Abstract
Xylanases from the pathogen fungus Chrysoporthe cubensis were produced under solid state fermentation (SSF) using wheat bran as carbon source. The enzymatic extracts were submitted to ion exchange (Q Sepharose) and gel filtration chromatography methods (Sephadex S-200) for purification. The xylanases were divided into three groups: P1 showed better performance at 60 °C and pH 4.0, P2 at 55 °C and pH 3.0, and P3 at 80 °C and pH 3.0. Oat spelt xylan was the best substrate hydrolyzed by P1 and P3, while beechwood xylan was better degraded by P2. Carboxymethyl cellulose (CMC) and p-nitrophenyl-β-d-xylopyranoside (p-NPβXyl) were not hydrolyzed by any of the xylanases. The K M ’ or K M values, using oat spelt xylan as substrate, were 2.65 mg/mL for P1, 1.81 mg/mL for P2, and 1.18 mg/mL for P3. Xylobiose and xylotriose were the main xylooligosaccharides of oat spelt xylan degradation, indicating that the xylanases act as endo-β-1,4-xylanases. Xylanases also proved to be efficient for hydrolysis of sugarcane bagasse when used as supplement of a commercial cocktail due to the increase of the reducing sugar release.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic biomass is the world’s most abundant natural source of carbohydrates, consisting mainly of cellulose, hemicellulose, and lignin [1]. However, the difficulty of access to the plant cell wall by the hydrolytic enzymes results in low degradation of the polysaccharides and their subsequent conversion into fermentable sugars. The cellulose and hemicellulose represent around 40–50 and 20–30% of the plants’ dry weight, respectively, and they constitute the polysaccharides which can be hydrolyzed to smaller sugars that will be fermented to produce ethanol. Ethanol produced from lignocellulosic biomass is especially important for decreasing dependence on fossil fuels [2, 3]. Xylanases (EC 3.2.1.8) are glycosyl hydrolases that catalyze hydrolysis of the β-1,4-xylosidic linkages from xylan backbone, generating soluble sugars [4, 5]. Most xylanases belong to two main families: GH10 and GH11. Family 10 is characterized by xylanases that show a larger structure, is more complex, with an (β/α)8 (TIM) barrel topology, and in some cases, is highly glycosylated. Family 11 is constituted by smaller (<30-kDa) xylanases, which are more structurally consistent, with a jellyroll topology, no glycosylation, and more specificity for xylan [6–9]. Xylanases have been used for biotechnological purposes in food and biofuel industries [8, 10, 11], for production of xylooligosaccharides and fermentable sugars, as prebiotics, and for bioethanol generation [12, 13].
Filamentous fungi, bacteria, and some yeasts are the main producers of xylanases [14]. Fungi of the genus Penicillium, Aspergillus, and Trichoderma are known to secrete hydrolytic enzymes of commercial interest, including xylanases [15]. Published studies by our research group revealed that the phytopathogen fungus Chrysoporthe cubensis secretes a variety of hydrolytic enzymes, especially xylanases, which show high activity and thermostability [16]. Furthermore, it was observed that cellulases and hemicellulases secreted by the fungus C. cubensis are more efficient in saccharification of sugarcane bagasse than the commercial cocktail Multifect® CL [16].
In the present study, we demonstrate high-level xylanolytic enzyme production by the pathogen fungus of C. cubensis LPF-1. The xylanases were produced, purified, characterized, and tested for their applicability in enzymatic saccharification to generate useful products.
Materials and Methods
Culture Conditions and Xylanase Production by Chrysoporthe cubensis
The fungus C. cubensis used in this study was maintained in the culture collection of the Department of Plant Pathology, BIOAGRO, Federal University of Viçosa, Brazil.
The inoculum was prepared by growing the fungus under submerged fermentation in 250-mL Erlenmeyer flasks containing 100 mL of mineral medium with the following composition, in grams per liter: glucose, 10.0; NH4NO3, 1.0; KH2PO4, 1.0; MgSO4, 0.5; and yeast extract, 2.0. Each flask was inoculated with 10 agar plugs cut out of a 5-day-old colony of C. cubensis grown on PDA plates and incubated in a rotary shaker for 5 days, at 150 rpm and 28 °C. The culture obtained was aseptically homogenized with Polytron® and immediately used to inoculate the solid culture media.
