Abstract
Combination therapy is considered a viable strategy to overcome the resistance to chemotherapeutics. Survivin as a member of the inhibitor of apoptosis protein (IAP) family, which is involved in resistance to various drugs. We investigated the role of combination therapy in downregulating survivin and increasing drug’s efficacy in MDA-MB-231 cells. MTT assay and DAPI staining were applied to study the anti-proliferative activity and apoptosis response of the agents. Real-time RT-PCR and Western blot analysis were applied to study survivin mRNA and protein. Our findings showed that combined treatment of cells with docetaxel and vinblastine reduces survivin expression and consequently decreases the IC50 value of docetaxel from 70 to 5 nM (p < 0.05). Furthermore, combination therapy with deguelin, a survivin inhibitor, exerted a considerable enhancement in synergistic efficacy of docetaxel and vinblastine (p < 0.05). Survivin downregulation may thus be considered a potential strategy in increasing the efficacy of chemotherapeutics in cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the second leading cause of cancer death in women after lung cancer. Systemic chemotherapy is the most common modality among other therapeutic strategies for breast cancer especially in the case of metastasis [1]. Different types of chemotherapeutic agents, including anthracyclines, taxanes, and vinca alkaloids are administered as first- and second-line treatments in patients with advanced breast cancer [2]. Mitotic disrupting agents including vinblastine exert dynamic instability in spindle microtubules and cause mitotic arrest followed by apoptosis [3]. In contrast to destabilizing agents, taxanes including docetaxel stabilize microtubules. Docetaxel is currently being used in the treatment of breast, lung, gastroesophageal, and more recently prostate cancers [4–6]. The binding of docetaxel to the β-tubulin subunits prevents microtubule depolymerization and leads to G2/M arrest. The G2/M arrest in turn, results in cell death by phosphorylation of bcl-2 [7], dysregulation of signal transduction pathways [8], or induction of cell cycle perturbations [9].
Various studies have reported resistance to docetaxel and vinblastine in breast cancer cell lines, including MDA-MB-231 [10, 11]. Different molecular mechanisms can be involved in induction of chemoresistance, including alterations in metabolism of the chemotherapeutics [12], alterations in the dynamics of microtubules [13], DNA methylation [14], and binding of the chemotherapeutic agents to microtubules [15], and overexpression of multidrug resistance gene product, P-glycoproteins [16], and inhibitor of apoptosis protein (IAP) family genes [17, 18].
Survivin is a 16-kD bifunctional protein with 142 amino acids involved in cell division and caspase inhibition. Survivin is the smallest member of the IAP family, which is involved in the resistance of certain tumors to chemotherapeutics [19]. This protein plays a key role in cancer initiation, tumor progression, and resistance to various chemotherapeutics, including taxanes and vinca alkaloids [20–22]. Survivin works within chromosomal passenger complex by interacting with other proteins, including inner centromere protein, borealin, and Aurora B and acts as the regulator of Aurora B kinase during mitosis [23]. Although the involvement of survivin in regulation of the mitotic checkpoints has been intensively studied, the fundamental mechanisms of this protein in chemoresistance still remain unclear [24].
Combination therapy with several chemotherapeutic agents provides several advantages: different phases of the cell cycle can be affected by multiple drugs resulting in synergism, dose-dependent side effects can be reduced, and since lower concentration of each agent would be used, the possibility of drug resistance will be reduced and the patient quality of life will increase [25–28]. Indeed, combination protocols suggest clinically significant survival advantage in comparison with monotherapy [29]. Combination therapy protocols rely fundamentally on experimental data produced from in vitro and in vivo studies [30].
In this study, we investigated the efficacy of single and combined incubation of MDA-MB-231 breast cancer cells with vinblastine and docetaxel. We also studied the role of survivin in induction of chemoresistance against docetaxel and vinblastine by comparing survivin expression in single and combined incubation of cancer cells with these chemotherapeutic agents. We also determined the efficacy of these drugs in induction of apoptosis when we inhibited survivin activity by deguelin. Results from our study indicated that combined incubation of cancer cells with vinblastine and docetaxel elevates the efficacy of docetaxel in induction of apoptosis by decreasing survivin expression. Inhibition of survivin activity increased the sensitivity of the cells to each agent or their combination. Clinical translation of this protocol suggests that combination therapy increases the efficacy of each agent alone in induction of apoptosis. We also predict that identifying patients who express high survivin activity and then inhibiting this activity could provide an important adjuvant for improving the efficacy of docetaxel and vinblastine in cancer treatment.
