Abstract
Different integrated systems with a bi-enzymatic biosensor, working with two different methods for ethanol detection—flow injection analysis (FIA) or sequential injection analysis (SIA)—were developed and applied for ethanol extracted from gasohol mixtures, as well as for samples of alcoholic beverages and fermentation medium. A detection range of 0.05–1.5 g ethanol/l, with a correlation coefficient of 0.9909, has been reached when using FIA system, working with only one microreactor packed with immobilized alcohol oxidase and injecting free horseradish peroxidase. When using both enzymes, immobilized separately in two microreactors, the detection ranges obtained varied from 0.001 to 0.066 g ethanol/l, without on-line dilution to 0.010–0.047 g ethanol/l when a 1:7,000 dilution ratio was employed, reaching correlation coefficients of 0.9897 and 0.9992, respectively. For the integrated biosensor SIA system with the stop–flow technique, the linear range was 0.005–0.04 g/l, with a correlation coefficient of 0.9922.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ethanol is a biofuel extensively used nowadays as one of the most interesting substitutes for petroleum. In Brazil, since 1975, an important government program has introduced ethanol as automotive biofuel, mostly obtained from sugar-cane fermentation. Ethanol may be used in cars working with the hydrated form (95wt% ethanol), or mixed with gasoline as anhydrous ethanol in a proportion varying from 22% to 25% (v/v), or in the new flex-fuel models, thus evidencing the need to find ways of measuring correctly ethanol’s level in biofuel quality control. Ethanol analysis is also required for monitoring and control of an alcoholic fermentation bioprocess, in food industries and in clinic and forensic areas. High-sensitivity analytical systems, using enzymatic reactions and aiming to achieve a high confidence and fast response signal, have been applied to quantify low ethanol concentrations [1]. Among those, flow injection analysis (FIA) is a very promising technique for being reliable, reproducible, reagent-saving and readily automated. FIA systems have also been applied for ethanol detection either in alcoholic beverages as for gasohol mixtures, using sequential enzymatic microreactors, packed with enzymes such as alcohol oxidase and horseradish peroxidase, immobilized on chitosan or glass beads [2–4]. A real alternative to FIA systems is the sequential injection analysis (SIA) technique due to its more versatile sample-handling capability [5]. SIA systems were successfully employed in bioprocess automation and control, working either with free or immobilized enzymes [6–9]. For ethanol analysis, biosensor systems have also been reported, although working with an expensive NAD+ co-factor-dependent enzyme [7, 9–10]. In this work, different integrated biosensor analysis systems have been developed to obtain a colorimetric method to analyze ethanol in diluted samples. The advantages of using optical determinations, which avoid interferences on the response signal caused by the electrochemical mechanism on the electron transference, through bio and/or mediator molecules to the electrode surface, have been aggregated to achieve an efficient analytical device [11]. The colorimetric biochemical reaction results in a coloured stable product, which could be detected in accordance with Lambert–Beer law. Other advantages of working with FIA and SIA methods should be mentioned, such as the economic aspects inherent to the reduction of reagent and sample volumes required for each analysis [12].
Materials and Methods
Chemicals
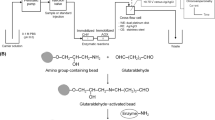
All reagents were analytical grade and have been acquired from Sigma Chemical, St. Louis, MO, USA, unless otherwise informed. Alcohol oxidase (AOD, Sigma) and horseradish peroxidase (HRP, Toyobo of Brazil) were immobilized separately on aminopropyl glass beads treated with 2.5% (v/v) glutaraldehyde, as previously described [3]. The composition of the indicator solution was: 4-aminophenazone 0.395 g/l and phenol 0.875 g/l, prepared in a 0.1 M sodium phosphate buffer solution (pH 7), which was also used as carrier [3, 13]. Reactions were carried out at room temperature (20°C). The ethanol samples were diluted with 0.1 M sodium phosphate buffer solution (pH 7).
Enzymatic Reactions

The resulting coloured product, monoimino-p-benzoquinone-4-phenazone, has been detected with a spectrophotometer at 470 nm.
