Abstract
A haloalkaliphilic bacterium was isolated from salt-enriched soil of Mithapur, Gujarat (India) and identified as Bacillus agaradhaerens Mi-10-62 based on 16S rRNA sequence analysis (NCBI gene bank accession, GQ121032). The bacterium was studied for its α-amylase characteristic in the presence of organic solvents. The enzyme was quite active and it retained considerable activity in 30% (v/v) organic solvents, dodecane, decane, heptane, n-hexane, methanol, and propanol. At lower concentrations of solvents, the catalysis was quite comparable to control. Enzyme catalysis at wide range of alkanes and alcohol was an interesting finding of the study. Mi-10-62 amylase retained activity over a broader alkaline pH range, with the optimal pH at 10–11. Two molars of salt was optimum for catalysis in the presence of most of the tested solvents, though the enzyme retained significant activity even at 4 M salt. With dodecane, the optimum temperature shifted from 50 °C to 60 °C, while the enzyme was active up to 80 °C. Over all, the present study focused on the effect of organic solvents on an extracellular α-amylase from haloalkaliphilic bacteria under varying conditions of pH, temperature, and salt.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Starch is one of the most abundant polymers in nature, and amylase plays a central role in its utilization. Amylase is produced by eukaryotes, prokaryotes, and archaea displaying its diverse nature [43]. The amylase is significant in many industrial processes, such as starch liquefaction, pulp process, and detergent making [22, 32, 43]. In addition, food and starch processing industries require its huge quantity [42]. Therefore, with its involvement in many industries, amylase has emerged as the key enzyme of biotechnological significance. Haloalkaliphiles are an attractive group of extremophiles, having an ability to survive under saline and alkaline conditions [8, 20]. With these feature, such organisms provide unique system for investigating biocatalysis under a multitude of extremities.
Halophilic proteins are stable at high salt concentrations due to their innate habitat at saline environment and special arrangement of amino acids [11, 23, 26, 30]. With comparatively larger number of negatively charged amino acids on surface, halophilic proteins display hydrophobic characteristics in contrast to non-halophilic proteins. This feature is beneficial to avoid precipitation of enzyme as well as to maintain structural flexibility with organic solvents [30]. Noticeably, haloalkaliphilic proteins need high salt concentration for activity and stability [9], and majority get unfolded and inactivated at less than 1–2 M NaCl or KCl [26]. High salt concentration creates a hydrophobic environment and thus, haloalkaliphilic enzymes are suitable for biocatalysis under non-aqueous conditions.
While many amylases are reported to be coming from microbial sources, only a few haloalkaliphilic bacteria are known in this context. In particular, studies on the organic solvent tolerance among haloalkaliphilic α-amylase are nearly non-existent.
Due to exceeding boundaries of biotechnology, requirement of variety of organic solvent-tolerant enzymes has enhanced. Demand of organic solvent-tolerant amylases is greatly enhanced due to their significance in clinical, medicinal, and analytical sectors. This present work focused on the catalytic potential of haloalkaliphilic amylase in the presence of organic solvents.
Material and Methods
Microorganism and Culture Conditions
Haloalkaliphilic sp. Mi-10-62 was isolated from saline soil collected from the seashore near Mithapur (Latitude 22.28°N, Longitude 69.4, 60°E) in Gujarat, western cost of India, by Dr. Mital Dodia as part of her PhD research in the Laboratory of Prof. Satya P. Singh, Saurashtra University, Rajkot, Gujarat (India). For screening of amylase-producing isolates, actively growing cultures were inoculated on starch agar plates (grams/liter: starch, 2; yeast extract, 3; peptone, 5; NaCl, 100; pH 8–10; and agar, 30) and incubated at 37 °C for 24–48 h. Amylase-producing bacteria were identified, and Mi-10-62 was selected on the basis of relative enzyme secretion for further study. Based on 16S rRNA gene sequencing, Mi-10-62 was phylogenetically nearest to Bacillus agaradhaerens. The accession number of the submitted 16S rRNA gene sequence from Mi-10-62 is GQ121032.
