Abstract
Bacillus atrophaeus’s spores are used as biological indicators to monitor sterilization processes and as a Bacillus anthracis surrogate in the development and validation of biosafety methods. The regular use of biological indicators to evaluate the efficiency of sterilization processes is a legal requirement for health services. However, its high cost hinders its widespread use. Aiming at developing a cost-effective inoculum medium, soybean molasses and nutrient-supplemented vinasse were evaluated for their effectiveness in solid-state fermentation (SSF). In biomass production, the results demonstrated that all tested compositions favor growth by providing the nutritional demands of the microorganism. Optimum casein peptone and soybean molasses concentration (1.0%, 2.5%, or 4.0%) was determined by a 2(2–0) factorial experimental design. The results have showed a positive influence of peptone on biomass production. In order to define peptone final concentration (4.0% or 6.0%), a 22 factorial experimental design was used. An optimized medium containing 4.0% soybean molasses and 4.0% casein peptone was similar in performance to a synthetic control medium (tryptone soy broth) in dry-heat thermal-resistant spore production by SSF. An experiment performed under optimum SSF conditions resulted in 1.9 × 1010 CFU g−1 dry matter with D 160 °C = 5.2 ± 0.2 min.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Preventing infection in patients undergoing dental or medical treatment is important in order to avoid human suffering and diminish health care costs. One aspect in the prevention of infection in health care facilities is the effective sterilization of tools and instruments [1]. Health service current guidelines on infection control practices recommend regular biological monitoring of sterilizers to avoid infections, and their regular use is a legal requirement for health services. Nevertheless, its high cost hinders its widespread use.
Bacillus atrophaeus’s spores are used as biological indicators in tests to assess anti-septic and sterilizing products [2, 3] in order to monitor the low temperature steam (up to 121 °C), dry heat, ethylene oxide, hydrogen peroxide, UV radiation, and plasma sterilization processes [4–6]; determine the efficiency of consumption water treatment systems [7]; and substitute Bacillus anthracis in the development and validation of biosafety methods [8].
The first stage of the sporulation process is the preparation of the culture starter—or inoculum—in order to obtain high biomass production. As a seed culture, spores have some advantages over vegetative cells inoculum because they are relatively easy to count and store while showing minimal chances in viability and other properties [9]. Davies et al. [10] have demonstrated that spores coming from spores’ solutions present greater thermal resistance in relation to those produced from vegetative cells. The United States Pharmacopeia [4] standardizes the use of the synthetic medium—tryptone soy broth (TSB) or soybean casein digest broth—for the production of biological indicators and to recover unheated and heated spores. This chemically defined medium contains casein digest peptone (soybean digest peptone), sodium chloride, and dibasic potassium phosphate as nutrient sources.
In industrial fermentation, the goal is to obtain a low-cost, easily available, and high cell productivity medium [11–13]. Complex media usually give higher fermentation yields at lower costs. The agro-industrial by-products and residues are common raw materials used in fermentation processes. Studies with sugar cane molasses, soy molasses, and corn-steep liquor have presented positive results in relation to the Bacillus sp. biomass productivity [14–17].
The soybean molasses is a by-product of the high-protein concentrate soybean meal production, with high concentration of sugars, 57% dry weight (approximately 65% mono- and disaccharides; and 35% oligosaccharides, mainly raffinose, 5–7%; and 30–32% stachyose), nitrogen, and other macro- and micronutrients [18–20]. Soy molasses vinasse is a residue of soy alcohol production whose utilization brings about positive results in the prevention of environmental pollution.
The B. atrophaeus strain’s capacity to adapt and use several nutrients as energy sources was reported by Neves et al. [21]; and Li et al. [22] have demonstrated that Bacillus strains can grow in the presence of raffinose, galactose, and sucrose as a carbon source. Sella et al. [23] have suggested that soybean molasses can be used as low-cost substrate to obtain high sporulation yield of B. atrophaeus for industrial production by solid-state fermentation (SSF) using sugar cane bagasse as support.