For enzyme production via solid state fermentation (SSF), 250-mL Erlenmeyer flasks were used, containing the following, in grams per liter, NH4NO3, 1.0; KH2PO4, 1.5; MgSO4, 0.5; CuSO4, 0.25; and extract yeast, 2.0, and the following trace elements, in milligrams per liter: MnCl2, 0.1; H3BO4, 0.75; Na2MoO4, 0.02; FeCl3, 1.0; and ZnSO4, 3.5. Wheat bran was used as the carbon source, maintaining the final moisture of 60%. The flasks were autoclaved at 120 °C for 20 min. Then, 8 mL of inoculum (containing 1.5 × 107 spores/mL) was added to 37.5 g of wet mixing and maintained at 28 °C for 7 days. Enzymes secreted during SSF were extracted with 50 mM sodium acetate buffer, pH 5, at a ratio of 10:1 (buffer/dry substrate), under agitation of 150 rpm for 60 min. The mixture was filtered and centrifuged at 15,000×g for 10 min at 4 °C. The supernatant was stored at −20 °C for subsequent enzymatic analysis.
Purification of Xylanases
Fast protein liquid chromatography (FPLC) (ÄKTA system, GE Healthcare Life Sciences, Uppsala, Sweden) was used for xylanase purification.
The enzymatic extract was loaded onto a Q Sepharose anion exchange column (5.0 × 5.0 cm, GE Healthcare), equilibrated with 50 mM sodium acetate buffer, pH 5. Elution was performed at the flow rate of 4 mL/min, with a linear gradient formed with 40 mL of 50 mM sodium acetate buffer and 40 mL of the same buffer containing 1 M NaCl. Fractions containing xylanase activity were pooled and subjected to a Sephacryl S-200 gel filtration column (16/60, GE Healthcare) equilibrated with 25 mM sodium acetate buffer, pH 5. The proteins were eluted with 130 mL of the same buffer at a flow rate of 1 mL/min. The active fractions were pooled for posterior analysis.
Zymogram and SDS-PAGE
Zymogram was performed in SDS-PAGE, according to the method described by Laemmli [17], with some modifications and using the Mini-PROTEAN II system (Bio-Rad). Ten microliters of sample was applied in a 10% (w/v) polyacrylamide gel containing 0.2% of birchwood xylan as substrate. The visualization of the enzymes was performed according to the method described by Falkoski et al. [16].
SDS-PAGE was performed using a 12% (w/v) polyacrylamide gel with a 5% stacking gel, according Laemmli [17], and gel was silver stained.
Protein Determination
Protein concentration was determined by the Coomassie Blue binding method using bovine serum albumin (Sigma) as the standard [18].
Enzymatic Assays
All enzymatic assays were carried out in 100 mM sodium acetate buffer, pH 5, at 50 °C, and in triplicate. Xylanase activity was determined using xylan from birchwood (1.25% w/v final concentration) as substrate. The enzymatic reaction was initiated by the addition of 100 μL of the appropriately diluted enzyme solution to 400 μL of xylan diluted in buffer. The reaction was incubated for 15 min, stopped by addition of 0.5 mL of 3,5-dinitrosalicylic acid (Sigma), and immediately boiled for 5 min [19]. The absorbance was measured at 540 nm, in a Thermo Scientific Multiskan GO (Waltham, MA, USA) spectrophotometer, to determine the release of reducing sugars. Xylose (Sigma) was used as the standard. One unit of xylanase activity (U) was defined as the amount of enzyme that releases 1 μmol of reducing sugar per minute, under the assay conditions.
The endoglucanase activity was determined as described before, except that carboxymethyl cellulose (CMC—at 1% w/v final concentration) was used as substrate.