Amylase Production and Enzyme Assay
From the activated culture of Mi-10-62 (A540, 1.0), 5% was inoculated into starch medium (grams/liter: starch, 2; yeast extract, 3; peptone, 5; NaCl, 100; pH 9) and incubated at 37 °C. Culture was harvested after 12 h, standardized for maximum amylase production, and centrifuged at 10,733 RCF (×g) for 15 min at 4 °C. The cell-free extract was used as crude enzyme preparation. The crude enzyme was precipitated by ammonium sulfate (75% saturation, w/v), and the precipitate was suspended in a minimum volume of 20 mM NaOH–borax buffer (pH 10). This preparation was treated as partially purified enzyme.
Amylase activity was measured by estimating reducing groups released from starch, by the reduction of 3,5-dinitrosalicylic acid (DNS), as described by Bernfeld [3]. Enzyme sample (0.5 ml) was added to 1-ml (2%, w/v) starch prepared in NaOH–borax buffer (20 mM, pH 10) and was incubated at 37 °C for 20 min. One milliliter of DNS reagent (grams/liter: DNS, 10; sodium potassium tartarate, 300; and sodium hydroxide, 16) was added to the mixture and kept in boiling water bath for 10 min. After cooling, the mixture was diluted with 8-ml distilled water, and absorbance was measured at 540 nm. One unit of amylase activity was defined as 1 μg of maltose liberated by the enzyme from starch per minute.
Organic Solvents
Methanol, butanol, propanol, n-hexane, heptane, decane, and dodecane, with log Pow values as 0.82, 0.9, 0.25, 3.9, 4.66, 5.7, and 6.6, respectively, were selected to assess their effect on the catalytic potential of Mi-10-62 amylase.
Effect of Organic Solvents on Catalysis of Amylase
Amylase activity of crude and partially purified enzyme was measured in a reaction mixture containing 0.5-ml enzyme and 1-ml starch solution (2%, w/v) prepared in NaOH–borax buffer (20 mM, pH 10) and 10%, 20%, and 30% (v/v) of methanol, propanol, n-hexane, butanol, heptane, decane, and dodecane. Enzyme assay was further carried out as described above. Controls for each set were also carried out simultaneously.
Effect of pH on Amylase Catalysis
Effect of pH on crude and partially purified amylase was examined by carrying out an enzyme assay at different pH in the presence of various organic solvents. The buffers (20 mM) were Tris-HCl (pH 8–9.5), NaOH–borax (pH 9.5–10), and glycine–NaOH (pH 8–12). The enzyme was incubated with 10%, 20%, and 30% (v/v) of organic solvents along with the respective buffers.
Effect of NaCl on Amylase Activity
To assess the influence of NaCl and organic solvents, in conjunction on crude and partially purified amylase, the reaction mixtures were supplemented with 0.5–4 M NaCl, and amylase assay were carried out at 37 °C with 10%, 20%, and 30% (v/v) of the solvents.
Effect of Temperature on Amylase Catalysis
The temperature profile for amylase activity was examined in the presence of propanol by incubating the assay reaction mixtures at different temperatures, 40–80 °C. The amylase activity was determined as mentioned above.
Results
The present study describes the characterization of amylase from haloalkaliphilic bacteria in response to organic solvents. Only limited citations are available for the catalytic potential of haloalkaliphilic bacteria [14, 16, 37], and majority of the reports are from Soda Lakes and Dead Sea. The present report assumes significance as the amylase is described from the haloalkaliphilic bacteria dwelling in saline habitat of the western coast in Gujarat, India. Partial purification of the enzyme was achieved by ammonium sulfate precipitation with 3.9-fold purification, specific activity of 1,246.6 U/mg and 34.87% yield.