The conditions under which sporulation takes place, including inoculum development conditions, determine spore characteristics such as heat resistance and germination properties [24–27]. The difficulty in developing an ideal inoculum medium lies not just in inducing high-yield spores at sporulation step. The real challenge is to produce large quantities of heat-resistant spores.
Aiming at developing a cost-effective inoculum medium, nutrient-supplemented soybean molasses and vinasse were evaluated for their effectiveness in SSF. Their component concentrations were optimized in order to obtain a similar performance to that of a synthetic control medium (TSB) in dry-heat thermal-resistant and high-yield spore production.
Materials and Methods
Bacterial Strain
B. atrophaeus ATCC 9372, Bach-1403349, was obtained from a standard strain supplied by the Instituto Nacional de Controle de Qualidade em Saúde (INCQS/MS, Brazil).
Selection and Optimization of Starter Culture Medium
Inoculum Medium Selection
Seed culture growth was tested in different agro-industrial by-products and residue media. TSB was used as a reference medium. Soybean molasses (∼75°Brix) was composed of total sugar 50.8 g%, protein 5.1 g%, lipids 0.3 g%, and pH 6.1 and was diluted 1/50 and 1/60.
The soybean vinasse (∼11°Brix) was composed of the following: total sugar 8.3 g%, protein 1.3 g%, lipids 1.9 g%, and pH 4.7. In medium no. 5, it was clarified and diluted 1/15. The medium compositions are (1) soybean molasses 1.7%; casein peptone, 1.8%; (2) soybean vinasse, 6.7%; beef extract, 2.3%; (3) soybean molasses, 2.0%; (4) soybean molasses, 1.7%; and (5) soybean vinasse, 6.7%.
The media were prepared in distilled water, filtered through cotton, adjusted to final pH 7.2 ± 0.1, and autoclaved at 121 °C for 15 min. They were kept at 2–8 °C until inoculation. The soybean molasses and vinasse came from IMCOPA Company (Araucaria, PR, Brazil). For inoculum preparation, 100.0 μL of spore suspension—batch: 01/06-CPPI—was inoculated in three tubes, each with 30.0 mL of tested medium. These media were incubated for 18 h at 36 °C.
Inoculum Media Optimization
A 2(2–0) factorial experimental design was used to select the suitable casein peptone and soybean molasses concentration, with replications of center point and experiments, as shown in Table 1.
The second stage of optimization consisted of a 22 factorial experimental design aiming at defining the casein peptone final concentration (4.0% or 6.0%), as shown in Table 2.
Inoculum Effect in SSF
SSF
Sugarcane bagasse support was obtained from COCAMAR, Cianorte-PR, Brazil. The milled residue was washed once in tap water and twice in distilled water. The washed bagasse was dried on trays for 24 h at 90 °C in an air oven [28]. The dry bagasse was sieved to obtain 0.84 to 1.18 mm particles size (mesh between nine and 14).
Five grams of dried sugar cane bagasse were placed into a 250 mL Erlenmeyer flask. The added substrates consisted of soybean molasses 2.0%, supplemented with sporulation inductor salts (K2HPO4·H2O, 0.005 g%; MnSO4·H2O, 0.004 g%; CaCl2·6H2O, 0.004%; MgSO4·7H2O, 0.005 g%). The initial substrate pH was adjusted to 8.0, and the initial moisture content was adjusted at 93% (dried sugar cane bagasse 1.0 mm mean particle size, that is, the maximum substrate absorption at 121 °C). The flasks were autoclaved at 121 °C for 15 min. The inoculum size was 3% (v/v substrate). Two batches of optimized inoculum medium and one from a control medium (TSB) were used. SSF was carried out at 36 °C for 9 days.
Sporulation Control Agar Medium
Culture was grown in Roux flasks containing 400.0 mL of medium, autoclaved at 121 °C for 15 min. Sporulation was carried out at 36 °C for up to 14 days, using spore suspension as seed for TSB inoculum. The medium pH was adjusted to 7.2 ± 0.2.
The sporulation agar had the following composition: yeast extract 0.8 g%, nutrient broth 0.4 g%, MnSO4·4H2O 0.005 g%, CaCl2·6H2O 0.005 g%, and agar 3.0 g%.