Materials and Methods
Materials
Docetaxel (20 mg, Taxotere®) and vinblastine (VBL; 10 mg) were purchased from Sanofi-Aventis (Paris, France) and Gedeon Richter Ltd (Budapest, Hungary), respectively. RPMI-1640 medium and penicillin/streptomycin were provided from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) was obtained from Invitrogen (Auckland, New Zealand). Primers were purchased from MWG Biotech (Ebersberg, Germany). RNA isolation kit (RNX-Plus) was obtained from CinnaGen Co. (Tehran, Iran), and REVERTA-L RT reagents kit was purchased from Central Research Institute of Epidemiology of Russia (Moscow, Russia). Power SYBR® Green PCR Master Mix (5 ml) was obtained from Applied Biosystems (Warrington, UK). 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; 5 mg) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Anti-survivin and Anti-beta actin (mAbcam 8226) antibodies were purchased from Abcam (Cambridge, MA, USA). Anti-mouse IgG (H&L) HRP-conjugated secondary antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Nitro cellulose membrane was provided from Millipore Corporation (Billerica, MA, USA). Enhanced chemiluminescence (ECL) kit was purchased from Amersham Biosciences (Freiburg, Germany). Prestained protein ladder was obtained from Fermentas (Hanover, MD, USA).
Cell Culture
Human breast cancer MDA-MB-231 cells were obtained from Pasteur Institute Cell Culture Collection (Tehran, Iran). Cells were grown in RPMI 1640 containing 10 % FBS and 100 units/ml penicillin/ streptomycin and incubated at 37 °C in 5 % CO2.
Single Therapy
To determine the drug efficacy in induction of apoptosis after a single exposure, MDA-MB-231 cells were seeded in 96-well plates with seeding density of 12,000 cells/well. Then, increasing concentrations of vinblastine (up to 50 μM) and docetaxel (up to 1 μM) were applied. MDA-MB-231 cells were incubated with media containing the agents for 24, 48, and 72 h.
Combined Treatment
To determine the effects of vinblastine/docetaxel combinations in cell death, MDA-MB-231 cells were seeded at the density of 12,000 cells/well in 96-well plates. Subsequently, the cells were incubated with variable concentrations of docetaxel and vinblastine.
MTT Assay
The media in each well was replaced with 200 μl fresh media containing 50 μl of MTT. Then the cells were incubated for 4 h at 37 °C. After incubation period, media/MTT mixture was removed and 200 ml of DMSO plus 25 ml of Sorenson’s glycine buffer (0.1 M glycine and 0.1 M NaCl, pH 10.5) were added to each well. The absorbance of each well was measured at 570 nm after shaking for 10 min, employing a microplate reader (Biotek, ELx 800, USA). MTT solution with DMSO (without cells and medium) was used as blank control.
Determination of IC50 of Vinblastine and Docetaxel Against MDA-MB-231
Plots of cytotoxicity index (% CI = (1 − (ODtreated/ODcontrol)) × 100) versus different concentrations of each chemotherapeutic agents were drawn. IC50 was determined from each plot by calculating the slop and intercept.
Calculation of the Combination Index
The cytotoxicity of docetaxel/deguelin and vinblastine/deguelin combinations was calculated using Combination Index (CI) given by the formula below:
Where, D 1 x is dose of drug 1 alone; D 1 is dose of drug in combination with drug 2; D 2x is dose of drug 2 alone; D 2 is dose of drug 2 in combination with drug 1; and α = 0 for mutually exclusive or 1 for mutually nonexclusive modes of drug action.
DAPI Staining
DAPI is known to form fluorescent complexes with natural double-stranded DNA. Binding of DAPI to DNA enhances its fluorescence strongly. DAPI staining was performed as we established previously [31]. Cells were seeded in 6-well plates, and after single and combination treatment for 48 h, cells were fixed with 4 % paraformaldehyde. After 15 min, cells were washed with phosphate-buffered saline (PBS and then permeabilized with 0.1 % Triton-X-100 for 10 min. Cells then were stained with DAPI (1:500 dilution in PBS) for 10 min. Nuclei were considered to have the normal or apoptotic phenotype. Apoptotic nuclei were identified by the condensed chromatin gathering at the periphery of the nuclear membrane or a total fragmented morphology of nuclear bodies. Triplicate samples were prepared for each treatment and at least 300 cells were counted in random fields for each sample and apoptotic nuclei were identified.
RNA Isolation and RT-PCR
Cells were harvested 24 h after incubation with different concentrations of drugs and lysed using lysis buffer, RNX-PLUS™ (RN7713C) CinnaGen Co., according to manufacturer protocol. RNA pellet was dissolved in DEPC-treated water, quantified by optical density measurement (A260/A280 ratio) with NanoDrop 1000 Spectrophotometer (Wilmington, DE, USA), checked the quality by agarose gel electrophoresis, and stored at −70 °C. cDNA synthesis was done using REVERTAA-L (RT reagents kit).