Amylase Catalysis in the Presence of Organic Solvents
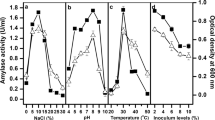
The crude and partially purified preparations of Mi-10-62 α-amylase were quite active in the presence of the solvents. Amylase activity, without any solvent, was considered 100% activity. The enzyme was noticeably active up to 30% (v/v) of propanol, n-hexane, heptane, decane, and dodecane. With alcohols and lower alkenes, however, relatively reduced activity was evident in partially purified enzyme. Catalysis in the presence of 10% (v/v) hexane, heptane, decane, and dodecane was comparable to control (Fig. 1a). While with 10% (v/v) dodecane, the activity was nearly the same as that of the control: the enzyme retained 50% activity in the presence of 10% (v/v) butanol. With 0.8 log Pow value, butanol is highly toxic for living organisms and their macromolecule, and therefore, it is quite an interesting feature of this enzyme to be substantially active in its presence. Partially purified amylase with 30% (v/v) dodecane, decane heptane, n-hexane, methanol, and propanol exhibited 72%, 68%, 65%, 64%, 36%, and 31% residual activities, respectively (Fig. 1b). However, with 30% (v/v) butanol, the amylase activity was totally lost. Amylase in crude form retained 79%, 78%, 71%, 61%, 56%, 47%, and 42% residual activities with dodecane, decane, heptane, hexane, propanol, methanol, and butanol, correspondingly. Activity of control was considered as 100% for calculating residual activity.
Effect of pH on the Catalysis of Amylase
Effect of pH on amylase was assessed in the presence of popanol and dodecane, where the enzyme was active in alkaline pH 8–12. The activity at pH 10, without any solvent, was considered as 100%. At several combinations of pH and solvent concentrations, the residual activities were monitored. Crude amylase retained 56%, 46%, and 45% residual activities with 10%, 20%, and 30% (v/v) propanol at pH 8. The loss of activities of partially purified enzyme under similar conditions of pH and solvents were quite comparable to those of crude preparation (Fig. 2a). The enzyme had 60%, 51%, and 45% residual activities in crude form, and 46%, 42%, and 36% in partially purified preparation with the tested concentrations of dodecane (Fig. 2b).
At ph 9, the crude amylase exhibited 57%, 50%, and 30% residual activities, while the partially purified enzyme retained 73%, 62%, and 40% activities in the presence of 10%, 20%, and 30% (v/v) of propanol. At the same concentrations of dodecane, the crude and partially purified enzymes had 75%, 60%, and 55%, and 55%, 49%, and 40% residual activities, respectively (Fig. 2b).
At optimum pH 10, 86%, 77%, and 64% activities for crude and 79%, 68%, and 62% activities for partially purified enzymes were obtained with tested concentrations of propanol. However, in the presence of dodecane, comparatively higher residual activities of 90%, 85%, and 78%, and 80%, 70%, and 63% were recorded for crude and partially purified enzymes, respectively.
Amylase was quite efficient at pH 11 with dodecane. At pH 11, the enzyme activities for crude were 70%, 58%, and 42%, while partially purified enzyme had 57%, 43%, and 32% activities in the presence of 10%, 20%, and 30% (v/v) solvent, respectively. At pH 12, the activities were quite negligible for both tested solvents. Effect of pH on amylase catalysis in the presence of solvents is presented in Fig. 2a, b.
Effect of Salt on the Catalysis of Mi-10-62 Amylase
Effect of 0.5–4 M NaCl on Mi-10-62 amylase catalysis was examined in the presence of 10–30% (v/v) propanol and dodecane. With both solvents, a change in the pattern of salt profile was evident. For crude and partially purified enzyme, the salt optima were 3 and 2 M NaCl, respectively. Crude amylase with 0.5 M salt and 10%, 20%, and 30% (v/v) propanol and dodecane had 43%, 37%, and 32%,, and 39%, 36%, and 30% residual activities, respectively. For partially the purified enzyme, compared to the control, the activities were 43%, 40%, and 37% with propanol and 44%, 41%, and 38% residual activity with dodecane, respectively.
At 1 M salt, better enzyme activity was observed with both tested solvents. As compared to the activity at 0.5 M salt, nearly twofold enzyme activities were recorded at 1 M salt. With 1 M salt, the activities of crude enzyme were 76%, 73%, and 64% at tested concentrations of propanol and 78%, 72%, and 46% residual activities with dodecane. With the same concentrations of salt and propanol, activities of partially purified enzyme were 74%, 71%, and 64%, while with dodecane, 80%, 76%, and 67% residual activities were recorded. Salt at 2 M was optimum for partially purified enzyme, exhibiting 94%, 89%, and 78% residual activities with propanol and 97%, 92%, and 87% residual activities with the tested concentrations of dodecane. With further increase in salt, partially purified enzyme resulted in loss of activity (Fig. 3a, b). NaCl at 3 M was optimum for crude amylase, resulting in 111%, 97%, and 77%, and 104%, 97%, and 89% residual activities with propanol and dodecane, respectively. At 4 M salt, decreased activities were evident with both solvents.