Spore Suspension Preparation
Spores were detached from the agar medium with a sterile glass rod and collected in cold, sterile 0.02 M calcium acetate solution adjusted to pH 9.7 with 0.14% calcium hydroxide solution. In SSF, the fermented mass was mixed with 100.0 mL of 0.02 M calcium acetate solution with Tween 80 (0.01%) pH adjusted to 9.7 and sterile glass beds for 1 h. The mixtures were filtered through cotton and gauze tissue and subsequently centrifuged three times at 2,500 rpm for 20 min at 4 °C [29, 30]. Spore’s suspensions kept in milk bottles were subjected to a heat shock (80 °C, 10 min), which is lethal to vegetative cells but not to spores. Spores suspensions were stored at 4 °C.
Viable Spores Count
Serial decimal dilutions in distilled sterile water were prepared from spore suspensions, and 50.0 μL of each dilution was inoculated in duplicate in tryptone soy agar plate’s surface. Plates were incubated overnight at 36 °C. The plate count made it possible to calculate colony-forming units per milliliter (CFU mL−1). For CFU per gram dry matter determination:
Biological Indicator System Preparation
About 100 sterile filter papers (48.0 g/m2 strips, size 1.0 cm × 2.0 cm) were soaked in 500.0 mL of 0.02 M calcium acetate solution pH 11, adjusted with 0.14% calcium hydroxide solution, and kept at room temperature for about 18 h. The strips were spread on trays and dried at 45.0 °C in a heated air oven [29]. The trays were packed in sealed plastic papers bags, autoclaved at 121 °C for 15 min, and dried at 45 °C in a heated air oven for about 24 h. They were stored at room temperature. The spore suspensions were homogenized by vortex, and 10.0 μL of it was dispensed on each strip. The inoculated strips were dried at 45 °C in a heated air oven for about 24 h. Each strip was put into a 7.0-mL sterile glass vial, rubber stoppered, and sealed with aluminum seal. Prepared biological indicators (BIs) were stored at 4 °C. Population assays on carrier strips were done using the glass bead method: Five inoculated strips were placed in sterile screw cap with glass beads and 10.0 mL of sterile distilled water, vortexed for 6 min, and counted as described in “Viable Spores Count” section.
Dry-heat Resistance Performance Test
The D value is defined as the time it takes to reduce the spore population by 90% or one log at a specified set of conditions. D value was performed by fraction negative analysis, limited Spearman–Kaber method. Dry-heat exposure conditions were 160 ± 2 °C at 15, 20, 25, 30, 35, and 40 min in a table-top heated air oven. Ten units per exposure were used. Following exposure, samples were cultured in a recovery growth medium containing TSB, 3.0%; CaCl2·6H2O, 0.018%; soluble starch, 0.1%; and bromotimol blue, 0.0015%. They were incubated at 36 °C for 48 h.
For D value determination: \(D_{160\;^\circ {\text{C}}} = \frac{{U_{sk} }}{{\log N_0 + 0.2507}}\)
Where:
- N 0 :
-
Initial number of organisms on BI
- U sk :
-
Spearman–Kaber heating time/dosage estimate
Where:
- U k :
-
First heating time with all units negative
- r i :
-
Number of replicate units negative
The survival/kill times were determined by the following formula:
95% confidence limits were calculated for all determinations.
Statistical Analysis
Regression variance analysis, estimated parameters, and respective confidence intervals at significance levels were calculated by the SGWIN program (StatGraphic Plus for Windows version 5.0, Statistical Graphics Co., 2000). The fractional factorial experimental designs were done through Statistic 5.0 (Statsof Inc., 1984).
Results
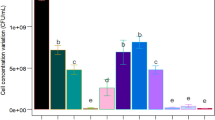
Different culture medium formulations were tested to evaluate the B. atrophaeus growth. The results, shown in Fig. 1, demonstrate that all tested mediums—formulated with soybean agro-industrial by-products and residues—were capable of promoting the microorganism growth, even adding no supplements. The addition of complementary carbon and nitrogen sources to soybean vinasses did not increase biomass production. Only the addition of peptone extract to soybean molasses allowed an increase of approximately 1 log in the biomass.
