Abstract
Light oil from pyrolysis, which accounts for ∼10 % carbon yield of the starting biomass, is a complex aqueous product that is difficult to utilize and usually discarded. This work presents the feasibility of light oil as a sole carbon source to support the growth of Rhodococcus opacus (R. opacus) that in turn accumulate triacylglycerols as biodiesel feedstock. Two types of bacteria (R. opacus PD630 and DSM 1069) were selected in this study. Research results showed that after short adaption periods both strains can grow well on this complex carbon source, as proved by the consumption of oligomers and monomers in light oil. Lipid content by R. opacus PD630 and DSM 1069 was observed up to 25.8 % and 22.0 % of cell dry weight, respectively. Palmitic and stearic acids were found to be the predominant fatty acids in these bacterial cells. In addition, the light oil-based lipid production can be enhanced by reducing the pH value from 7 to 4, especially in case of DSM 1069.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Current petroleum resources are predicted to be sufficient only for the next 50 years given the projected economic and population growth; consequently, research on renewable fuels such as biodiesel has attracted considerable interest by society [1]. Biodiesel is mainly obtained from plant oils, animal fats, and algal oils via transesterification with a primary alcohol (e.g., methanol) in the presence of a catalyst leading to fatty acid methyl esters (FAMEs) [2–5]. This method usually suffers from the high cost of raw materials which generally accounts for around 60–75 % of operational expenses [1]. Therefore, biorefinery research has focused on new approaches to solve this problem, and recently, the utilization of single cell oils (SCOs) obtained from heterotrophic oleaginous microorganisms for biodiesel production has seen increasing attention [6, 7]. SCOs consist of triacylglycerols (TAGs) which are fatty acid triesters of glycerol. Special bacteria, primarily belonging to the actinomycetes group, are capable of biosynthesis and storage of TAGs by utilizing a wide range of carbon sources such as sugars, organic acids, and alcohols [8–11]. Rhodococcus opacus (R. opacus) PD630 has been intensively studied over the past few years because the lipid accumulated in its cells can reach 80 % of the cell dry weight [12]. It has been reported that PD630 can grow on alkanes, phenylalkanes, or non-hydrocarbon substrates as sole carbon source to accumulate lipid [9]. Another strain known as R. opacus DSM 1069 can degrade coniferyl alcohol and lignin-related methoxylated compounds [13]. Recent studies with this strain growing on lignin model compounds proved that oleaginicity can be approached using aromatic substances [14].
Pyrolysis of biomass, such as softwoods, hardwoods, and grasses, is a promising method to utilize these lignocellulosic resources for production of biofuels and chemicals. The process of pyrolysis involves a thermal decomposition process at elevated temperatures (i.e., 400–600 °C) under an inert environment. The liquid products from pyrolysis are known as pyrolysis oils which frequently separate into two immiscible phases by density difference: heavy oil and light oil [15]. By weight, heavy oil is the main product and there are multiple studies focusing on its upgrading to replace diesel and gasoline, typically relaying on catalytic hydrogenation [16, 17]. In contrast, the light oil is usually viewed as waste stream and few utilization studies have been conducted to date. Although a large quantity of water (>40 wt%) exists in this fraction, it still contains organic compounds accounting for one third the weight of heavy oil. The organic compounds include various acids, methanol, and some aromatic-structured compounds [18, 19]. Thus, research should be conducted to make full use of this fraction. As mentioned above, oleaginous Rhodococci such as PD630 and DSM 1069 are known to accumulate lipids while existing on diverse carbon sources [8–14]. So hypothetically, the low molecular weight water-soluble substances in light oil can represent an economic carbon source for the metabolism of digesting bacterial cells that in turn produce and accumulate lipids as biodiesel feedstock.
In this study, the possibility of lipid accumulation in R. opacus PD630 and DSM 1069 was investigated by using the components from light oil as sole carbon source. Two different pH values were selected to induce fat built-up. Switchgrass was chosen as the starting material for pyrolysis because of its broad cultivation properties. The growth of bacterial cells was monitored during the course of the experiments. The chemical changes in light oil and product formation are also described in detail.