Temperature Optima of Mi-10-62 in Presence of Propanol
Figure 4 displays the effect of temperature on the catalysis of Mi-10-62 amylase in the presence of propanol. Optimum temperature for crude amylase was 50 °C, which shifted to 60 °C in the presence of propanol. At 20% (v/v) propanol, the partially purified enzyme retained a comparable activity as control with the enhanced temperature optima. At 30% (v/v) solvent, the enzyme retained nearly 50% of the residual activity.
Discussion
Extremozymes have attracted considerable attention due to their potential to meet industrial demands for enzymes with multitude of extremities. High salinity, alkaline conditions, and non-aqueous medium are some of the examples of extremity for biocatalysis. However, only limited literature is available on the enzymes from haloalkaliphilic bacteria with respect to non-aqueous biocatalysis [12, 15, 17, 21, 29]. Some intracellular and extracellular enzymes from extremely and moderately halophilic and haloalkaliphilic bacteria and actinomycetes have been isolated and characterized, which might have potential applications in food, chemical, pharmaceutical, leather, tanning, paper pulp, and waste-treatment industries [5, 19, 27, 34, 35, 41, 44]. The studies on haloalkaliphilic amylase with respect to their tolerance against organic solvent have not been investigated in great deal. However, some haloalkaliphilic archaea, actinomycetes, and their relationship with organic solvents have been investigated during the recent years [38–40]. It is well reported that enzymes are inactivated in the presence of organic solvents, and catalytic activities in non-aqueous environment are generally lower than those in aqueous system [33, 36].Therefore, it was quite interesting to study an amylase from haloalkaliphilic bacteria in a non-aqueous medium. A haloalkaliphilic amylase reported in this study was screened against seven organic solvents: methanol, propanol, n-hexane, butanol, heptane, decane, and dodecane. The amylase displayed varying responses against these solvents. Catalysis of Mi-10-62 amylase in the presence of butanol was an interesting feature of the study. Catalysis with 20% (v/v) water, miscible and immiscible alcohols, and alkane indicated the robust nature of the enzyme. At concentrations above 20% (v/v), varying effects were observed.
Mi-10-62 α-amylase was active over a wide range of pH 8–11, the optimum being at 10. These values are marginally higher than those reported for an amylase from an alkaliphilic Bacillus sp. [18] and significantly higher than those reported for another amylase from Halobacterium salinarum [13]. Other organisms, such as thermophilic and halotolerant bacteria, Halothermothrix orenii, are reported to have an amylase with optimal activities in the similar range of pH [28]. Halophilic enzymes, in general, are not stable in low salt concentrations because of ionic charges and salt-dependent structural stability [1, 7, 26, 31]. Therefore, an increase of activity with salt concentrations is a common feature of the halophilic enzymes [10].
The α-amylase from Mi-10-62 in the present study displayed an upward shift in activity with salt from 0.5 to 2 M. Salt affects the binding between the enzyme and substrate (starch). Most of the halophilic and haloalkaliphilic enzymes are inactivated at NaCl or KCl concentrations below 2 M [6]. As described earlier, the amylase activity in Mi-10-62 increased with increasing salt concentrations, indicating an overall effect of salt on the reaction. The enzyme was quite active with 1 M NaCl even in the presence of solvents. The optimum catalysis at comparatively low salt and its behavior to retain activity with broader range of salt concentrations in the presence of solvents is quite relevant to haloalkaliphilic bacteria. The findings are quite comparable to a moderately halophilic and aerobic bacterium, Halomonas meridiana [4]. The Mi-10-62 amylase had differential effects in response to salt when crude and partially purified enzymes were compared. While 2 M salt was optimal for partially purified enzyme, the crude preparation required 3 M for maximal activity.