The β-xylosidase activity was determined using p-nitrophenyl-β-d-xylopyranoside (p-NPβXyl) as substrate. The mixture contained 125 μL of the synthetic substrate solution (4 mM final concentration) and 375 μL of diluted enzyme solution. The reaction was incubated for 15 min at 50 °C and stopped by addition of 0.5 mL sodium carbonate solution (0.5 M). Absorbance was measured at 410 nm, and the amount of pNP released was calculated according to a standard curve. One unit of enzyme activity (U) was defined as the amount of enzyme that releases 1 μmol pNP per minute under the assay conditions.
Enzyme Characterization
To determine the optima pH and temperature profiles, the enzymatic reactions were carried out at different pH values (from 2.0 to 8.0) using McIlvaine’s buffer system [20] and various temperatures (from 20 to 90 °C), respectively. For thermostability evaluation, the enzymes were incubated at 50, 60, 70, and 80 °C, and aliquots were taken at determined intervals to have their residual activity measured. For pH stability evaluation, the enzymes were preincubated in McIlvaine’s buffer for 30 min at 5 °C, and their residual activity was measured at pH 4.
Substrate specificity was determined with synthetic and natural substrates: 1.25% (w/v) birchwood, beechwood, and oat spelt xylan (Sigma), 1.0% (w/v) CMC (Sigma), and 4 mM p-NPβXyl (Sigma).
The kinetic parameter K M (or K M ’, for apparent K M) was determined by varying the substrate concentration and oat spelt xylan and measuring the rates of the reactions. The calculations were performed using the software SigmaPlot version 10.0.
Synergism Between Xylanases
To investigate the presence of synergy between the xylanases of the fungus C. cubensis, the theoretical activities were calculated based on the following equation: (% enzyme1 × enzyme activity1) + (% enzyme2 × enzyme activity2). This theoretical value was compared to the actual measured activity, and synergism was expressed as a percentage of theoretical activity. The same amount of enzyme in units per milliliter was ensured.
Compositional Analysis of Hydrolytic Products
The composition of hydrolytic products from 2% (w/v) oat spelt xylan degradation by the xylanolytic enzymes of C. cubensis was analyzed on a silica gel plate (Sigma-Aldrich) by thin-layer chromatography (TLC).
The reaction mixtures contained 150 μL of purified enzyme and 300 μL of 2% (w/v) oat spelt xylan suspension prepared in 0.1 M sodium acetate buffer, pH 4, in the ratio 2:1 (xylan suspension/enzyme sample), wherein control contained only buffer and xylan suspension. The mixtures were incubated at 50 °C for 12 h, and 25-μL aliquots of the hydrolytic products were spotted onto the TLC plate. The TLC was developed with n-propanol/acetic acid/water in the ratio 1:1:0.1 (v/v), and the plate was sprayed with a staining solution of 1% (w/v) α-naphthol and 10% (v/v) phosphoric acid in absolute ethanol. The plate was heated at 120 °C in an oven for 10 min. Xylose, xylotriose, and xylotetraose (Megazyme, Bray, Co. Wicklow, Ireland) were used as standards.
Biomass Saccharification
Sugarcane bagasse was obtained from the Jatiboca mill, Urucânia, MG, Brazil, and submitted to alkaline pretreatment according to the method described by Falkoski et al. [16].
The enzymatic saccharification of alkali-pretreated sugarcane bagasse was performed in 125-mL Erlenmeyer flasks at an initial solid concentration of 2% dry matter (w/v) in 15 mL of 50 mM sodium acetate buffer, pH 5. Enzyme loading was specified as 10 FPase units of the commercial mixture Multifect® CL (Genencor International Inc., Rochester, NY, USA) per gram of biomass. Sodium azide (10 mM) and tetracycline (40 mg/mL) were added to inhibit microbial contamination. Four treatments were tested: one saccharification experiment with the commercial cocktail Multifect® CL, as a control, and three saccharification experiments performed with the same commercial cocktail supplemented with three different xylanases from C. cubensis, separately. Ten units per milliliters of each purified xylanase was added for the supplementation. The reactions were carried out in a shaker at 250 rpm and 50 °C for 72 h. Samples (1.0 mL) were taken from the reaction mixture at different time intervals and centrifuged at 10,000×g for 5 min. The supernatant was immediately frozen for tests. Analysis of hydrolysis efficiency was determined by the measurement of reducing sugars, as described by Miller [19], and glucose concentration was determined by a commercial kit (Doles, Brazil), in which the principle is based on the glucose oxidase reaction.