Real-time PCR
The iQ5 Optical System (Bio-Rad Laboratories, Inc., CA, USA) was utilized for performing all amplification reactions in a total volume of 25 μl. Each well contained 1 μl of cDNA, 5.75 μM of each primer, and 12.5 μl of 2× Power SYBR Green PCR Master Mix. The internal control was the constitutively expressed housekeeping human glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Primers for human survivin were as follows: sense, 5′GACCACCGCATCTCTACATTC-3′; antisense, 5′-TGCTTTTTATGTTCCTCTATGGG-3′, and for human GAPDH were as follows: sense, 5′-ACAGTCAGCCGCATCTTCTT-3′; antisense, 5′-GACAAGCTTCCCGTTCTCAG-3′. Samples were assayed in triplicate on the 7500 Real-time PCR System (Applied Biosystems). Interpretation of the results was performed using the Pfaffle method and the CT values were normalized with respect to GAPDH expression.
Western Blot
To determine survivin protein level, Western blot analysis was carried out. The cells were treated with various concentrations of chemotherapeutic drugs for 48 h and washed three times with cold PBS. Cell lysates was obtained by incubating with lysis buffer (10 mM Tris, pH 8, 1 mM EDTA, 1 % NP40, 0.1 % SDS plus protease inhibitor cocktail Tablet (Roche)) on ice for 30 min. Cell debris was removed from lysates by centrifugation at 13,000×g for 20 min. The protein concentrations were measured at 280 nm using a nanodrop spectrophotometer (ND-1000 Wilmington, DE, USA). Equal amounts of protein lysate of each sample (50 μg) were electrophoretically separated on 12.5 % of SDS-PAGE gel and transferred to polyvinylidene difluoride (PVDF) membrane (Millipore; Billerica, MA). The membrane was blocked with 5 % nonfat dry milk for 1 h at room temperature and then incubated with the anti-survivin antibody (1:1,000) in 1× TBS containing 0.01 % Tween-20 buffer overnight at 4 °C. The membrane was washed three times with TBST-20 buffer and incubated with HRP-conjugated secondary antibody for 1 h at room temperature. After washing, the protein bands were detected using ECL Plus detection system on X-ray films (Fuji Photo Film Co., Ltd., Tokyo, Japan) according to the manufacturer’s instruction. Then the membrane were treated with antibody stripping buffer (SDS, 2 %; mercaptoethanol, 0.1 M; and Tris–HCl, 50 mM, pH 7] and incubated with anti-actin antibody (1:3,000 dilution) and secondary antibody for control. The results of Western blot were quantified using the band densitometry analysis with ImageJ software. The intensity of each protein was compared with that of β-actin, and relative intensity ratios were calculated.
Statistical Analysis
Results were presented as means from three independent experiments. Statistical analysis was performed using SPSS software through ANOVA or student t tests. p < 0.05 was considered as statistically significant.
Results
Anti-proliferative Effects of Vinblastine and Docetaxel on MDA-MB-231 Cells
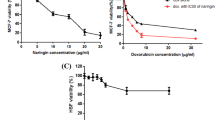
The effects of vinblastine and docetaxel in induction of apoptosis on breast cancer cells were evaluated through two different methods: MTT assay and DAPI staining. We first incubated the cells with increasing concentrations of each chemotherapeutic agent in different incubation times (24, 48, and 72 h) to determine the optimal and IC50 concentrations for each agent. Both agents showed significant anti-proliferative activity in a dose- and time-dependent manner (p < 0.05) (Fig. 1).
Docetaxel concentration over 100 nM, revealed no significant change in cytotoxicity in MDA-MB-231 cells. The effective concentration range of vinblastine in induction of apoptosis was 1 to 100 μM. There was no significant increase in cytotoxicity when vinblastine concentration was increased over 100 μM. The IC50 value for vinblastine and docetaxel after 48 h incubation was 8 μM and 70 nM, respectively (Fig. 1a).
To study the effects of these chemotherapeutic agents in induction of apoptosis, morphological examination using DAPI staining was applied. DAPI is known to form fluorescent complexes with double-stranded DNA. Minimum 300 cells were examined for each treatment, and the percentage of apoptotic nuclei, intensely stained, fragmented nuclei, and condensed chromatin, was calculated. Our results from DAPI staining after 48-h incubation of the cells with the agents showed a percentage of apoptotic cells of up to 20 % with docetaxel and up to 62 % with vinblastine (Fig. 1b).
Docetaxel and Vinblastine Elevated Survivin Expression
We evaluate the expression of survivin mRNA in MDA-MB-231 cells by real-time RT-PCR. Total RNA was extracted from the cells treated with variable concentrations of docetaxel (0–100 nM) and vinblastine (0–50 μM) after 24-h incubation. The highest survivin mRNA level was shown in the cells treated with 50 nM of docetaxel or 10 μM of vinblastine (Fig. 2a, b) (p < 0.001).
Western blot analysis was applied to determine survivin protein expression. For this purpose, cells were incubated with docetaxel (50 nM) and vinblastine (10 μM) for 48 h, then total protein was extracted using RIPA buffer. Our results showed a significant increase in survivin protein when the cells were incubated with docetaxel (Fig. 2d).