Material and Methods
Chemicals, Strains, and Materials
All chemicals and materials were obtained from VWR (West Chester, PA, USA) or Sigma Aldrich (St. Louis, MO, USA) and used as received. R. opacus DSM 1069 and PD630 (DSMZ 44193) strains were purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ, www.dsmz.de). The switchgrass used in this work was corresponded to the Kanlow variety (Panicum virgatum var. Kanlow). This switchgrass was seeded in 2000 at the University of Georgia Plant Sciences Farm, near Watkinsville, GA (33_520 N; 83_320 W), and was harvested in 2007, dried, and stored near 0 °C.
Equipment and Process of Pyrolysis
Pyrolysis experiments were conducted using the method introduced by Ben and Ragauskas [15] with little modification. Switchgrass was ground to a 0.84-mm particle size by Wiley mill prior to feeding into a pyrolysis tube with a quartz sample boat. Being flushed with N2 gas at a flow rate up to 500 mL/min, the pyrolysis tube was placed into a preheated furnace at 600 °C. Two condensers, which were immersed in liquid N2, were used to accumulate the outflow from pyrolysis. After pyrolysis, the reaction tube was taken out from the furnace and cooled to room temperature under constant N2 flow. The condensers were then removed from liquid nitrogen and the pyrolysis oil was collected. The light oil was acquired by decantation and filtered through a 0.22-μm membrane filter for subsequent fermentation experiment.
Culture Media
Two types of media were used during shake flask fermentations in case of both strains: a full media and a minimal media with adjusted carbon and nitrogen source concentrations. Full media were prepared according to DSMZ recommendations: DSMZ 65 for strain DSM 1069 and DSMZ 535 for PD630. Minimal media for DSM 1069 contained the following chemicals: 0.4 g KH2PO4, 1.6 g K2HPO4, 0.2 g MgSO4·7H2O, 0.015 g FeCl3, 0.5 mg MnSO4⋅H2O, 1.0 mg CuSO4·5H2O, 1.0 mg ZnSO4·7H2O, 0.5 mg CaCl2, 0.1 mg KCl, and 0.5 mg H3BO3 per liter distilled water [14]. In the case of PD630, it contained the following chemicals: 9.00 g Na2HPO4·12H2O, 1.50 g KH2PO4, 0.20 g MgSO4·7H2O, 1.2 mg FeNH4 citrate, 20 mg CaCl2, 2 ml Hoagland solution (Sigma, H2395), and 0.50 g NaHCO3 per liter distilled water [20]. According to the cell adaption or lipid accumulation phases, the concentration of nitrogen (DSM 1069, NH4NO3; PD630, NH4Cl) was adjusted to 0.1 or 0.05 % w/v and carbon resource was added (glucose, light oil 0.25–4 % w/v). The pH was adjusted to 4 and 7 with 2 M NaOH; the media was then sterile filtered before use.
Shake Tube and Flask Fermentations
At first, the bacterial cells in all cases were inoculated by using aerobic shaker tubes with 10.00 mL full media for 36 h when the OD600 reached ∼0.6. The cells were then centrifuged and washed three times with minimal media containing 0.1 % w/v nitrogen. The cells were resuspended in 10 mL minimal media and ∼1 mL was inoculated into flasks with 50 mL light oil media to start the adaption phase. After 24 h, cells were harvested by centrifugation, followed by suspending and cultivating in 200 mL light oil media which contained 0.05 % w/v nitrogen source. All fermentations were performed under 30 °C at 150 rpm.
Analysis Method
Cell Growth and Change of Dry Matter in Light Oil
After each sampling, cells were pelletized by centrifugation and separated from the supernatants. OD was measured through UV absorbance at 600 nm when the cells were washed and resuspended; subsequently, the pellets and the supernatant were freeze-dried and measured as cell dry weights (CDW) and dry matter weights (DMW), respectively. All analyses were done in three replicates.