Statistical Analysis of Data
The values of xylanase activities on different substrates were analyzed using OriginPro 7.0 software, performing analysis of variance (ANOVA) followed by Tukey’s test at a significance level of 5% (α = 0.05). The standard deviation was also calculated for all assays.
Results and Discussion
Xylanase Production and Purification
The fungus C. cubensis LPF-1 grown under solid state fermentation (SSF) using wheat bran as carbon source exhibited xylanase activity of 360.0 U/g when cultivated during 7 days. Wheat bran has been shown to be a good source for inducing xylanases when compared to other carbon sources, such as kraft pulp and corn cobs, since it contains high levels of hemicellulose in its composition [16]. Wheat bran also shows up as a good carbon source for SSF when compared to submerged fermentation (SmF), to maintain a balance in the carbon/nitrogen (C/N) ratio suitable for SSF [16].
Results of C. cubensis LPF-1 xylanase purification show that the enzymatic extract subjected to ion exchange chromatography resulted in the separation of three protein fractions with xylanase activity, which were called P1, P2, and P3. P1 was eluted before the NaCl gradient; P2 and P3 were eluted with 0.3 and 0.4 M NaCl, respectively. The Q Sepharose column is an anion exchanger; therefore, P1 exhibits a cationic character while P2 and P3 present an anionic character (Fig. 1a).
a Elution profile of the proteins (filled circles) and xylanase activity (open circles) produced by Chrysoporthe cubensis in Q Sepharose. The column was eluted with NaCl salt gradient (dashed lines). b Elution profile of the proteins (filled circles) and xylanase activity (open circles) produced by Chrysoporthe cubensis in Sephacryl S-200 gel filtration column (16/60)
The ion exchange chromatography promoted considerable specific activity enrichment of P1 (data not shown). The P2 and P3 fractions were submitted to gel filtration chromatography (Fig. 1b), which yielded a single active peak for each enzyme. This procedure resulted in a purification factor of 1.2 and 1.4 with 13.4 and 1.3% recovery of the original xylanase activity, respectively, for P2 and P3. Fractions with activity were pooled and stored at −20 °C for enzyme characterization. The ion exchange chromatography partially purified P1 enzymes and separated them from P2 and P3. The use of ion exchange chromatography has been described by several authors [21–23] for xylanase purification, also reported to be efficient when compared to other chromatography methods. The ion exchange chromatography in the Resource Q column followed by gel filtration chromatography in the Superdex™200 HR 10/30 column was reported to be useful by Chi et al. [24] for purification of the xylanase from Bacillus sp. MX47, getting a purification factor of 29.8 and 6.8% recovery of the original xylanase activity.
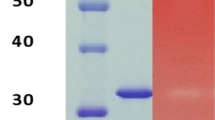
Figure 2a shows the zymogram analysis which reveals the presence of four xylanase bands on gel and confirms that the four isoforms were separated into three different peaks of activity after ion exchange chromatography. The xylanase molecular masses were already obtained by Falkoski et al. [16], being the enzymes present in P1 with 53.2 and 47.9 kDa, P2 xylanase with 19.2 kDa, and P3 xylanase with 37.3 kDa; this last value was not confirmed by this study (Fig. 2b). It is observed that P3 has a molecular mass of approximately 60 kDa, but it should be considered that Falkoski et al. [16] calculated the masses from the crude extract and under different conditions concerning denaturing agents. Figure 2b also shows that P2 has the predicted molecular mass and it was not possible to purify P1.