Combination of Docetaxel with Vinblastine Reduces Survivin Expression and Increases the Efficacy of Treatment in MDA-MB-231 Cells
Next, we chose combination of docetaxel/vinblastine to investigate whether combined protocols could decrease drug-induced upregulation of survivin and consequently enhance the efficacy of drugs. Results from real-time RT-PCR demonstrated that survivin mRNA level decreased significantly upon combination treatment of docetaxel (50 nM) and vinblastine (10 μM) (p < 0.001) (Fig. 2c). Furthermore, Western blot analysis showed that combined treatment of cells with docetaxel/vinblastine decreases the survivin protein expression significantly (Fig. 2d).
To investigate whether combination therapy could enhance the antitumor effects of docetaxel, the inhibitory effects of docetaxel/vinblastine combinations were compared with single therapy. We applied 5, 10, and 50 μM of vinblastine along with variable concentrations of docetaxel (5, 10, 50, and 100 nM). Combination treatment increased cytotoxicity of docetaxel up to 30 % after 48 h exposure (Fig. 3a). IC50 value of docetaxel decreased from 100 to 5 nM when combined with 5 μM of vinblastine (p < 0.05).
DAPI staining results indicated that combination of docetaxel and vinblastine could enhance the apoptotic nuclei significantly. Combination of docetaxel (10 nM) with vinblastine 50 μM) increased the percentage of apoptotic cells from 19 to 65 %. As well, 100 nM of docetaxel in combination with 50 μM of vinblastine increased the percentage of apoptotic cells up to 57 % (Fig. 3b; Table 1).
Inhibition of Survivin Activity Induces Apoptosis in MDA-MB-231 Cells
We first examined the role of survivin in proliferation and cell viability by applying deguelin, a survivin inhibitor. The best effective dose of deguelin was in a range of 0.01–10 μM. The growth inhibition of 50 % was seen by 1 μM deguelin after 72 h (p < 0.05). No considerable enhancement of cytotoxicity was noted at concentrations beyond 50 μM of deguelin (Fig. 3c).
Deguelin Downregulates Survivin mRNA and Protein
To examine the effects of deguelin on the expression of survivin in MDA-MB-231 cells, survivin mRNA and protein levels were determined by real-time RT-PCR and Western blot analyses, respectively. Real-time RT-PCR showed a dose-dependent decrease in survivin expression after 48 h treatment of MDA-MB-231 cells with increasing concentrations of deguelin. Treatment of the cells with 0.01–10 μM deguelin markedly reduced the levels of survivin mRNA (p < 0.001) (Fig. 4a). Further treatment of cells with 0.01 μM deguelin for 2 days markedly decreased the expression of survivin protein (Fig. 2d).
To further investigate the role of deguelin in enhancement of docetaxel and vinblastine efficacy, we examined combining effect of docetaxel/deguelin and vinblastine/deguelin on cellular proliferation.
Deguelin Increases the Sensitivity of MDA-MB-231 Cells to Docetaxel and Vinblastine
Deguelin may contribute to the efficacy of chemotherapy by blocking survivin expression. Previous studies have demonstrated that treatment of human cancer cells with chemotherapeutic agents induces upregulation of survivin [32, 33], which is associated with decreased drug sensitivity [34]. Therefore, in this study, we had two speculations: whether docetaxel and vinblastine induce upregulation of survivin in MDA-MB-231 cells and combination of deguelin with either docetaxel or vinblastine increases the rate of apoptosis in human MDA-MB-231 cells.
However, incubation of the cells with docetaxel or vinblastine alone increased survivin mRNA levels in MDA-MB-231 cells; applying deguelin (0.01 μM) along with docetaxel (50 nM) or vinblastine (10 μM) decreased survivin gene expression significantly (p < 0.001) (Figs. 4b and 5c). Western blot analysis verified these results in the protein level; combination of docetaxel (50 nM) or vinblastine (10 μM) with deguelin (0.01 μM) decreased survivin protein significantly (Fig. 2d).
Next, we examined whether inhibition of survivin expression could alter the apoptotic response and chemosensitivity of the cells. Although 0.01, 0.1, 1, and 10 μM deguelin did not have a tangible effect on growth inhibition and apoptosis after 24 h, it inhibited the expression of survivin. Even these levels of deguelin markedly enhanced the effects of docetaxel and vinblastine in combination treatments of MDA-MB-231 cells. A 24-h treatment of MDA-MB-231 cells with docetaxel (10 nM) in the presence of 0.01 μM deguelin resulted in reduction of cell viability compared with that of docetaxel-treated cells by 24 %. The combination of vinblastine (10 μM) with deguelin (10 μM) significantly reduced cell viability from 73 to 65 % (p < 0.05) (Fig. 5a).
