibre, 18, 100184.
Chen, M., Bowman, M. J., Dien, B. S., Rausch, K. D., Tumbleson, M. E., & Singh, V. (2014). Bioresource technology autohydrolysis of Miscanthus x giganteus for the production of xylooligosaccharides (XOS): kinetics, characterization, and recovery. Bioresource Technology, 155, 359–365.
Ferraz, A., Baeza, J., Rodrigues, J., & Freer, J. (2000). Estimating the chemical composition of biodegraded pine and eucalyptus wood by DRIFT spectroscopy and multivariate analysis. Bioresource Technology, 74, 20–12.
Masarin, F., Gurpilhares, D. B., Baffa, D. C., Barbosa, M. H., Carvalho, W., Ferraz, A., et al. (2011). Chemical composition and enzymatic digestibility of sugarcane clones selected for varied lignin content. Biotechnology for Biofuels, 4(1), 55.
Xu, F., Sun, J. X., Liu, C. F., & Sun, R. C. (2006). Comparative study of alkali- and acidic organic solvent-soluble hemicellulosic polysaccharides from sugarcane bagasse. Carbohydrate Research, 341(2), 253–261.
Brienzo, M., Carvalho, W., & Milagres, A. M. F. (2010). Xylooligosaccharides production from alkali-pretreated sugarcane bagasse using xylanases from Thermoascus aurantiacus. Applied Biochemistry and Biotechnology, 162, 1205–1195.
Bailey, M. J., Biely, P., & Poutanen, K. (1992). Interlaboratory testing of methods for assay of xylanase activity. Journal of Biotechnology, 23(3), 257–270.
Tan, L. U. L., Mayers, P., & Saddler, J. N. (1987). Purification and characterization of a thermostable xylanase from a thermophilic fungus Thermoascus aurantiacus. Canadian Journal of Microbiology, 33, 689–692.
Hartree, E. F. (1972). Determination of protein: a modification of the Lowry method that gives a linear photometric response. Analytical Biochemistry, 48(2), 422–427.
Sluiter, J. B., Ruiz, R. O., Scarlata, C. J., Sluiter, A. D., & Templeton, D. W. (2010). Compositional analysis of lignocellulosic feedstocks. Review and description of methods. Journal of Agricultural and Food Chemistry, 58(16), 9043–9053.
Masarin, F., Cedeno, F. R. P., Chavez, E. G. S., Oliveira, L. E., Gelli, V. C., & Monti, R. (2016). Chemical analysis and biorefinery of red algae Kappaphycus alvarezii for efficient production of glucose from the residue of the carrageenan extraction process. Biotechnology for Biofuels, 9(1), 122.
Roldán, I. U. M., Mitsuhara, A. T., Desajacomo, J. P. M., Oliveira, L. E., Gelli, V. C., Monti, R., Sacramento, L. V. S., & Masarin, F. (2017). Chemical, structural and ultrastructural analysis of waste from the carrageenan and sugar-bioethanol processes for future bioenergy generation. Biomass and Bioenergy, 127, 233–243.
Parajó, J. C., Garrote, G., Cruz, J. M., & Domingues, H. (2004). Production of xylooligosaccharides by autohydrolysis of lignocellulosic materials. Trends in Food Science and Technology, 15(3-4), 115–120.
Vena, P. F., García-Aparicio, M. P., Brienzo, M., Görgens, J. F., & Rypstra, T. (2013). Effect of alkaline hemicellulose extraction on kraft pulp fibers from eucalyptus grandis. Journal of Wood Chemistry and Technology, 33(3), 157–173.
Sun, R. C., Tomkinson, J., Ma, P. L., & Liang, S. F. (2000). Comparative study of hemicelluloses from rice straw by alkali and hydrogen peroxide treatments. Carbohydrate Polymers, 42(2), 111–122.
Ford, E. W., & Publishers, E. S. (1986). Comparative structural studies of lignin-carbohydrate complexes from Digitaria decumbens (Pangola Grass) before and after chlorite delignification. Carbohydrate Research, 47, 101–117.
Converse, A. O., Ooshima, H., & Burns, D. S. (1990). Kinetics of enzymatic hydrolysis of lignocellulosic materials based on surface area of cellulose accessible to enzyme and enzyme adsorption on lignin and cellulose - scientific note. Applied Biochemistry and Biotechnology, 24–25, 67–73.
Figueiredo, F. C., Carvalho, A. F. A., Brienzo, M., Campioni, T. S., & de Oliva-Neto, P. (2017). Chemical input reduction in the arabinoxylan and lignocellulose alkaline extraction and xylooligosaccharides production. Bioresource Technology, 228, 164–170.
Nieto-Domínguez, M., Eugenio, L. I., York-Durán, M. J., Rodríguez-Colinas, B., Plou, F. J., Chenoll, E., Pardo, E., Codoñer, F., & Martínez, M. J. (2017). Prebiotic effect of xylooligosaccharides produced from birchwood xylan by a novel fungal GH11 xylanase. Food Chemistry, 232, 105–113.
Aachary, A. A., & Prapulla, S. G. (2009). Value addition to corncob: production and characterization of xylooligosaccharides from alkali pretreated lignin-saccharide complex using Aspergillus oryzae MTCC 5154. Bioresource Technology, 100(2), 991–995.
Availability of Supporting Data
We provide support if necessary data is needed for publication of the article.
Funding
The Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP (contract number 2018/06241-3) funded this work.
Author information
Authors and Affiliations
Contributions
TDTM and FSPPN performed hemicellulose extraction, chemical analysis, and enzymatic hydrolysis analyses of samples and data interpretation, and reviewed the manuscript. ABN participated in the production and availability of enzymatic extract containing xylanases (Verdartis-Brazil), data interpretation and reviewed the manuscript. RM FM participated in the design of the study and data interpretation and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Statement of Novelty
The originality and new contributions of this paper involve the extraction of hemicellulose and the production of xylooligosaccharides (XOS) from a by-product of Eucalyptus (EB) from the processes of pulp. The EB is currently oxidized to produce steam and/or electric energy, but the material is rich in polysaccharides (cellulose and hemicellulose) which contain low energy efficiency in its oxidation. Thus, the use of the polysaccharide fraction to produce high value-added bioproducts (such as XOS) would be a way of taking advantage of the hemicellulose fraction. The enzymatic hydrolysis of extracted hemicellulose was performed with different enzymatic extracts containing xylanase activities, including xylanases from Bacillus subtilis expressed by Escherichia coli.
Highlights
• A by-product from Eucalyptus was obtained in the pulp industry
• The hemicellulose of the Eucalyptus by-product was extracted
• The chemical composition of the by-product and hemicellulose were determined
• The extracted hemicellulose was enzymatically hydrolyzed and converted to xylooligosaccharides
Electronic Supplementary Material
ESM 1
(DOCX 110 kb)
Rights and permissions
About this article
Cite this article
Mafei, T.D.T., Neto, F.S.P.P., Peixoto, G. et al. Extraction and Characterization of Hemicellulose from Eucalyptus By-product: Assessment of Enzymatic Hydrolysis to Produce Xylooligosaccharides. Appl Biochem Biotechnol 190, 197–217 (2020). https://doi.org/10.1007/s12010-019-03076-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03076-0