The optimal temperature (55–60 °C) for Mi-10-62 amylase was quite comparable with the enzyme from Halobacterium salinarum [13]. However, the enzyme was active at higher temperatures, and with 20% v/v propanol, it retained significant activity up to 70 °C. Comparable to our studies, the enzyme from an alkaliphilic Bacillus sp. also exhibited the optimal temperature at 60 °C [18]. Other halophilic enzymes, such as NAD and NADP glutamate dehydrogenases from Halobacterium salinarum displayed maximal activity at 70 °C, with higher temperature stability [2]. The temperature profiles and stability were quite comparable to a thermophilic amylase from Thermus sp. AMD33, which had an optima at 70 °C [24], or with a halophilic and thermophilic bacteria Halothermothrix orenii, with an optima at 65 °C [28]. The thermophilic nature of our enzyme was also reflected by a shift in temperature optima to a higher range. The high optimal temperatures for enzymatic catalysis in halophilic organisms may be considered an adaptive feature, as these enzymes have to endure in their natural salt environments, such as slatterns exposed to intense sunlight. The thermophilic nature has been further reported for several halophilic enzymes [25, 28].
In conclusion, the enzyme described in the present report highlighted several features quite similar to those found in other halophilic enzymes, including salt-dependent activity. Further, the temperature profile and thermal stability closely resembled to features reflected in thermophilic organisms. The findings on the haloalkaliphilic extracellular α-amylase with respect to its catalysis and enzymatic stability under multitude of extremities, salt, temperature, and organic solvents, would enrich the knowledge on non-aqueous enzymology, broadening the prospects of biocatalysis.
References
Bonete, M. J., Camachmo, L., & Cadenaes. (1987). International Journal of Biochemistry, 19, 1149–1155.
Bonnete, F., Madern, D., & Zaccai, G. (1994). Journal of Molecular Biology, 244, 436–447.
Bernfeld, P. (1951). Interscience Publ., NY, 379.
Coronado, M. J., Carmen, V., Mellado, E., Tegos, G., Drainas, C., Nieto, J. J., et al. (2000). Microbiology, 146, 861–868.
Costa, D. A., Santos, M. S., & Galinski, E. A. (1998). Advances in Biochemical Engineering/Biotechnology, 61, 117–153.
Camacho, M. L., Brown, R. A., Bonete, M. J., Danson, M. J., & Hough, D. W. (1995). Isocitrate dehydrogenases from Haloferax volcanii and Sulfolobus solfataricus: enzyme purification, characterization and N-terminal sequence. FEMS Microbiology Letters, 134(1), 85–90.
Danson, M. J., & Hough, D. W. (1997). Biochemistry and Physiology, 117A, 307–312.
Dodia, M. S., Bhimani, H. G., Rawal, C. M., Joshi, R. H., & Singh, S. P. (2008). Bioresource Technology, 99, 6223–6227.
Dodia, M. S., Rawal, C. M., Bhimani, H. G., Joshi, R. H., Khare, S. K., & Singh, S. P. (2008). Journal of Industrial Microbiology and Biotechnology, 35(2), 121–132.
Dym, O., Mevarech, M., & Sussman, J. L. (1995). Science, 267, 1344–1346.
Eisenberg, H., Mevarech, M., & Zaccai, G. (1992). Advances in Protein Chemistry, 43, 1–62.
Eichler, J. (2001). Biotechnology Advances, 19, 261–278.
Good, W. A., & Paul, A. H. (1970). Journal of Bacteriology, 104(1), 601–603.
Gimenez, M. I., Studdert, C. A., Sanchez, J., & De Castro, R. (2000). Extremophiles, 4, 181–188.
Gupta, A., Roy, I., Patel, R. K., Singh, S. P., Khare, S. K., & Gupta, M. N. (2005). Journal of Chromatography. A, 1075, 103–108.
Herrera, S. K., Studdert, C., Sanchez, J., & De Castro, R. (1997). Journal of Basic Microbiology, 7, 313–322.
Herbert, R. A. (1992). Trends in Biotechnology, 10, 395–401.
Igrashi, K., Hatada, Y., Hagihara, H., Saeki, K., Takaiwa, M., Uemura, T., et al. (1998). Applied and Environmental Microbiology, 64, 3282–3289.