The combined effects of docetaxel and vinblastine along with deguelin on cell proliferation were evaluated using isobolographic analysis method. The CI values ranged from 0.2 to 0.85 and 0.33 to 2 for docetaxel and vinblastine, respectively (Table 2). To evaluate apoptotic response to combination therapy of docetaxel or vinblastine with deguelin, the number of apoptotic cells stained with DAPI was calculated using florescent microscopy. Docetaxel (10 nM) induced 20 % apoptotic cells while in combination with deguelin (0.01 μM), and we observed up to 28 % of apoptotic cells. In addition, 54 % apoptotic cells were observed in the cells treated with vinblastine (10 μM); however, when we applied combination of vinblastine and deguelin (0.01 μM), no significant effect was observable; nevertheless, the total number of cells decreases markedly, which suggests nonapoptotic cell death (Fig. 5b).
Discussion and Conclusion
Despite much development in drug industry and production of novel chemotherapeutics, resistance to currently available chemotherapeutic agents is still a complicated and multifactorial bottleneck in cancer treatment. Chemoresistance is responsible for cancer recurrence, relapse, and metastasis [35, 36]. Molecular mechanisms of chemoresistance are not completely understood yet [10]. Over the past two decades, investigating the apoptotic dysregulation in cancer cells has been a demanding research area. Defective apoptosis often results from changes in induction of the extrinsic and intrinsic pathways or through alternative pathways of cell death, such as autophagy, mitotic catastrophe, or necrosis. Thus, an increase in our understanding of the mechanisms by which cancer cells evade apoptosis and its links to drug resistance can help improve the efficacy of chemotherapeutics and possibly help develop molecular-targeted pro-apoptotic therapies for cancer treatment [37].
The aim of this study was to examine whether combining chemotherapeutics can change the sensitivity of cancer cells to drugs and to explore the potential role of survivin in this process. Overexpression of survivin is linked with chemoresistance and in some groups of patients, is associated with a poor prognosis and treatment outcome [38, 39]. Because of the crucial role of survivin in the development and growth of solid tumors and hematologic malignancies, many attempts have been made to develop therapeutic survivin inhibitors to increase the efficacy of existing chemotherapeutic agents [40–43].
In this work, we investigated the anti-proliferative and apoptotic effects of docetaxel (up to 100 nM) and vinblastine (up to 200 μM). Cytotoxic effects of these agents were determined using MTT assay and DAPI staining.
Treating MDA-MB-231 cells with docetaxel (100 nM) and vinblastine (50 μM) showed a percentage of cell death after 48 h of up to52 % with docetaxel and 78 % with vinblastine in MTT assay. Percentage of apoptotic nuclei by DAPI staining was almost 13 and 65 % for the same concentrations of docetaxel and vinblastine, respectively. The discrepancies between the results from MTT assay and DAPI staining suggest that cells probably died also by mitochondria-independent and nonapoptotic mechanisms of cell death. Another possible explanation would be that DAPI staining determines the percentage of the cells only in the attached cells to the dish [31].
Survivin gene and protein expression analyses further revealed that docetaxel and vinblastine-induced cytotoxicity can be strongly antagonized by survivin activity. Survivin has a fundamental role in resistance to chemotherapy in tumor cells [44], and its expression is also correlated with chemoresistance in ovarian and prostate cancers [45]. In breast cancer patients, a higher survivin level is associated with poor prognostic factors, including high histological grade and tumor cell proliferation [46]. While survivin is normally found at low levels in normal cells, it is upregulated in many tumor cells [47, 48]. Although some studies argue that survivin inhibits caspase activity directly [49], others dispute this issue [50]. Dohi et al. [51] have suggested that only the survivin released from mitochondria and not survivin that may be expressed in the cytosol before the death stimulus, can inhibit apoptosis, while Wheatley et al. have brought those results under question [24]. Nevertheless, many publications have shown the ability of upregulated survivin levels in protecting cells against multiple apoptosis-inducing agents, including docetaxel [24, 52]. This might reflect the fact that the anti-apoptotic function of survivin is only observed when the level of this protein is elevated in tumor cells and in response to chemotherapeutic agents.
While survivin has a controversial role as an inhibitor of apoptosis, it has an undisputed role as a mitotic regulator [53]. Survivin facilitates and contributes to mitosis due to its role as a member of the chromosomal passenger complex family. In early phases of mitosis, the chromosomal passenger complex moves to chromosome arms and accumulates at centromeres until metaphase. In this phase, the complex contributes to mitotic checkpoint, chromosome biorientation, and assembly of the mitotic spindle. In anaphase, the complex relocates to the spindle midzone and then to the midbody during telophase and is required for cytokinesis [54].