B. atrophaeus growth in different soybean agro-industrial by-product and residue media: (TSB-control) = 1.2 × 108 CFU mL−1; 1 soybean molasses + peptone = 7.6 × 10 7 CFU mL−1; 2 soybean vinasse + beef extract = 3.9 × 106 CFU mL−1; 3 soybean molasses 1/50 (v/v) = 4.2 × 106 CFU mL−1; 4 soybean molasses 1/60 (v/v) = 1.9 × 106 CFU mL−1; 5 soybean vinasse = 4.3 × 107 CFU mL−1
The growth box plot analyses (Fig. 2) indicate that formulations with colony counts equal or above 1.5 107 CFU mL−1 (median) can substitute the standard medium. Although two formulated media follow this criterion, the results indicate that the best medium is number 1 (soybean molasses supplemented with peptone), which presents colony counts similar to those of the control medium.
The 2(2–0) factorial experimental design results were submitted to analysis of variance (ANOVA) statistical analysis (Table 3). The F ratio, which in this case equals 25.0989, is a ratio of the between-group estimate to the within-group estimate. Since the p value of the F test is below 0.05, there is a statistically significant difference between the means of the five variables at the 95.0% confidence level. To determine which means are significantly different from the others, a Fisher’s least significant difference (LSD) procedure was applied.
The LSD plot analyses (Fig. 3) indicate that only medium no. 1 (soybean molasses 4.0% and peptone casein 4.0%) presents a significant statistical difference.
The Pareto chart (Fig. 4) was used to plot the estimated effects and interactions in decreasing order of importance. Its analyses confirm the casein peptone positive influence in biomass production and show a possible interaction casein peptone × soybean molasses concentration influence.
The increase of the peptone casein concentration to 6.0% did not positively affect biomass production (Table 4).
The main effects plot of biomass (Fig. 5) presents 4.0% (w/v) for casein peptone concentration optimum value and 4.0% (v/v) for soybean molasses concentration optimum value.
The spore yield and the D value of the spore population obtained from the different inoculum media are shown in Table 5. Yields (spores g−1 dry matter) ranging from 1.1 × 109 to 1.2 × 1010 for control media and from 1.2 × 1010 to 1.9 × 1010 for optimized complex inoculum medium were recorded. The highest spores yields (1.9 × 1010 CFU g−1dry matter) were obtained from the optimized complex inoculum medium.
The dry-heat resistance D value results varied from up to 5.1 ± 0.2 min to less than 5.5 ± 0.3 min for spores from different inoculum media.
Sporulation results Box-and-Whisker plot analysis (Fig. 6) indicates that complex-optimized medium given total spores count statistically equal to TSB inoculum medium and better than sporulation agar medium control.
The D 160 °C-value results were submitted to ANOVA statistical analysis (Table 6). The F ratio, 0.46, is a ratio of the between-group estimate to the within-group estimate. The p value of the F test is greater than or equal to 0.05, indicating that there is no statistically significant difference between the means of the five media at the 95.0% confidence level.
The LSD plot (Fig. 7) graphically demonstrates that all media had the same thermal resistance performance.
Discussion
The relationship between sporulation media and spore yield, thermal resistance, and germination index has been widely demonstrated [9, 29]; however, there are few studies about the inoculum media influence. Olson and Nottingham [31] have demonstrated that the thermal resistance of spores depends on the quality of vegetative cells and that spores produced from stationary phase vegetative cells are more sensitive to heat due to their long exposition to toxic metabolites in the culture medium.
Thermal resistant spores used as a sterilization bioindicator are usually produced on synthetic agar medium surface inoculated with vegetative cells from TSB or other synthetic nutritive commercial broth, aiming at obtaining less variation in resistance spores characteristics [32], although the industrial use of defined culture medium is relatively expensive. Complex natural nutrients had been studied as a source for high-level growth factors and trace elements and a mixture of free amino acids for exponential growth of the Bacillus sp.