Enzyme Characterization
The optima pH values presented by the enzymes were 4.0 for P1 and 3.0 for P2 and P3 (Fig. 3a). Activities higher than 60% were found for P1 in the pH values comprising between 3.0 and 6.0; for P2 and P3, high activities were found in the pH values from 2.0 to 4.5. Optima pH values close to those found for xylanases from C. cubensis were reported for the xylanase from Penicillium glabrum, which showed maximum activity when incubated at pH 3.0 [25], and for the xylanase from Penicillium expansum which presented optimum activity at pH 5.5 [22]. Xylanases with higher performance at acid pH values are widely used in biotechnological processes such as production of animal feed and clarification and maceration of juices and wines, as well as in the food and bioenergy industries [25, 26], which indicates that the xylanases purified from the crude extract of C. cubensis could be used for these purposes.
The analysis of pH stability revealed that the xylanases from C. cubensis were able to regain their native conformation, even at pH values distant from the optimum, displaying more than 80% of their activity after incubation for 30 min in a pH range of 2.0–8.0 (Fig. 3b–d). These results show that C. cubensis xylanases are highly stable in a wide pH range.
The xylanases from C. cubensis achieved maximal substrate hydrolysis at 60 °C for P1, 55 °C for P2, and 80 °C for P3 (Fig. 4a). P1, P2, and P3 maintained up to 80% of the initial activity between 50 and 70, 35 and 60, and 65 and 80 °C, respectively. Optimum temperature values close to those found for the xylanases from C. cubensis were described for the purified xylanase from Fomitopsis pinicola, which showed optimal activity at 70 °C [27], and the xylanase from Paenibacillus sp. NF1 which presented 60 °C as optimum temperature [26].
Figure 4b, c shows that the purified P2 xylanase maintained approximately 66% of its original activity after 12 h of preincubation at 50 °C and 50% after 24 h of incubation at the same temperature. This indicates that P2 is therefore more stable than P1 at this temperature. The half-lives of P1 and P2 xylanases at 50 °C were 2.7 and 17.2 h, respectively. At 60 and 70 °C, P1 and P2 xylanases exhibited little or no activity, indicating that these xylanases presented greater structural instability when exposed to high temperatures. P3 xylanase maintained approximately 62% of its original activity after 6 h of preincubation and approximately 32% after 12 h at 70 °C. At 60 °C, the P3 enzyme lost only 30% of its activity after 36 h of preincubation. At 80 °C, this enzyme was unstable (Fig. 4d). The half-life of P3 xylanase at 70 °C was 6.4 h. In general, we could assume that the xylanases from the fungus C. cubensis are thermostable enzymes and they can be applied for industrial processes requiring temperatures between 50 and 70 °C, such as food and biofuel industries.
Activities of the xylanases against several substrates are shown in Fig. 5. The purified xylanases were able to hydrolyze birchwood, beechwood, and oat spelt xylans (Fig. 5a), and all soluble substrates. For P1 and P3, the best activities were found against oat spelt xylan. For P2, the beechwood xylan was better degraded. Xylanases were unable to hydrolyze p-NPβXyl and CMC substrates (Fig. 5b); thus, these enzymes do not have β-xylosidase and endoglucanase activities. Similar results were observed by Kamble and Jadhav [23] in their studies about the purification of a Bacillus sp. xylanase produced by SSF.
Hydrolysis of several substrates with xylanases from Chrysoporthe cubensis. a Natural substrates and b synthetic substrates. Analysis of variance (ANOVA) followed by Tukey’s test at a significance level of 5% (α = 0.05). Asterisk, the treatment differs from the control (CE) and from the other treatments. Double asterisks, the treatment differs from the control (CE) but not from the other treatments
The K M and K M ’ values were determined by the Michaelis-Menten curve using oat spelt xylan as substrate. The K M ’ value was 2.65 mg mL−1 for P1, and K M values were 1.81 and 1.18 mg mL−1 for P2 and P3, respectively. Therefore, K M or K M ’ values found for the xylanases from C. cubensis are in accordance with the values described in the literature which are 1.55 [28], 3.49, and 2.1 mg mL−1 [29], all using oat spelt xylan as substrate.
Synergism Between Xylanases
Since C. cubensis presents a complex xylanolytic system, the ability of each xylanase fraction to promote a synergistic effect on each other was evaluated. However, no synergistic effect was observed between these enzymes (data not shown). The observed activities corresponded to 80% of the theoretical values for all tested possibilities. This fact suggests that the xylanases could present the same mechanism for the hydrolysis of xylan, which was later confirmed by TLC analysis (Fig. 6).