In this study, we showed a significant increase in survivin gene expression level of up to 9-fold when the cells were incubated with docetaxel or vinblastine alone, which is consistent with other studies that showed treatment of cancer cells with single microtubule inhibitor agents increases survivin expression [32]. These increases in survivin expression are probably because of G2/M arrest induced by docetaxel or vinblastine [55, 56]. Expression of survivin is cell cycle dependent with a robust increase at the G2/M phase of cell cycle [57].
Our findings demonstrated that the observed chemoresistance can be negated by combination therapy. Furthermore, adjuvant therapy using survivin inhibitors can help enhance the tumor cell response to drugs in vitro.
It is known that the contribution of survivin to cancer cell survival depends on the therapeutic agent used and possibly type of cancer cells. For example, in lung cancer, Normura et al. showed that survivin contributed to cisplatin resistance by inhibition of apoptosis [58]. Zhang et al. also demonstrated that adenovirus-mediated inhibition of survivin has the potential to sensitize human prostate cancer cells to paclitaxel [59]. Survivin is also required for stable checkpoint activation in paclitaxel-treated HeLa cells [60]. In that study, downregulation of survivin by siRNA abolished the ability of the cells to sustain paclitaxel-induced mitotic arrest [60]. However, downregulation of survivin alone could not induce massive death in HeLa cells [60]. Interestingly, survivin-induced resistance to microtubule destabilizers, such as Vinca alkalodis, cochicine, and combretastatin A-4-related compounds in cancers has rarely been reported in the past [61].
In this study, we showed that deguelin induces apoptosis in MDA-MB-231 cells. Xiang-Hong et al. reported that deguelin selectively provokes apoptosis in human breast cancer but not with normal mammary epithelial and fibroblast cells [41]. Genoveva et al. also demonstrated the growth inhibitory effects of deguelin in several breast cancer cell lines with the highest growth suppression in the MDA-MB-231 cells [62], which is consistent with results from our study. We also found that various concentrations of deguelin (0.01–10 μM) significantly inhibited the expression of survivin gene and protein in MDA-MB-231 cells. Xiang-Hong found that only relatively low concentrations of deguelin (≥10 nM) significantly inhibit the levels of both survivin gene and protein in SK-BR3 and MCF-7 cells. The observed discrepancy is likely due to differences in genomic properties of studied cell lines. MDA-MB-231 cells are the most aggressive and highly proliferating cells [63]. Furthermore, p53 which is widely believed to be a negative regulator of survivin expression [64], which is mutated in MDA-MB-231 cells [65], and the expression of survivin is much higher in MDA-MB-231 compared with MCF-7 and SK-BR3 cells.
In our study, although incubation of MDA-MB-231 cells with 0.01–10 μM deguelin markedly reduced survivin levels, only 2–19 % of growth inhibition was found in the tumor cells. A significantly higher level of cell growth inhibition (20 %) was detected when we incubated the cells with combined concentrations of deguelin with docetaxel (10 nM) and vinblastine (10 μM). The synergistic effects of these combined treatments were shown by calculation of CI. As expected, deguelin considerably blocked docetaxel and vinblastine-induced upregulation of survivin.
Here, we demonstrated that downregulation of survivin by deguelin restored sensitivity to docetaxel and vinblastine in MDA-MB-231 cells. These results suggested that survivin plays an important role in the sensitivity and resistance of MDA-MB-231 cells to the microtubule inhibitor agents, docetaxel and vinblastine.
In conclusion, our results demonstrate that combination treatment of docetaxel and vinblastine increases the efficacy of each agent in induction of apoptosis in breast cancer cells. This work identifies that inhibition of survivin activity could improve the treatment outcomes of cancers by docetaxel and vinblastine and possibly other chemotherapeutic agents in patients with high survivin expression.
References
Siegel, R., Naishadham, D., & Jemal, A. (2013). CA: A Cancer Journal for Clinicians, 63(1), 11–30.
Crown, J., Dieras, V., Kaufmann, M., von Minckwitz, G., Kaye, S., Leonard, R., Marty, M., Misset, J. L., Osterwalder, B., & Piccart, M. (2002). Lancet Oncology, 3, 719–727.
Lee, E. A., Keutmann, M. K., Dowling, M. L., Harris, E., Chan, G., & Kao, G. D. (2004). Molecular Cancer Therapeutics, 3, 661–669.
Petrylak, D. P. (2000). Seminars in Oncology, 27, 24–29.
Shepherd, F. A., Fossella, F. V., Lynch, T., Armand, J. P., Rigas, J. R., & Kris, M. G. (2001). Seminars in Oncology, 28, 4–9.
Figgitt, D. P., & Wiseman, L. R. (2000). Drugs, 59, 621–651.
Haldar, S., Basu, A., & Croce, C. M. (1997). Cancer Research, 57, 229–233.
Wang, S., Guo, C. Y., Castillo, A., Dent, P., & Grant, S. (1998). Biochemical Pharmacology, 56, 635–644.