Lipids
Approximately 3 mg of lyophilized cells were collected and dissolved in 1.00 ml CHCl3, and then 0.85 ml methanol and 0.15 mL concentrated H2SO4 were added for transesterification which was conducted at 100 °C for 140 min. Subsequently, deionized water (1 mL) was added, and the samples were shaken vigorously for 1 min. After phase separation, the organic phase containing FAMEs was removed and stored in a freezer until gas chromatography–mass spectrometry (GC/MS) measurements were accomplished.
An Agilent 7890AGC system equipped with MS and Agilent HP-5MS was used for the measurements of FAMEs. Helium, 19.7 cm/s, was used as a carrier gas, and 2 μL samples were split injected (20:1); the temperature of oven was set to 50 °C and kept for 5 min, then was elevated by 15 °C/min until 325 °C and kept for 10 min. A 37-compound FAME mix from Sigma (CRM47885) prepared in dichloromethane at 0.1, 0.25, and 0.5 mg/mL concentrations was used as external standards. Accordingly, FAME contents were calculated in milligrams per milliliter, and these values present approximate total lipid contents, with a standard deviation ≤3.56 %. These FAMEs were identified using Agilent’s NIST08 library with above 95 % compound m/z spectrum similarity.
Characterization of Light Oil
All nuclear magnetic resonance (NMR) spectral data reported in this study were recorded with a Bruker Avance/DMX 400 MHz NMR. Quantitative 31P NMR was acquired after in situ derivatization of the samples using 4.0 mg dry matter from light oil with 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane (TMDP) in a solution of pyridine/CDCl3(1.6:1 v/v), chromium acetylacetonate (relaxation agent), and endo-N-hydroxy-5-norbornene-2,3-dicarboximide(NHND, internal standard). 31P NMR was set at inverse-gated decoupling pulse sequence 90° pulse angle, 25 s pulse delay, and 128 scans at room temperature with a LB of 4.0 Hz. For 13C-NMR sample preparation, 0.5 mL light oil was put into one tube with DMSO in a separated tube. 13C-NMR worked at inverse-gated decoupling sequence, 200 ppm sweep width, 90° pulse angle, 5 s pulse delay, and 6000 scans.
Results and Discussion
Preliminary Experiments
R. opacus PD630 and DSM 1069 can grow on diverse substrate. However, their growth was influenced by the content of carbon supply considerably. Thus, preliminary experiments were conducted to determine the optimal ratio of light oil to minimal media for bacterial utilization. In the present study, the two strains were incubated in the mixtures of light oil and minimal media at different proportions to make the dry matter concentrations varying from 0.25 to 4 % w/v. Moreover, due to the previous work in our group confirming that low pH contributed to the variation of fatty acid distribution [14], pH 4 and 7 were set for both strains to compare the results. Subsequent growth of the bacteria monitored by OD600 was shown in Fig. 1. The results can be best described by the typical logarithmic model:
Where A is the constant depending on the strain and substrate, and T is the growing time. The evaluation of A value, maximum OD values, and correlation coefficient are shown in Table 1. Compared with PD630, DSM 1069 can use light oil for proliferation with better efficiency. Increasing the carbon concentration was beneficial to the growth of both the strains. However, little difference occurred when the concentration increased from 2 to 3 % w/v, which means the growth of cells was not enhanced by further increase of concentration. When the concentration increased to 4 % w/v, the growth of cells began to decrease, which was due to the high concentration of toxic substances (e.g., furfural and acetic acid) in light oil that can influence the bacterial physiological activity [21]. As a result, we selected the concentration of 2 % w/v for the large-scale oil accumulation fermentations to minimum the toxic effect on bacteria.