Results of the present study confirm that soybean molasses and vinasse supply the necessary nutrients for the B. atrophaeus culture growth at inoculum stage, even adding no supplements. The best growth was obtained by increasing initial complex nitrogen concentrations with peptone addition.
The concentration of soybean molasses and the peptone effect on cell growth was examined, and the results show an optimum concentration of 4% (w/v) for both. These nutrient concentrations produced vegetative cell biomass ranging from 8.4 × 107 to 2.1 × 1010 CFU mL−1 and the control media from 1.1 × 108 to 2.4 × 108 CFU mL−1 in 18-h culture (exponential growth phase), producing a colony count difference of less than 1 log, and were thus considered statistically equal.
Only calibrated spores can be used to determine the sterilization capacity of heat treatments. In the evaluation of sterilization processes, the D value is routinely used as a measure of microorganism resistance. The D value, survival time, and kill time results (from 5.1 ± 0.2 to 5.2 ± 0.2 min, from 25.1 ± 1.0 to 25.5 ± 1.0 min, and from 55.9 ± 2.2 to 56.7 ± 2.2 min, respectively) were above the typical characteristics for commercially supplied BI systems: D value from 1.0 to 3.0 min; survival time from 4 to 14 min, and kill time from 10 to 32 min [33]. This could be explained by the kind of packing used (glass vial), which makes heat penetration difficult, allowing a better evaluation of the dry-heat sterilization process whose recommended exposition time is 120 min.
The spore yield obtained from the different inoculum media and the dry-heat resistances (D value) showed variability within the ANSI/AAMI/ISO 11138 [34] recommendations: Spores population should be replicated within 50% to 300% of the claims of manufacturers, and resistance (D value) should be within ±20%.
Soybean molasses is a very low-cost agro-industrial by-product, which appears not to have been studied for Bacillus atropheus thermal-resistant spore production.
Conclusions
The present results demonstrate that soybean molasses with peptone have a performance similar to that of a synthetic control medium, indicating an economically attractive alternative for dry-heat biological indicator inoculum production. Its choice as inoculum medium will also have to take into account the easiness and the guarantee of the supply, incidental costs (transport, conservation), and homogeneity of the lots, reducing the costs of the standardization analyses.
Complementary researches will have to be carried out to evaluate the interference of inoculum produced from this medium in the formed spore thermal resistance and viable stability.
References
Association For The Advanced Of Medical Instrumentation/AAMI (2005) ST 40:2004
Blakistone, B., Chuyate, R., Kautter, D. Jr., Charboneau, J., & Suit, K. (1999). Food Protection, 62, 262–267.
Penna, T. C., Mazzola, P. G., & Martins, A. M. (2001). BMC Infectious Diseases, 1, 16.
U.S. P. XXIX (2005). Biological indicator for dry-heat sterilization, paper strip. In: The United States Pharmacopeia. 29th rev. Rockville, MD.
Bayliss, C. E., & Waites, W. M. (1979). Journal of Applied Bacteriology, 47, 263–269.
Whitbourne, J. E., & Reich, R. R. (1979). Journal of the Parenteral Drug Association, 33, 132–143.
Gale, E. P., Pitchers, R., & Gray, P. (2002). Water Research, 36, 1640–1648.
Weber, D. J., Sickbert-Bennett, E., Gergen, M. F., & Rutala, W. A. (2003). JAMA, 289, 1274–1277.
Hodges, N. A., Melling, J., & Parker, S. J. (1980). Journal of Pharmacy and Pharmacology, 32, 126–130.
Davies, F. L., Underwood, H. M., Perkins, A. G., & Burton, H. (1977). Thermal death kinetics of Bacillus stearothermophilus spores at ultra high temperatures. 1 Laboratory determination of temperature coefficients. Journal of Food Technology, 12, 115–119.
Junge, H., Krebs, B., & Kilian, M. (2000). Pflazenschutz-Nachrichten Bayer, 1, 94–104.
Moraes, I. (2001). In: Biotecnologia Industrial. 3. LIMA et al. Ed.Blucher Ltda, São Paulo. 200–217.
