Abstract
Pancreatic cancer is a malignant tumor with the worst prognosis among all cancers. At the time of diagnosis, surgical cure is no longer a feasible option for most patients, thus early detection of pancreatic cancer is crucial for its treatment. Metabolomics is a powerful new analytical approach to detect the metabolome of cells, tissue, or biofluids. Here, we report the application of 1H nuclear magnetic resonance (NMR) combined with principal components analysis to discriminate pancreatic cancer patients from healthy controls based on metabolomic profiling of the serum. The metabolic analysis revealed significant lower of 3-hydroxybutyrate, 3-hydroxyisovalerate, lactate, and trimethylamine-N-oxide as well as significant higher level of isoleucine, triglyceride, leucine, and creatinine in the serum from pancreatic cancer patients compared to that of healthy controls. Our data demonstrate that the subtle differences in metabolite profiles in serum of pancreatic cancer patients and that of healthy subjects as a result of physiological and pathological variations could be identified by NMR-based metabolomics and exploited as metabolic markers for the early detection of pancreatic cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is a malignant tumor that accounts for only 2% of all cases of cancer worldwide, but it has the worst prognosis among all kinds of cancers, with a 5-year survival rate of less than 3%. In the USA, pancreatic cancer is the fourth leading cause of cancer mortality. It is estimated that over 37,000 patients will be diagnosed with and 34,000 patients will die of pancreatic cancer annually [1]. The high mortality rate is largely due to the insidious symptoms and the difficulty in early identification. At the time of diagnosis, surgical cure is no longer a feasible option for most patients. Only 10–25% of pancreatic cancer cases are candidates for surgical cure [2]. When the diameter of tumor is less than 1 cm, the patients have a 5-year survival rate of 70–100%, but when the diameter of tumor is less than 2 cm, the patients have a 5-year survival rate of 19–41%. Thus, the best treatment option for pancreatic cancer is resection at the early stage.
Unfortunately, because of unnoticeable symptoms and deep location, pancreatic cancer often starts silently without symptoms at its early stage but progresses quite rapidly and has a poor prognosis. The clinical diagnosis of pancreatic cancer is mainly dependent on imaging examination, such as magnetic resonance imaging and computed tomography, endoscopic retrograde cholangiopancreatography and endoscopic ultrasound. However, the specificity and sensitivity of these modalities are not good enough for a tumor with a diameter of less than 2 cm.
Nuclear magnetic resonance (NMR) spectroscopy is one of the most powerful analytical tools that can provide a wealth of metabolite information on biological samples under physiological and pathological conditions. It has been successfully applied in the study of endogenous metabolic changes in biofluids such as urine, serum, and saliva caused by diseases or drug toxicity [3]. The comparison of metabolic profiles between cancer and normal subjects has the potential to allow clinicians to detect pancreatic cancer at its early stage for early diagnosis of pancreatic cancer.
In this study, proton NMR (1H NMR) was employed to investigate the metabolic features in the serum from human pancreatic cancer patients and healthy controls. The differences in metabolite profiles in serum of pancreatic cancer patients and that of healthy subjects as a result of physiological and pathological variations were identified, and the significance for early detection of pancreatic cancer was analyzed.
Material and Methods
Sample Collection
Both pancreatic cancer patients and healthy subjects were included in the study. Seventeen pancreatic cancer patients (aged from 49 to 68 years old, 7 male and 10 female) were recruited and who underwent general surgery, and 23 healthy subjects (aged from 45 to 71 years, 9 male and 14 female) were volunteers who underwent routine medical examination. To be eligible for the study, all subjects had to complete a questionnaire in which they confirmed their continued good health and they did not take any regular medication. Exclusion criteria included hypertension and acute illness within 2 weeks before sample collection. The study protocol was approved by the Ethics Committee of Fujian Medical University Union Hospital, and all subjects gave written informed consent.
1H NMR Spectroscopy
Serum samples (400 μl) were mixed with a 200-μl phosphate buffer solution (0.2 M Na2HPO4/0.2 M NaH2PO4, pH 7.4, 100% D2O) to minimize variations in pH. A 0.3-mM 2,2-dimethyl-2-silapentane-5-sulfonic acid (DSS) was used as an internal reference standard at δ 0.0. The mixture was piped into 5-mm NMR tubes. The 1H NMR spectra of the serum samples were acquired on Varian NMR system 500 MHz spectrometer equipped with triple resonance probe. The probe temperature was set to 298 K, and the 90° pulse lengths were calibrated individually for each sample. For the serum samples, a Carr–Purcell–Meiboom–Gill spin-echo pulse train was incorporated with the relaxation time (2nτ) 100 ms and the echo time (τ) 250 μs. A total of 256 scans with a spectral width of 5 kHz were collected for all NMR spectra. All the signals were zero filled to 16 k before regular Fourier transformation.