Dry Matter Loss, Bacterial Growth, and Lipid Production
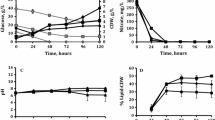
In the present work, the experiments included two stages. The first stage is for the adaption of bacteria in light oil with high nitrogen minimal media (0.1 % w/v). After that, the cells were collected and then transferred into low nitrogen minimal media (0.05 % w/v) to begin the second stage for TAG anabolism. Figures 2 and 3 show the growth and fatty acid content (% w/w of CDW) of the two strains as well as dry matter weight changes of substrate in the minimal media with low nitrogen concentration. Bacteria in all cases were found to grow well on the light oil as characterized by CDW that increased during the whole fermentation accompanied by the loss of dry matter in light oil, especially under the lower pH condition. At the end of fermentation, the CDW of PD630 and DSM1069 reached 0.82 and 0.90 g/L, respectively, at pH 4, which was 10.81 and 9.76 % higher than those at pH 7. The lipid peaks (measured in FAMEs) of PD630 and DSM 1069 occurred at 16 h, with the maximum values of 25.8 and 22.0 % w/w of CDW, respectively, which exceeded the reported oleaginous level of 20 % of CDW [22]. Moreover, it is noteworthy that as the pH increases from 4 to 7 it resulted in a 15.1 and 23.2 % drop in fatty acid content of PD630 and DSM 1069, respectively. These results indicated that PD630 and DSM 1069 can grow under acidic environment as proposed in our previous study [14], which found that cell number rose ∼5 times while pH reduced to 3.19 and lipid contents changed almost in parallel. The reason for the promoting effect of low pH value on cell growth and lipid accumulation might be associated with better environment for substrate conversion, membrane transport, or other metabolic regulatory parameters [23]. However, the detailed mechanism needs further research. Simultaneously, at the end of the experiment, the highest decrease of dry matter reached 26.0 and 42.5 % for PD630 and DSM 1069, respectively (at pH = 4). These results showed that although high water content (40 ∼ 80 wt%) obstructed the exploration and utilization of light oil, it provided a suitable environment for bacterial growth. When the whole light oil (including water) was taken into consideration, only small amount of the light oil was consumed; however, the fact that more than 26 % of dry matter can be utilized by R. opacus is of significance to provide a novel method for this by-product reuse and also to avoid environmental contamination. These results above showed that the bioconversion of light oil into lipid by oleaginous Rhodococci is possible. Interestingly, when the weight of the dry matter in the light oil decreased to minimum, the lipid content reached the peak, and then dropped dramatically with the gradual increase of cell growth during the course of the experiment. Consistent with the results of the previous work [14], this study also demonstrated that the two strains can consume their lipids for cell growth during the later stage.
Variations of black circle cell dry weight (CDW), white circle dry matter weight (DMW), and black down-pointing triangle FA content (% w/w of CDW) of DSM 1069 during the fermentation for lipid accumulation, a at pH = 4 and b at pH = 7. Values are the means and the standard deviations from three replicates
Variations of black circle cell dry weight (CDW), white circle dry matter weight (DMW), and black down-pointing triangle FA content (% w/w of CDW) of PD630 during the fermentation for lipid accumulation, a at pH = 4 and b at pH = 7. Values are the means and the standard deviations from three replicates
Chemical Changes of Light Oil
Many works have been done by using GC/MS, TGA, or FTIR to analyze the pyrolysis oil. However, pyrolysis oil is a complex mixture including more than 300 different oxygenated organic compounds [18]. GC can only monitor approximately 40 wt% of the complex bio-oils, and FTIR is restricted to qualitative analysis [15]. In contrast, NMR can provide more detailed chemical information of the entire and intact light oil because of its higher resolution. It has been reported that quantitative characterization of hydroxyl functional groups by phosphitylation with 2-chloro-4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaphospholane has been applied to a variety of oils including coal pyrolysis condensates, bio-oil from pyrolysis of kraft lignin, and bio-oils form pyrolysis of biomass [15, 24, 25]. In this study, phosphorus derivatization following by 31P NMR analysis was utilized to provide the insight into the fundamental chemistry changes of the light oil at pH 4 before and after the bacterial modification process. Based on the 31P NMR spectrum, the changes of the contents of phosphitylated hydroxyl functional groups were summarized in Table 2.