The synergistic action of enzymes is described in literature mainly for enzymes involved in the hydrolysis of the main chain of xylan, while debranched enzymes promote a more efficient hydrolysis of hemicellulose [30, 31]. Visser et al. described a blend of enzymatic extracts with complementing activities to obtain a more ample enzyme hydrolysis spectrum [32]. C. cubensis and Penicillium pinophilum extracts were able to improve the hydrolysis of sugarcane bagasse, especially when the ratio 50:50 was used. The synergistic effect observed was mainly due to the action of the enzymes endoglucanase and xylanase, for degradation of hemicellulose fractions, which facilitates cellulose hydrolysis.
Compositional Analysis of Hydrolytic Products
TLC analysis showed that the main hydrolysis products are composed by xylose, xylobiose, and xylotriose (Fig. 6), suggesting that C. cubensis has a great potential to produce fermentable sugars and xylooligosaccharides. As expected, the control was not hydrolyzed. A similar profile of xylooligosaccharides was reported for the xylanase from Geobacillus thermodenitrificans TSAA1 [33]. The presence of xylooligosaccharides indicates hydrolysis of internal linkages of the xylan backbone, with purified enzymes classified as endo-β-1.4-xylanases. The lower xylooligosaccharides generated from xylan hydrolysis are advantageous for application in the prebiotics industry, an emerging beneficial system for human intestinal health, and the xylooligosacharides may increase with a higher hydrolysis time [33, 34].
Biomass Saccharification
Sugarcane bagasse was selected for saccharification since it is considered the main feedstock produced in Brazil. Moreover, it is a rich source of fermentable sugars [35]. When using alkaline pretreatment, lignin is preferably removed, which facilitates the access of cellulose and hemicellulose, optimizes the process of biomass saccharification, and minimizes unproductive adsorption of enzymes on lignin [16, 36].
The addition of each xylanase fraction as supplement on the saccharification processes showed to be more efficient (Fig. 7). As the commercial cocktail Multifect® CL is composed mainly of cellulases, the supplemented xylanases helped to promote hydrolysis of the hemicellulose fraction and, consequently, there was an increase in the concentration of reducing sugars. Glucose concentration did not differ between the treatments which was already expected (data not shown). The results suggest that the xylanases from C. cubensis can be used in biomass conversion processes to be applied in second-generation ethanol production.
Conclusion
Xylanases from C. cubensis LPF-1 were produced, partially purified (P1), or purified (P2 and P3) and characterized. These enzymes present promise for industrial applications, mainly food and biofuel industries. The results indicate that the xylanases may be used for generation of xylooligosaccharides and fermentable sugars from abundantly available agro-residues. Therefore, the xylanolytic genes may be screened to construct genetically engineered strains aiming for better results.
References
Bon, E. P. S., Ferrara, M. A., & Corvo, M. L. (2008). Enzimas em biotecnologia: produção, aplicações e mercado. Rio de Janeiro: Interciência: UFRJ: CAPES: FAPERJ: FCT (Portugal).
Sangkharak, K., Vangsirikul, P., & Janthachat, S. (2011). Isolation of novel cellulase from agricultural soil and application for ethanol production. International Journal of Advanced Biotechnology and Research, 2, 230–239.
Zhang, J. H., et al. (2011). Comparison of the synergistic action of two thermostable xylanases from GH families 10 and 11 with thermostable cellulases in lignocellulose hydrolysis. Bioresource Technology, 102, 9090–9095.
Motta, F., Andrade, C., Santana, M. (2013). A review of xylanase production by the fermentation of xylan: classification, characterization and applications. In: Chandel, A. K. E Silva, S. S. (Eds). Sustainable degradation of lignocellulosic biomass—techniques, applications and commercialization: InTech, cap. 10.
Polizeli, M. L. T. M., Rizzatti, A. C. S., Montir, R., Terenzi, H. F., Jorge, J. A., & Amorim, D. S. (2005). Xylanases from fungi: properties and industrial applications. Applied Microbiology and Biotechnology, 67(5), 577–591.