Jogi, C., Joshi, R. H., Dodia, M. S., & Singh, S. P. (2005). Journal of Cell and TissueResearch, 5(2), 439–444.
Joshi, R. H., Dodia, M. S., & Singh, S. P. (2008). Biotechnology and Bioprocess Engineering, 13, 552–559.
Karan, R., Singh, S. P., Kapoor, S. M., & Khare, S. K. (2010). New Biotechnology. doi:10.1016/j.nbt.2010.10.007.
Kadziola, A., Sogaard, M., Svensson, B., & Haser, R. (1998). Journal of Molecular Biology, 278, 205–217.
Lanyi, J. K. (1974). Bacteriological Reviews, 38, 272–290.
Nakamura, T., Syukunobe, Y., Sakarai, T., & Idota, T. (1993). Milchwissenschaft, 48, 11–14.
Marhuenda-Egea, F., & Bonete, M. J. (2002). Current Opinion in Biotechnology, 13, 385–389.
Madern, D., Ebel, C., & Zaccai, G. (2000). Extremophiles, 4, 91–98.
Mehta, V. J., Thumar, J. T., & Singh, S. P. (2006). Bioresource Technology, 97, 1650–1654.
Mijts, B. N., & Patel, B. K. C. (2001). Extremophiles, 5, 61–69.
Madigan, M. T., & Marrs, B. L. (1997). Scientific American, 276(4), 66–71.
Mevarech, M., Frolow, F., & Gloss, L. M. (2000). Biophysical Chemistry, 86, 155–164.
Martınez-Espinosa, R. M., Lledoo, B., Frutos Marhuenda-Egea, C., Dıaz, S., & Marıa Jose, B. (2009). Extremophiles, 13, 785–792.
Machius, M., Wiegand, G., & Huber, R. (1995). Journal of Molecular Biology, 246, 545–559.
Ogino, H., & Ishikawa, H. (2001). Journal of Bioscience and Bioengineering, 91, 109–116.
Patel, R. K., Dodia, M. S., & Singh, S. P. (2005). Process Biochemistry, 40, 3569–3575.
Patel, R. K., Dodia, M. S., Joshi, R. H., & Singh, S. P. (2006). World Journal of Microbiology and Biotechnology, 22(4), 375–382.
Ru, M. T., Dordick, J. S., Reimer, J. A., & Clark, D. S. (1999). Biotechnology and Bioengineering, 63(2), 233–241.
Studdert, C. A., Seitz, M. K. H., Gilv, M. I. P., Sanchez, J. J., & De Castro, R. (2001). Journal of Basic Microbiology, 41, 375–383.
Saraiva, J., Oliveira J., Hendrickx, M. (1996). Lebensmittel-Wissenschaft und-Technologie, 29, 310–315.
Tadamasa, F., Toru, M., Akinobu, E., Akira, I., & Usami, R. (2005). Extremophiles, 9, 85–89.
Thumar, J. T., & Singh, S. P. (2009). Journal of Industrial Microbiology and Biotechnology, 36, 211–218.
Thumar, J. T., & Singh, S. P. (2007). Journal of Chromatography B, 854, 198–203.
Vihinen, M., Peltonen, T., Iitia, A., Suominen, I., & Mantsala, P. (1994). Protein Engineering, 7, 1255–1259.
Upadek, H., & Kottwitz, B. (1997). Surfactant science series (pp. 203–212). New York: Marcel Dekker.
Wejse, P. L., Ingvorsen, K., & Mortensen, K. K. (2003). Extremophiles, 7, 423–431.
Acknowledgment
Financial assistance from the University Grants Commission (New Delhi, India) and Saurashtra University, Rajkot (India) is acknowledged. Mr. Sandeep Pandey is grateful to the UGC, New Delhi for the Research Fellowship in Sciences for Meritorious Students.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandey, S., Singh, S.P. Organic Solvent Tolerance of an α-Amylase from Haloalkaliphilic Bacteria as a Function of pH, Temperature, and Salt Concentrations. Appl Biochem Biotechnol 166, 1747–1757 (2012). https://doi.org/10.1007/s12010-012-9580-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9580-4