The data showed that in original light oil the aliphatic hydroxyl groups are the major components amounting up to 58 %, followed with C-5 condensed hydroxyl groups and guaiacyl which are 14 and 11 %, respectively. It also contained a small amount of catechol, p-hydroxy-phenyl groups, and acid hydroxyl groups. Different from the 31P NMR spectrum of lignin from switchgrass [26]; in this study, the C-5 condensed phenolic content is about 1.3 times that of guaiacyl phenolic content, indicating the formation of condensed structure during the thermal treatment. Furthermore, the current chemical makeup in light oil was more complicated than that from Kraft lignin which exhibited fewer signals that were easily being identified as methanol (δ147.9 ppm), catechol (δ138.9 ppm), and acetic acid (δ134.6 ppm) [15] depending on the starting materials. With NMR signals being compared before and after fermentation, the aliphatic hydroxyl groups reduced obviously from initial 4.71 to 3.14 and 3.78 mmol/g inoculated with PD630 and DSM 1069, respectively. Simultaneously, more than half of the C-5 condensed hydroxyl groups were eliminated, proving that not only can bacteria digested the aliphatic components in light oil, but also they can attack the aromatic-structural compounds. As expected, guaiacyl, catechol, and p-hydroxy-phenyl groups also decreased at the end of fermentation. In particular, when bacteria were growing under acidic environment, the concentration of the total hydroxyl group contents (acid-OH not included) in light oil with PD630 and DSM 1069 reduced by 43 and 28 %, respectively, higher than that of 23 and 24 % obtained at pH 7 (data not shown), which was consistent with DMW results. Additionally, results showed that acid-type components in light oil were unable to be utilized by selected bacteria in this study. The increase of this part at the end of the experiment might be caused by (1) acids produced from bacterial metabolism [27] and (2) oxidation of the alkyl-OH substituents [10].
To further explore the changes of the functional groups in light oil, more detailed analysis of the degradation products was accomplished using 13C NMR. Based on the chemical shift assignment range of pyrolysis oil reported by Ben and Ragauskas [15], the integration results of 13C NMR analysis for light oil are tabulated in Table 3. The data showed that compared with the functional groups in original light oil, the aliphatic signals with PD630 and DSM 1069 decreased by 29.29 and 9.48 % after 108 h, while the aromatic signals increased by 9.04 and 4.97 %, respectively. Moreover, the carbonyl or carboxyl content with PD630 and DSM 1069 rose apparently from 22.40 to 28.91 and 27.63 %. These results together with 31P NMR observations confirmed that the bacteria can grow on this complex carbon source by consuming various oligomers and monomers. In this process, bacteria released degrading enzymes to react with these aromatic structural compounds, involving the cleavage of linkage between aryl rings and the oxidation and shortening of the side chain. Then, these fractions in light oil can be converted into archetypal substrates (e.g., protocatechuate) for bacterial utilization through β-ketoadipate (β-KAP) pathway [28, 29], leaving the more polymerized and recalcitrant compounds behind.