Biely, P., Vrsanska, M., Tenkanen, M., & Kluepfel, D. (1997). Endo-b-1,4-xylanase families: differences in catalytic properties. Journal of Biotechnology, 151–166.
Kulkarni, N., Shendye, A., & Rao, M. (1999). Molecular and biotechnological aspects of xylanases. FEMS Microbiology Reviews, 23, 411–456.
Subramaniyan, S., & Prema, P. (2002). Biotechnology of microbial xylanases: enzymology, molecular biology, and application. Critical Reviews in Biotechnology, 22, 33–64.
Dodd, D., & Cann, I. K. (2009). Enzymatic deconstruction of xylan for biofuel production. Global Change Biology Bioenergy, 18, 2–17.
Butt, M. S., Tahir-Nadeem, M., Ahmad, Z., & Sultan, M. T. (2008). Xylanases in baking industry. Food Technology and Biotechnology, 46(1), 22–31.
Huang, J., Chen, D., Wei, Y., Wang, Q., Li, Z., Chen, Y., Huang, R. (2014). Direct ethanol production from lignocellulosic sugars and sugarcane bagasse by a recombinant Trichoderma reesei strain HJ48. The Scientific World Journal, ID. 798683.
Buckeridge, M. S., et al. (2012). Ethanol from sugarcane in Brazil: a ‘midway’ strategy for increasing ethanol production while maximizing environmental benefits. Global Change Biology Bioenergy, 4, 119–126.
Lafond, M., Tauzin, A., Desseaux, V., Bonnin, E., Ajandouz, H., & Giardina, T. (2011). GH10 xylanase D from Penicillium funiculosum: biochemical studies and xylooligosaccharide production. Microbial Cell Factories, 10, 20.
Fitzpatrick, M., Champagne, P., Cunningham, M. F., & Whitney, R. E. (2010). A biorefinery processing perspective: treatment of lignocellulosic materials for the production of value-added products. Bioresource Technology, 101, 8915–8923.
Van Den Brink, J., Maitan-Alfenas, G. P., Zou, G., Wang, C., Zhou, Z., Guimarães, V. M., & de Vries, R. P. (2014). Synergistic effect of Aspergillus niger and Trichoderma reesei enzyme sets on the saccharification of wheat straw and sugarcane bagasse. Biotechnology Journal, 9, 1329–1338.
Falkoski, D. L., Guimarães, V. M., de Almeida, M. N., Alfenas, A. C., Colodette, J. L., & de Rezende, S. T. (2013). Chrysoporthe cubensis: a new source of cellulases and hemicellulases to application in biomass saccharification processes. Bioresource Technology, 130, 296–305.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Bradford, M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Miller, G. L. (1959). Use of dinitrosalicycilic acid reagent for determianiton of reducing sugars. Analytica Chemistry, 31, 426–430.
Mcllvaine, T. C. (1921). A buffer solution for colorimetric comparison. Journal of Biological Biochemistry, 49, 183–186.
Monti, R., Cardello, L., Custódio, M. F., Goulart, A. J., Sayama, A. H., & Contiero, J. (2003). Production and purification of an endo-1,4-b-xylanase from Humicola grisea var. thermoidea by electroelution. Brazilian Journal of Microbiology, 34(2), 124–128.
Querido, A. L. S., Coelho, J. L. C., Araujo, E. F., & Chaves-Alves, V. M. (2006). Partial purification and characterization of xylanase produced by Penicillium expansum. Brazilian Archives of Biology and Technology, 49, 474–480.
Kamble, R. D., Jadhav, A. R. (2012). Isolation, purification, and characterization of xylanase produced by a new species of Bacillus in solid state fermentation. International Journal of Microbiology, ID 683193.
Chi, W. J., Park, D. Y., Chang, Y. K., & Hong, S. K. (2012). A novel alkaliphilic xylanase from the newly isolated mesophilic Bacillus sp. mx47: production, purification, and characterization. Applied Biochemistry and Biotechnology, 168(4), 899–909.