Monteiro, S. M., Clemente, J. J., Henriques, A. O., Gomes, R. J., Carrondo, M. J., & Cunha, A. E. (2005). Biotechnology Progress, 21, 1026–1031.
Youkong, C., Dechmahitkul, W., & Mekvichitsaeng, P. (2004). Available from http://knowledge.biotec.or.th/doc_upload/2004113153453.doc. Accessed July 26, 2006.
Fadel, M., & Sabour, M. (2002). Journal of Biological Sciences, 2, 16–120.
Cesare-Vidaurre, T., Campos, E., & Castro-Gomez, R. J. H. (1997). Anais do VII Mexican Congress of Biotechnology and Bioengineering, Mexico.
Luna, C. L., Mariano, R. L. R., & Souto-Maior, A. M. (2002). Brazilian Journal of Chemical Engineering, 19, 133–140.
Cegla et al. (2005). United States Patent 6,913,771.
Siqueira, P. F. (2006). Master of Sciences Thesis, Federal University of Paraná/Universities of Provence and of the Mediterranean Sea, Curitiba, Brazil.
Machado, R. (1999). Master Thesis, Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
Neves, L.C. M., Oliveira, K. S., Kobayashi, M. J., & Penna, T. C. V. (2006). 28th Symposium on Biotechnology for Fuels and Chemicals. Nashville, EUA.
Li, X., Yang, L., Yan, P., Zuo, F., & Jin, F. (1997). Letters in Applied Microbiology, 24, 1–4.
Sella, S. R. B. R., Vandenbergue, L. P. S., Medeiros, A. P., & Soccol, C. R. (2007). Anais XVI Simpósio Nacional de Bioprocessos—2007. Curitiba. Brazil. Cd. FES 520.
Vries, Y. P., Atmadja, R. D., Hornstra, L. M., deVos, W. M., & Abee, T. (2005). Applied and Environmental Microbiology, 71, 3248–3254.
Feavers, I. M., Foulkes, J., Setlow, B., Sun, D., Nicholson, W., Setlow, P., et al. (1990). Molecular Microbiology, 4, 275–282.
Raso, J., Barbosa-Canovas, G., & Swanson, B. G. (1998). Journal of Applied Microbiology, 85, 17–24.
Redmond, C., Baillie, L. W., Hibbs, S., & Moir, A. J. (2004). Microbiology, 150, 355–363.
Mohan, R., Chui, E. A., Biasi, L. A., & Soccol, C. R. (2005). Brazilian Archives of Biology and Technology, 48, 37–42.
Hoxey, E. V., Soper, C. J., & Davies, D. J. (1985). Journal of Applied Bacteriology, 58, 207–214.
Penna, T. C. V., Machoshvili, I. A., & Taqueda, M. E. S. (1996). PDA Journal of Pharmaceutical Science and Technology, 50, 227–237.
Olson, J. R., & Nottingham, P. M. (1980). Ecologia Microbiana de los Alimentos. Zaragosa, Acribia, p.1727.
Waites, W. M., & Bayliss, C. E. (eds.) (1980). In: Microbial growth and survival in extremes of environment. Applied bacteriology technical series. 15, 159–172. London: Academic.
USP 29 (2005). Biological Indicators for Sterilization. Available from www.pharmacopeia.cn/v29240/usp29nf24s0_c1035.html. Acessed July 25, 2007.
Association for the Advanced of Medical Instrumentation-ANSI/AAMI/ISO 11138 (1994). Sterilization of Health Care Products—Biological Indicators.
Acknowledgments
The present research was financially supported by the Secretaria de Estado da Ciência, Tecnologia e Ensino Superior—Fundo Paraná. The authors thank José Carlos Pereira Comink for the statistic revision and Neuza Araujo and Elza Retka for the laboratory support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sella, S.R.B.R., Dlugokenski, R.E.F., Guizelini, B.P. et al. Selection and Optimization of Bacillus atrophaeus Inoculum Medium and its Effect on Spore Yield and Thermal Resistance. Appl Biochem Biotechnol 151, 380–392 (2008). https://doi.org/10.1007/s12010-008-8206-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8206-3