NMR Data Reduction and Principal Component Analysis
The collected NMR data were phased, and the baseline data were corrected manually using the software MestRe-C 4.8 (http://www.mestrec.com). The chemical shifts were referenced to the DSS at δ 0.0. Every spectrum was segmented into regions of 0.04 ppm width in the range of 0.2–9.8 ppm. The region of 4.6–5.0 ppm of water signal was excluded for serum spectra. The remaining spectral segments were scaled to total integrated area of each spectrum to account for the differences in concentration. Principal component analysis (PCA) was carried out using the program we wrote.
Results
1H NMR Spectroscopy
The representative spectra for 1H NMR analysis of serum samples from pancreatic cancer patients and healthy controls were shown in Fig. 1. The main serum metabolites were assigned according to literature [4].
Principal Component Analysis of 1H NMR Spectroscopy
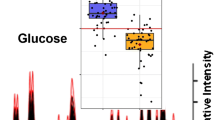
Multivariate statistical modeling using PCA was performed on the 1H NMR spectra of serum samples. The score plot (Fig. 2a) derived from PCA demonstrated a clear discrimination between the metabolome of pancreatic cancer patients and healthy group. Furthermore, loadings plot analysis revealed that the discrimination was mainly attributed to the metabolites such as isoleucine, triglyceride, leucine, creatinine, lactate, 3-hydroxybutyrate, 3-hydroxyisovalerate, and trimethylamine-N-oxide (TMAO; Fig. 2b, Table 1).
The spectroscopic profiling of these two groups revealed obvious alterations in metabolite levels. The score plot of PCA showed clear separation of the pancreatic cancer group from the healthy group, indicating the discrepancy in metabolomes between these two classes.
Discussion
In the present study, we performed metabolomic profiling of serum from human pancreatic cancer patients and healthy controls using 1H NMR spectroscopy and principal component analysis. The results demonstrated that significant lower level of 3-hydroxybutyrate, 3-hydroxyisovalerate, lactate, and TMAO and significant higher level of isoleucine, triglyceride, leucine, and creatinine were observed in serum from pancreatic cancer patients, suggesting that these metabolites in the CCM region [4] can be used as metabolic markers for the discrimination of pancreatic cancer patients from healthy subjects.
3-Hydroxybutyrate can be synthesized from 3-hydroxybutyric acid, which is a chiral compound with two enantiomers, d-3-hydroxybutyric acid and l-3-hydroxybutyric acid. 3-Hydroxybutyric acid level is increased in ketosis. In humans, beta-hydroxybutyrate is synthesized in the liver from acetyl-CoA and can be used as an energy source by the brain when blood glucose is low [5].
3-Hydroxyisovalerate is derived from isovaleryl-CoA, a catabolic intermediate of leucine. Interestingly, a recent study suggested that modulation of the expression of leucine zipper tumor suppressor 2 has direct effects on the proliferation of various cancer cells [6]. Thus, the significant decrease of 3-hydroxyisovalerate level in the serum may suggest the development of pancreatic cancer.
Isoleucine and leucine are essential amino acids. The increased levels of isoleucine and leucine in the serum of pancreatic cancer patients we detected may attribute to the metabolism obstacle of these amino acids in pancreatic cancer.
Trimethylamine-N-oxide is biosynthesized from trimethylamine. A decrease in TMAO level occurs upon the consumption of a lactovegetarian diet. Increased excretion of TMAO has been correlated with meat and fish ingestion or a diet with a high salt content [7]. The lower level of TMAO may be associated with the food tendency of pancreatic cancer patients.
Hypertriglyceridemia is crucially involved in obesity, which increases the risk of lung, rectal, and thyroid cancer. Clinical studies have clearly shown that obesity leads to increased severity of acute pancreatitis [8–11], and obesity appears to confer an increased risk of pancreatic cancer [12–15].
Our results showed that pancreatic cancer patients had a significantly higher serum creatinine level compared to controls. An increase in creatinine within the first 48 h is strongly associated with the development of pancreatic necrosis [16]. Histological necrosis is a simple, accurate, and reproducible predictor of postoperative outcome in pancreatic ductal carcinoma patients [17].
Lactate is the product of anaerobic glycolysis and is increased in hypoxia, ischemia, and poorly vascularized cancer. The significant increase in lactate level has been found in many human cancers [18–20]. Nevertheless, our results showed that the lactate level was decreased in the serum of pancreatic cancer patients. This discrepancy should be investigated in our future study.