Shen, S. C., Huang, T. S., Jee, S. H., & Kuo, M. L. (1998). Cell Growth and Differentiation, 9, 23–29.
Lapensee, E. W., Tuttle, T. R., Fox, S. R., & Ben-Jonathan, N. (2009). Environmental Health Perspectives, 117, 175–180.
Klement, G., Baruchel, S., Rak, J., Man, S., Clark, K., Hicklin, D. J., Bohlen, P., & Kerbel, R. S. (2000). Journal of Clinical Investigation, 105, R15–R24.
Kavallaris, M. (1997). Anti-Cancer Drugs, 8, 17–25.
Goncalves, A., Braguer, D., Kamath, K., Martello, L., Briand, C., Horwitz, S., Wilson, L., & Jordan, M. A. (2001). Proceedings of the National Academy of Sciences of the United States of America, 98, 11737–11742.
Kastl, L., Brown, I., & Schofield, A. C. (2010). International Journal of Oncology, 36, 1235.
Drukman, S., & Kavallaris, M. (2002). International Journal of Oncology, 21, 621–628.
Gottesman, M. M. (2002). Annual Review of Medicine, 53, 615–627.
Simstein, R., Burow, M., Parker, A., Weldon, C., & Beckman, B. (2003). Experimental Biology and Medicine, 228, 995–1003.
Fraser, M., Leung, B., Jahani-Asl, A., Yan, X., Thompson, W. E., & Tsang, B. K. (2003). Reproductive Biology and Endocrinology, 1, 66.
Cheng, J. Q., Jiang, X., Fraser, M., Li, M., Dan, H. C., Sun, M., & Tsang, B. K. (2002). Drug Resistance Updates, 5, 131–146.
Duffy, M. J., O’Donovan, N., Brennan, D. J., Gallagher, W. M., & Ryan, B. M. (2007). Cancer Letters, 249, 49–60.
LaCasse, E. C., Baird, S., Korneluk, R. G., & MacKenzie, A. E. (1998). Oncogene, 17, 3247.
Pellegrini, F., & Budman, D. R. (2005). Cancer Investigation, 23, 264–273.
Vader, G., Medema, R. H., & Lens, S. M. (2006). Journal of Cell Biology, 173, 833–837.
Wheatley, S. P., & McNeish, I. A. (2005). International Review of Cytology, 247, 35–88.
Aoudjit, F., & Vuori, K. (2012). Chemotherapy Research and Practice, 2012, 283181.
Kerbel, R. S., & Kamen, B. A. (2004). Nature Reviews Cancer, 4, 423–436.
Spector, S., & Spector, N. L. (2001). Compound for selective treatment of malignant cells by inhibiting cell cycle progression, decreasing Bcl2, and increasing apoptosis. Google Patents.
Ma, J., & Waxman, D. J. (2008). Molecular Cancer Therapeutics, 7, 3670–3684.
Miles, D., von Minckwitz, G., & Seidman, A. D. (2002). The Oncologist, 7, 13–19.
Zoli, W., Ricotti, L., Dal Susino, M., Barzanti, F., Frassineti, G. L., Folli, S., Tesei, A., Bacci, F., & Amadori, D. (1999). British Journal of Cancer, 81, 609–615.
Samadi, N., Gaetano, C., Goping, I., & Brindley, D. (2008). Oncogene, 28, 1028–1039.
Ling, X., Bernacki, R. J., Brattain, M. G., & Li, F. (2004). Journal of Biological Chemistry, 279, 15196–15203.
Peng, X. H., Cao, Z. H., Xia, J. T., Carlson, G. W., Lewis, M. M., Wood, W. C., & Yang, L. (2005). Cancer Research, 65, 1909–1917.
Tamm, I., Wang, Y., Sausville, E., Scudiero, D. A., Vigna, N., Oltersdorf, T., & Reed, J. C. (1998). Cancer Research, 58, 5315–5320.
Huanwen, W., Zhiyong, L., Xiaohua, S., Xinyu, R., Kai, W., & Tonghua, L. (2009). Molecular Cancer, 8, 125.
Samadi, N., Bekele, R., Capatos, D., Venkatraman, G., Sariahmetoglu, M., & Brindley, D. N. (2011). Biochimie, 93, 61–70.
Melet, A., Song, K., Bucur, O., Jagani, Z., Grassian, A. R., & Khosravi-Far, R. (2007). Programmed cell death in cancer progression and therapy. (pp. 47–79). Netherlands: Springer.
Fields, A. C., Cotsonis, G., Sexton, D., Santoianni, R., & Cohen, C. (2004). Modern Pathology, 17, 1378–1385.
Ikeguchi, M., Ueda, T., Sakatani, T., Hirooka, Y., & Kaibara, N. (2002). Diagnostic Molecular Pathology, 11, 33–40.
Schimmer, A. D., & Dalili, S. (2005). Hematology, American Society of Hematology. Educatio Program. 215–219.