The Integrated Biosensor Flow Injection Analysis Systems
The Flow Injection Analysis system consisted of Técniques Mesura Instrumentació (TMI) modules, a five-channel peristaltic pump, an eight-channel injection valve, an eight-channel distribution valve and a colorimeter, connected to an interface and to an IBM-personal computer (PC) microcomputer. Two sequential microreactors, made of acrylic, each one with 0.91 ml of void volume, with 3:1 length-to-diameter ratio, were used. Both enzymes were immobilized separately on glass beads (80–120 mesh) and packed in both microreactors, working in sequence. The beads were supported in the microreactor by a 110-mesh nylon screen and two rubber O-rings, having an external diameter of 11.4 mm. The FIA system was used for quantifying ethanol extracted from gasohol blends, working with different conditions: (1) one microreactor, packed with immobilized AOD and injection of free HRP and reagent solution; and (2) two microreactors with immobilized AOD and HRP and having different strategies for diluting the concentrated ethanol samples (off-line and on-line).
The Sequential Injections Analysis System of the Integrated Biosensors
The analyser has comprised interfaces of four modules (Easy Technologies, Cerdanyola del Vallés, Spain) connected by a RS-485 protocol and powered by a single 12V/2.5 A source. The system included a five-way eight-roller peristaltic pump (Model 1201/06-5-0), a 470 nm colorimeter (Model 1203/470/Z10), a module with two rotary valves of the three-port type (Model 1202/3) and a six-port rotary valve (Cheminert 4162510, Valco Instruments, Houston, TX, USA). The SIA system was made of polytetrafluoroethylene (PTFE) tubing (0.8 mm i.d.), connected to polyvinylchloride (PVC) fittings. One-meter length of PTFE tubing was used as a holding coil. Samples and reagent solutions were aspirated and fed to the system via the six-port rotary valve by two automatic micro-burettes (Crison MicroBU 2031, Alella, Spain) with two syringes, 1 ml and 0.5μl (Hamilton 1002 Teflon Luer Lock, Hamilton Bonaduz AG, Bonaduz, Switzerland). A PC via an RS-485/RS-232 interface controlled all the elements. Operations of the SIA analyser and data acquisition were PC-controlled using specific software developed in C++ language. The SIA system with two acrylic microreactors, having a 0.91-ml void volume each, was packed separately with the AOD- and HRP-immobilized enzymes. The SIA integrated system worked with the stop–flow technique, at the first AOD-immobilized microreactor. The time between sample frequency and linear range was settled in 120 s. The SIA system was used to measure ethanol content in alcoholic beverages and in fermented medium, working with the two microreactors described above and off-line sample dilutions.
Figure 1a and b shows the schematic structure of the FIA and SIA systems proposed in this work, respectively. Each system had been tested, after calibration, on both response signal repeatability and stability of the immobilized enzymes, when reused in successive analysis. The results were compared to gas chromatography and high-performance liquid chromatography, having shown good agreement [3, 4, 12].
a The FIA IV-I and II integrated biosensor systems; 1 concentrated sample solution, 2 diluted sample solution, 3 reagent solution, 4 eight-channel distribution valve, 5 peristaltic pump, 6 microreactors, 7 eight-way injection valve, 8 temperature chamber control, 9 colorimeter, 10 micro computer, 11 waste, 12 coil, 13 peristaltic pump for sample dilution, 14 phosphate buffer. b The SIA integrated biosensor system; 1, 2 and 3 phosphate buffer solution; 4 ethanol diluted sample; 5 reagent solution; 6 waste; 7 1,000-μl micro-burette; 8 500-μl micro-burette; 9 peristaltic pump; 10 two three-way valves; 11 six-channel distribution valve; 12 colorimeter; 13 serpentine; 14 AOD-immobilized microreactor; 15 HRP-immobilized microreactor, 16 computer; 17 Interface RS-232/RS-485
Results and Discussion
Five integrated FIA systems have been proposed to measure ethanol extracted from gasohol, and changes were conducted to introduce an on-line dilution process of the samples. The proposed SIA system was applied to quantify ethanol samples, which had been previously diluted.