Fatty Acid Profile and Lipid Yield
Oleaginous microorganisms could accumulate lipids when given nitrogen is limited. Simultaneous extraction and transesterification were used to obtain the fatty acids which were confirmed by GC analysis and a mass spectrometer detector. Figure 4 illustrated the fatty acid compositions of glucose and light oil (in two different initial pH) grown cells of R. opacus DSM 1069 and PD630 at point of time with maximal lipid yield. Results showed that different carbon source and cultivation conditions can evidently alter the fatty acid pattern of cellular lipid produced. The chain-length of major fatty acids in all cases varied between 15 and 18 carbon atoms including pentadecanoic acid (C15:0), palmitic acid (C16:0), palmitoleic acid (C16:1), heptadecanoic acid (C17:0), cis-10-Heptadecenoic acid (C17:1), stearic acid (C18:0), and oleic acid (C18:1). Among the primary fatty acids, the content of C16 (37.4 ∼ 42.5 %) and C18 (26.0 ∼ 42.7 %) was the most abundant components. However, it is noted that the production of palmitic and stearic acid extracted from cells used light oil as carbon source was promoted in different extent compared with that from glucose-growth cells. These two types of fatty acids are well-suited for biodiesel applications [7]. On the other hand, when using light oil as carbon source, the ratios of saturated fatty acids to unsaturated fatty acids produced from DSM 1069 were around 5:1 and 3:1 with pH 4 and 7 respectively, while the corresponding ratios in PD630 cells represented around 6:1 and 19:1. These results revealed that for DSM 1069, acidic environment can provoke the production of saturated fatty acids compared with neutral environment, whereas the situation is quite the reverse for PD630. However, due to the high content of saturated fatty acid (more than 75 %), great burning characteristics of the lipid-based biodiesel can be predicted in all cases [30].
Table 4 listed the maximum cell yield, fatty acid content, and lipid yield of the two strains in different substrate obtained from this study. It can be observed that in the case of PD630, although the low pH environment improved the maximum biomass and fatty acid content, the higher lipid yield was obtained in substrate with pH 7. However, acidic environment is more suitable for DSM 1069, in which the lipid yield of this strain can reach 0.117 g/L.
For biosynthesis of TAGs, diverse carbon sources including various sugars, alcohols, alkanes, organic acids, oils, and coal have been investigated [12, 16]. However, its commercialization was restricted by the cost of these expensive raw materials. Thus, the more cost-effective feedstocks such as agriculture and industrial nutritional waste have been considered as an alternative to support bacteria [9, 31]. Pyrolysis oil is the cheapest liquid product which can be made from lignocelluloses, the most inexpensive and abundant biomass in nature. The results obtained in this study proved that light oil can be utilized as a carbon source to feed bacteria for biodiesel production. However, many factors (such as nitrogen concentration, culture scale, and inhibitors) will affect the cell growth and lipid accumulation. Since the control glucose-based lipid yields in this study far lag behind current best fermentation with PD630 [32], optimization and scale-up need to be conducted to enhance the light oil-based SCOs production to satisfy in the actual biodiesel application. DSM 1069 was also proved with the ability to convert aromatic structural substrate into bio-oil in our previous research [14]. In the present study, this strain performed better lipid production capacity using light oil as carbon source.
Conclusions
This study confirmed that light oil can be treated as sole carbon source to support the growth of R. opacus and simultaneously induce the lipid production under nitrogen-limited conditions. The maximum lipid content observed in PD630 and DSM 1069 was 25.8 and 22 % of cell dry weight, which reached the level of oleaginicity. Reducing pH value was beneficial to the fatty acid production and distribution in bacterial cells, especially for DSM 1069, while palmitate and stearate were predominant fatty acids in all cases. This investigation proposed a novel approach to post treat the light oil, which also can provide more extensive feedstocks for biodiesel production.
References
Huang, G. H., Chen, F., Wei, D., Zhang, X. W., & Chen, G. (2010). Applied Energy, 87, 38–46.
Modi, M. K., Reddy, J. R. C., Rao, B. V. S. K., & Prasad, R. B. N. (2007). Bioresource Technology, 98, 1260–1264.
Chen, C. Y., Yeh, K. L., Aisyah, R., Lee, D. J., & Chang, J. S. (2011). Bioresource Technology, 102, 71–81.
Wyatt, V. T., Hess, M. A., Dunn, R. O., Foglia, T. A., Haas, M. J., & Marmer, W. N. (2005). American Oil Chemists Society, 82, 585–591.
Du, W., Xu, Y. Y., Zeng, J., & Liu, D. H. (2004). Biotechnology and Applied Biochemistry, 40, 187–190.
Zhu, L. Y., Zong, M. H., & Wu, H. (2008). Bioresource Technology, 99, 7881–7885.
Kosa, M., & Ragauskas, A. J. (2011). Trends in Biotechnology, 29, 53–61.