Knob, A., Beitel, S.M., Fortkamp, D., Terrasan, C.R.F., de Almeida, A.F. (2013). Production, purification, and characterization of a major Penicillium glabrum xylanase using brewer’s spent grain as substrate. BioMed Research International, ID 728735.
Zheng, H. C., Sun, M. Z., Meng, L. C., Pei, H. S., Zhang, X. Q., Yan, Z., Zeng, W. H., Zhang, J. S., Hu, J. R., Lu, F. P., & Sun, J. S. (2014). Purification and characterization of a thermostable xylanase from Paenibacillus sp. NF1 and its application in xylooligosaccharides production. Journal of Microbiology and Biotechnology, 24(4), 489–496.
Shin, K., Jeya, M., Lee, J., & Kim, Y. (2010). Purification and characterization of a thermostable xylanase from Fomitopsis pinicola. Journal of Microbiology and Biotechnology, 20(10), 1415–1423.
Taibi, Z., Saoudi, B., Boudelaa, M., Trigui, H., Belghith, H., Gargouri, A., & Ladjama, A. (2012). Purification and biochemical characterization of a highly thermostable xylanase from Actinomadura sp. strain cpt20 isolated from poultry compost. Applied Biochemistry and Biotechnology, 166(3), 663–679.
Vikramathithan, J., Ravikumar, S., Muthuraman, P., Nirmalkumar, G., Shayamala, S., & Srikumar, K. (2012). Purification and biochemical characterization of two major and thermophilic xylanase isoforms (T70 and T90) from xerophytic Opuntia vulgaris plant spp. Cellulose, 19, 1373–1383.
Gonçalves, T. A., Damásio, A. R., Segato, F., Alvarez, T. M., Bragatto, J., Brenelli, L. B., Citadini, A. P., Murakami, M. T., Ruller, R., Paes Leme, A. F., Prade, R. A., & Squina, F. M. (2012). Functional characterization and synergic action of fungal xylanase and arabinofuranosidase for production of xylooligosaccharides. Bioresource Technology, 119, 293–299.
Wongwisansri, S., Promdonkoy, P., Matetaviparee, P., Roongsawang, N., Eurwilaichitr, L., & Tanapongpipat, S. (2013). High-level production of thermotolerant β-xylosidase of Aspergillus sp. BCC125 in Pichia pastoris: characterization and its application in ethanol production. Bioresource Technology, 132, 410–413.
Visser, E. M., Falkoski, D. L., de Almeida, M. N., Maitan-Alfenas, G. P., & Guimarães, V. M. (2013). Production and application of an enzyme blend from Chrysoporthe cubensis and Penicillium pinophilum with potential for hydrolysis of sugarcane bagasse. Bioresource Technology, 144, 587–594.
Verma, D., Anand, A., & Satyanarayana, T. (2013). Thermostable and alkalistable endoxylanase of the extremely thermophilic bacterium Geobacillus thermodenitrificans TSAA1: cloning, expression, characteristics and its applicability in generating xylooligosaccharides and fermentable sugars. Applied Biochemistry and Biotechnology, 170, 119–130.
Wu, Q., Li, Y., Li, Y., Gao, S., Wang, M., Zhang, T., & Chen, J. (2013). Identification of a novel fungus, Leptosphaerulina chartarum SJTU 59 and characterization of its xylanolytic enzymes. PloS One, 8(9), e73729.
Goldemberg, J. (2007). Ethanol for a sustainable energy future. Scienc, 315, 808–810.
Ladisch, M., Mosier, N. S., Kim, Y., Ximenes, E., & Hogsett, D. (2010). Converting cellulose to biofuels. Biofuels, 106(3), 56–63.
Acknowledgments
We thank the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the financial support and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for providing scholarships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Sousa Gomes, K., Maitan-Alfenas, G.P., de Andrade, L.G.A. et al. Purification and Characterization of Xylanases from the Fungus Chrysoporthe cubensis for Production of Xylooligosaccharides and Fermentable Sugars. Appl Biochem Biotechnol 182, 818–830 (2017). https://doi.org/10.1007/s12010-016-2364-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2364-5