In summary, based on principal component analysis of the metabolomic profiling, pancreatic cancer patients and healthy controls could be well separated in all of the score plots as shown in Fig. 2. Although the number of samples in our study was limited, our results provide support for the potential application of 1H NMR for the investigation of metabolic features of serum derived from pancreatic cancer patients. Our data demonstrate that the differences in metabolite profiles in serum of pancreatic cancer patients and that of healthy subjects as a result of physiological and pathological variations could be identified by NMR-based metabonomics and exploited as metabolic markers for the early detection of pancreatic cancer.
References
Jemal, A., Siegel, R., Ward, E., Hao, Y., Xu, J., & Murray, T. (2008). Thun MJ. CA: A Cancer Journal for Clinicians, 58, 71–96.
Sener, S. F., Fremgen, A., Menck, H. R., & Winchester, D. P. (1999). Journal of the American College of Surgeons, 189, 1–7.
Bollard, M. E., Stanley, E. G., Lindon, J. C., Nicholson, J. K., & Holmes, E. (2005). NMR in Biomedicine, 18, 143–162.
Holmes, E., Foxall, P. J. D., Spraul, M., Farrant, R. D., Nicholson, J. K., & Lindon, J. C. (1997). Journal of Pharmaceutical and Biomedical Analysis, 15, 1647–1659.
Baran, E. T., Ozer, N., & Hasirci, V. (2002). Journal of Microencapsulation, 19(3), 363–376.
Kim, J. M., Song, J. S., Cho, H. H., Shin, K. K., Bae, Y. C., Lee, B. J., et al. (2011). Molecular and Cellular Biochemistry, 346(1–2), 125–136.
Kim, K. B., Yang, J. Y., Kwack, S. J., Park, K. L., Kim, H. S., Ryu, D. H., et al. (2010). Journal of Toxicology and Environmental Health. Part A, 73(21–22), 1420–1430.
DeWaele, B., Vanmierlo, B., Van Nieuwenhove, Y., & Delvaux, G. (2006). Pancreas, 32, 343–345.
Martinez, J., Johnson, C. D., Sanchez-Paya, J., de Madaria, E., Robles-Diaz, G., & Perez-Mateo, M. (2006). Pancreatology, 6, 206–209.
Papachristou, G. I., Papachristou, D. J., Avula, H., Slivka, A., & Whitcomb, D. C. (2006). Pancreatology, 6, 279–285.
Suazo-Barahona, J., Carmona-Sanchez, R., Robles-Diaz, G., Milke-Garcia, P., Vargas-Vorackova, F., Uscanga-Dominguez, L., et al. (1998). The American Journal of Gastroenterology, 93, 1324–1328.
Calle, E. E., Rodriguez, C., Walker-Thurmond, K., & Thun, M. J. (2003). The New England Journal of Medicine, 348, 1625–1638.
Giovannucci, E., & Michaud, D. (2007). Gastroenterology, 132, 2208–2225.
Michaud, D. S., Giovannucci, E., Willet, W. C., Colditz, G. A., Stampfer, M. J., & Fuchs, C. S. (2001). JAMA., 286, 921–929.
Patel, A. V., Rodriguez, C., Bernstein, L., Chao, A., Thun, M. J., & Calle, E. E. (2005). Cancer Epidemiology, Biomarkers and Prevention, 14, 459–466.
Muddana, V., Whitcomb, D. C., Khalid, A., Slivka, A., & Papachristou, G. I. (2009). The American Journal of Gastroenterology, 104(1), 164–170.
Hiraoka, N., Ino, Y., Sekine, S., Tsuda, H., Shimada, K., Kosuge, T., et al. (2010). British Journal of Cancer, 103(7), 1057–1065.
Brizel, D. M., Schroeder, T., Scher, R. L., Walenta, S., Clough, R. W., & Dewhirst, M. W. (2001). International Journal of Radiation Oncology, Biology, Physics, 51, 349–353.
Schwickert, G., Walenta, S., Sundfor, K., Rofstad, E. K., & Mueller-Klieser, W. (1995). Cancer Research, 55, 4757–4759.
Walenta, S., Chau, T. V., Schroeder, T., Lehr, H. A., Kunz-Schughart, L. A., Fuerst, A., et al. (2003). Journal of Cancer Research and Clinical Oncology, 129, 321–326.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
OuYang, D., Xu, J., Huang, H. et al. Metabolomic Profiling of Serum from Human Pancreatic Cancer Patients Using 1H NMR Spectroscopy and Principal Component Analysis. Appl Biochem Biotechnol 165, 148–154 (2011). https://doi.org/10.1007/s12010-011-9240-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9240-0