Alvarez, H., & Steinbüchel, A. (2002). Applied Microbiology and Biotechnology, 60, 367–376.
Gouda, M. K., Omar, S. H., & Aouad, L. M. (2008). World Journal of Microbiology and Biotechnology, 24, 1703–1711.
Alvarez, H. M., Mayer, F., Fabritius, D., & Steinbüchel, A. (1996). Archives of Microbiology, 165, 377–386.
Yang, L., Zhu, Z., Wang, W. H., & Lu, X. F. (2013). Bioresource Technology, 150, 1–8.
Alvarez, H. M., Kalscheuer, R., & Steinbüchel, A. (2000). Applied Microbiology and Biotechnology, 54, 218–223.
Eggeling, L., & Sahm, H. (1980). Archives of Microbiology, 126, 141–148.
Kosa, M., & Ragauskas, A. J. (2012). Applied Microbiology and Biotechnology, 93, 891–900.
Ben, H. X., & Ragauskas, A. J. (2011). Energy and Fuels, 25, 2322–2332.
Zhang, S., Yan, Y. J., Li, T. C., & Ren, Z. W. (2005). Bioresource Technology, 96, 545–550.
Vispute, T. P., Zhang, H., Sanna, A., Xiao, R., & Huber, G. W. (2010). Science, 330, 1222–1227.
Vispute, T. P., & Huber, G. W. (2009). Green Chemistry, 11, 1433–1445.
Ben, H. X., Mu, W., Deng, Y. L., & Ragauskas, A. J. (2013). Fuel, 103, 1148–1153.
Schlegel, H. G., Kaltwasser, H., & Gottschalk, G. (1961). Archives of Microbiology, 38, 209–222.
Huang, C., Zong, M. H., Wu, H., & Liu, Q. P. (2009). Bioresource Technology, 100, 4535–4538.
Ratledge, C., & Wynn, J. P. (2002). Advances in Applied Microbiology, 51, 45–51.
Harwood, C. S., & Parales, R. E. (1996). Annual Review of Microbiology, 50, 553–590.
Wroblewski, A. E., Lensink, C., Markuszewski, R., & Verkade, J. G. (1988). Energy and Fuels, 2, 765–774.
Fu, Q. R., Argyropoulos, D. S., Tilotta, D. C., & Lucia, L. A. (2008). Journal of Analytical and Applied Pyrolysis, 8, 60–64.
David, K., & Ragauskas, A. J. (2010). Energy and Environmental Science, 3, 1182–1190.
Bugg, T. D. H. (2012). Nature Chemistry, 5, 10–12.
Bugg, T. D. H., & Winfield, C. J. (1998). Natural Product Reports, 15, 513–530.
Wells, T., & Ragauskas, A. J. (2012). Trends in Biotechnology, 30, 627–637.
Knothe, G. (2005). Fuel Processing Technology, 86, 1059–1070.
Balasubramanian, L., Subramanian, G., Nazeer, T. T., Simpson, H. S., Rahuman, S. T., & Raju, P. (2011). Biotechnology and Applied Biochemistry, 58, 220–225.
Voss, I., & Steinbüchel, A. (2001). Applied Microbiology and Biotechnology, 55(5), 547–555.
Acknowledgments
Z. Wei is grateful to China Scholarship Council for awarding a scholarship under the State Scholarship Fund to pursue her study. This work will be used by Z. Wei for partial fulfillment of the degree requirement for her doctoral research at the College of Environmental Science and Engineering, Hunan University, Changsha, China. We also wish to acknowledge DOE (EE0006112) for their support via Synthetic Design of Microorganisms for Lignin Fuel.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wei, Z., Zeng, G., Kosa, M. et al. Pyrolysis Oil-Based Lipid Production as Biodiesel Feedstock by Rhodococcus opacus . Appl Biochem Biotechnol 175, 1234–1246 (2015). https://doi.org/10.1007/s12010-014-1305-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1305-4