Peng, X.-H., Karna, P., O'Regan, R. M., Liu, X., Naithani, R., Moriarty, R. M., Wood, W. C., Lee, H.-Y., & Yang, L. (2007). Molecular Pharmacology, 71, 101–111.
Zaffaroni, N., Pannati, M., & Diadone, M. G. (2005). Journal of Cellular and Molecular Medicine, 9, 360–372.
Bhatnagar, N., Li, X., Chen, Y., Zhou, X., Garrett, S. H., & Guo, B. (2009). Cancer Prevention Research, 2, 581–589.
Li, J., Liu, P., Mao, H., Wanga, A., & Zhang, X. (2009). Oncology Reports, 21, 1605–1610.
Pratt, M. A. C., Niu, M. Y., & Renart, L. I. (2006). Apoptosis, 11, 589–605.
Nassar, A., Lawson, D., Cotsonis, G., & Cohen, C. (2008). Applied Immunohistochemistry & Molecular Morphology, 16, 113–120.
Li, F., Yang, J., Ramnath, N., Javle, M. M., & Tan, D. (2004). International Journal of Cancer, 114, 509–512.
Engels, K., Knauer, S., Metzler, D., Simf, C., Struschka, O., Bier, C., Mann, W., Kovács, A., & Stauber, R. (2007). The Journal of Pathology, 211, 532–540.
Li, F., Ackermann, E. J., Bennett, C. F., Rothermel, A. L., Plescia, J., Tognin, S., Villa, A., Marchisio, P. C., & Altieri, D. C. (1999). Nature Cell Biology, 1, 461–466.
Verdecia, M. A., Huang, H., Dutil, E., Kaiser, D. A., Hunter, T., & Noel, J. P. (2000). Nature Structural & Molecular Biology, 7, 602–608.
Dohi, T., Beltrami, E., Wall, N. R., Plescia, J., & Altieri, D. C. (2004). Journal of Clinical Investigation, 114, 1117–1127.
Blanc-Brude, O. P., Mesri, M., Wall, N. R., Plescia, J., Dohi, T., & Altieri, D. C. (2003). Clinical Cancer Research, 9, 2683–2692.
Altieri, D. C. (2006). Current Opinion in Cell Biology, 18, 609–615.
Lens, S. M. A., & Medema, R. H. (2003). Cell Cycle, 2, 507–510.
Samadi, N., Bekele, R. T., Goping, I. S., Schang, L. M., & Brindley, D. N. (2011). PLoS One, 6, e20608.
Kobayakawa, J., Sato-Nishimori, F., Moriyasu, M., & Matsukawa, Y. (2004). Cancer Letters, 208, 59–64.
Li, F., Ambrosini, G., Chu, E. Y., Plescia, J., Tognin, S., Marchisio, P. C., & Altieri, D. C. (1998). Nature, 396, 580–584.
Nomura, T., Yamasaki, M., Nomura, Y., & Mimata, H. (2005). Oncology Reports, 14, 993.
Zhang, M., Latham, D. E., Delaney, M. A., & Chakravarti, A. (2005). Oncogene, 24, 2474–2482.
Carvalho, A., Carmena, M., Sambade, C., Earnshaw, W. C., & Wheatley, S. P. (2003). Journal of Cell Science, 116, 2987–2998.
Zaffaroni, N., & Daidone, M. G. (2002). Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 5, 65.
Murillo, G., Peng, X., Torres, K. E. O., & Mehta, R. G. (2009). Cancer Prevention Research, 2, 942–950.
Mertens-Talcott, S. U., Chintharlapalli, S., Li, X., & Safe, S. (2007). Cancer Research, 67, 11001–11011.
Zhu, N., Gu, L., Findley, H. W., Chen, C., Dong, J. T., Yang, L., & Zhou, M. (2006). Journal of Biological Chemistry, 281, 14711–14718.
Hui, L., Zheng, Y., Yan, Y., Bargonetti, J., & Foster, D. (2006). Oncogene, 25, 7305–7310.
Acknowledgments
The authors would like to acknowledge Dr. Tohidkia and Dr. Abdolalizadeh for their kind assistance in Western blot analysis and Mr. Sajjad Khani for contribution in Real-time PCR experiments. We are also grateful for the opportunity to work in Research Center for Pharmaceutical Nanotechnology in Tabriz University of medical sciences.
Conflict of interest
Authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Parisa Ghanbari and Mahsa Mohseni contributed equally to this.
Rights and permissions
About this article
Cite this article
Ghanbari, P., Mohseni, M., Tabasinezhad, M. et al. Inhibition of Survivin Restores the Sensitivity of Breast Cancer Cells to Docetaxel and Vinblastine. Appl Biochem Biotechnol 174, 667–681 (2014). https://doi.org/10.1007/s12010-014-1125-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1125-6