Table 1 shows the description of the different proposed integrated systems, namely, as FIA I, II, III, IV-I, IV-II and, finally, SIA-I. The adjustment of the FIA systems was reached, as proposed in this work, to amplify the response signal and to fit the data in a linear range. For the proposed FIA integrated biosensor systems, the flow rate that showed the best profile for the response signal was 3.6 ml/min. Seventy analyses of ethanol samples were measured with the immobilized AOD and free HRP integrated biosensor system FIA-I in 7 days [3]. The integrated biosensor FIA-II system worked with an immobilized HRP packed in a second microreactor that was adapted to the integrated system. A second peristaltic pump was included into the original FIA-II integrated system to dilute the sample by on-line automated control proceeding. A chamber was also adapted to the FIA-III integrated system to study the effect of the temperature on the bi-enzymatic reaction, as shown in Fig. 1a. The experiments were performed with FIA-IV-I and FIA-IV-II at controlled temperature of 25°C. For the integrated biosensor SIA-I system, the best profile of the response signal was obtained with 7.4 ml/min [12] with two microreactors working in series. The detection limit values have been calculated as three times the standard deviation of the background noise [12].
The detection range of 0.05 to 1.5 g ethanol/l, obtained with the system FIA-I, working with only one microreactor, was changed to 0.001 to 0.066 g ethanol/l when a second microreactor was introduced in the FIA-I line, packed with the immobilized HRP (integrated system II), to reuse both enzymes in successive analysis. That instrument permitted the analysis of low ethanol contents, in highly diluted solutions. The analysis cost will decrease once the immobilized enzymes could be reused, in successive measurements, always using the same immobilized lot. On the other hand, the enzymes lose the activity when they are reused in successive analyses [4].
Figure 2 shows the calibration curves obtained working with the proposed integrated biosensor FIA systems II, III and IV, including the SIA integrated system I. The calibration curves were adjusted to a hyperbolic correlation for the different configuration systems investigated. The original system FIA-I was modified to detect correctly the linear range and improve the accuracy and sensitivity of the analysis results. For the FIA and SIA systems proposed in this study, the linear range fitted circa 0.01 to 0.047 g ethanol/l, which enables to analyze properly low ethanol concentrations in samples from different origins.
The proposed integrated FIA and SIA biosensor systems permitted to obtain good response signal reproducibility, with a maximum relative error of 12% for system VI-1, working with on-line dilution (1:1,580) and 2% for the SIA integrated system I. A small change in analysis per hour was observed: 19 (h−1; FIA-I) and 15 (h−1; FIA-II, III, IV-I and IV-II), when a second peristaltic pump was used to dilute the real samples, working with an automated dilution system [3, 4]. For the SIA system, the sample frequency was around seven analyses per hour, working with an off-line dilution method and a time schedule, which included a cleaning colorimeter and stop–flow steps at the AOD-immobilized microreactor to increase the response signal value [12].
Figure 3 shows the stability of the immobilized enzymes for the SIA systems when testing the samples of standard ethanol solution, in which continuous and stop–flow strategies were compared. After 3 days, 60% of the bi-enzymatic activity has been retained for the stop–flow strategy, although 20% had been lost after the first day in the same maintaining conditions. When ethanol samples were analyzed using the SIA integrated system, 60 measurements were performed, and the immobilized AOD and HRP lots were reused during two consecutive days after a new calibration.
Figure 4 shows the influence of the temperature on the response signal working with the FIA system for 0.1 g/l ethanol samples. The results have shown that the absorbance value had decreased from 0.1105 ± 0.0007 at 25°C to 0.0313 ± 0.0015 at 45°C. The decrease of the absorbance values may be explained by enzyme inactivation, when the temperature was increased from 25°C to 45°C and by the reuse of the immobilized enzymes in successive analysis [4] during the course of the experiment.
The accuracy of the proposed methods was evaluated for real sample analysis. The results obtained for real samples made with FIA integrated system IV-I (extracted ethanol from gasohol mixtures) with on-line dilution and with SIA integrated system I (alcoholic beverages and fermented medium) with off-line dilution have agreed fairly with those obtained by using high-performance liquid chromatography (HPLC) standard method [12]. The samples of extracted ethanol were obtained from red gasohol and yellow gasohol mixtures received from different distributors. Slightly different values were obtained when compared to the nominal 25% v/v ethanol, claimed by vendors of commercial gasohol mixture samples, although a relative deviation of 12.7% was observed for a red gasohol (1:1,589) diluted sample [4]. In addition, low relative deviations were also obtained for alcoholic beverages when compared with the HPLC measurements, with a maximum value of 7.3% for white wine diluted samples and 4.7% for fermented medium, as presented in previous work [12] for measurements made with the SIA system. The low relative deviations found in this work were similar to those observed with amperometric and colorimetric ethanol biosensors reported in the literature [7, 9–10].
Conclusions
A highly advantageous and robust method for ethanol analysis was developed working with a continuous injection flowing integrated system (FIA) and with a stop–flow sequential injection integrated system (SIA). A change on FIA-I system sensitivity, which worked with one microreactor for the immobilized AOD and free HRP, was obtained when a second microreactor, for the immobilized HRP (FIA-II) was introduced into the integrated system. Ethanol was analyzed using an on-line dilution system, showing good reproducibility and reliability (FIA-III, FIA-IV-I and FIA-IV-II). An economy on the analytical method was observed when the immobilized enzymes were reused in several analyses. In addition, savings on reagent and sample volumes were achieved for the sequential injection analysis proposed system (SIA-I), working with a stop–flow strategy into the AOD-immobilized microreactor. No pretreatment of the samples was needed; a simple dilution for the adequate linear range was enough to analyze 60 ethanol samples from different origins.
References
Patel, P. D. (2002). Trends in Analytical Chemistry, 21, 96–115.
Taniai, T., Sukurragawa, A., & Okitani, T. (2001). Journal of Association of Official Analytical Chemists International, 84(5), 1475–1483.
Alhadeff, E. M., Salgado, M. A., Pereira, N., Jr., & Valdman, B. (2004). Applied Biochemistry and Biotechnology, 113, 125–136.
Alhadeff, E. M., Salgado, M. A., Pereira, N., Jr., & Valdman, B. (2005). Applied Biochemistry and Biotechnology, 121, 361–372.
van Staden, J. F., & Stefan, R. I. (2004). Talanta, 64, 1109–1113.
Surribas, A., Cos, O., Montesinos, J. L., & Valero, F. (2003). Biotechnology Letters, 25, 1795–1800.
Lapa, R. A. S., Lima, J. L. F. C., & Pinto, I. V. O. S. (2003). Food Chemistry, 81, 141–146.
Masini, J. C., Rigobello-Masini, M., Salatino, A., & Aidar, E. (2001). Latin American Applied Research, 31, 463–468.
Niculescu, M., Erichsen, T., Sukharev, V., Kereny, Z., Csregi, E., & Schuhmann, W. (2002). Analytica Chimica Acta, 463, 39–51.
Segundo, M. A., & Rangel, A. O. S. S. (2002). Analytica Chimica Acta, 458, 131–138.
Coi, M. M. F. (2004). Microchemical Acta, 148, 107–132.
Alhadeff, E. M., Salgado, M. A., Cos, O., Pereira, N., Jr., Valero, F., & Valdman, B. (2007). Applied Biochemistry and Biotechnology, 136–140, 17–26.
Salgado, A. M., Folly, R. O. M., Valdman, B., Cós, O., & Valero, F. (2000). Biotechnology Letters, 22, 327–330.
Acknowledgments
We acknowledge the support received from the BI-EURAM Project of the Alpha Program-EU. We also would like to thank Toyobo of Brazil for gently donating the horseradish peroxidase. We also acknowledge LABCOM-EQ/UFRJ and Refinaria de Petróleos Manguinhos, for donating, respectively, the different samples of gasohol mixture and gasoline A.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alhadeff, E.M., Salgado, A.M., Cós, O. et al. Integrated Biosensor Systems for Ethanol Analysis. Appl Biochem Biotechnol 146, 129–136 (2008). https://doi.org/10.1007/s12010-007-8130-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-007-8130-y