Abstract
In this work, the effect of adaptation on P. stipitis fermentation using acid-pretreated corn stover hydrolyzates without detoxification was examined. Two different types of adaptation were employed, liquid hydrolyzate and solid state agar adaptation. Fermentation of 12.5% total solids undetoxified acid-pretreated corn stover was performed in shake flasks at different rotation speeds. At low rotation speed (100 rpm), both liquid hydrolyzate and solid agar adaptation highly improved the sugar consumption rate as well as ethanol production rate compared to the wild-type strains. The fermentation rate was higher for solid agar-adapted strains compared to liquid hydrolyzate-adapted strains. At a higher rotation speed (150 rpm), there was a faster sugar consumption and ethanol production for both the liquid-adapted and the wild-type strains. However, improvements in the fermentation rate between the liquid-adapted and wild strains were less pronounced at the high rotation speed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Economically feasible processes for biomass conversion to ethanol requires the fermentation of the sugars generated in the pretreatment and hydrolysis steps. In agricultural residues and hardwoods, xylose constitutes about 45% of the total sugars, and therefore xylose conversion to ethanol is important for high yields. Dilute acid hydrolysis of cellulosic biomass generates inhibitory compounds such as furfural, hydroxymethyl furfural, and acetic acid [1]. These inhibitors affect the ability of yeasts to ferment the hydrolyzates, and therefore a detoxification step is usually included in fermenting acid hydrolyzates [1, 2]. Detoxification may include the use of chemicals [3] and may require additional process steps [4, 5], which add up to the total process costs.
Previous studies have identified Pichia stipitis as a good candidate for xylose fermentation of agricultural residues that have been subjected to an acid-catalyzed pretreatment process [6–8]. An issue with using this microorganism for fermentation is that the pretreated slurries reportedly have been found to be inhibitory to the growth of P. stipitis at high substrate concentrations, reducing the sugar consumption as well as ethanol production rates [9–11]. Ethanol production rates have also been linked to the availability of oxygen to P. stipitis during fermentation of synthetic media and pretreated lignocellulosic slurries because of the microaerophilic nature of the yeast during ethanol metabolism [12–15].
As an alternative to detoxification methods, improvement in P. stipitis ability to grow and ferment in acid-pretreated slurries (through conditioning or adaptation) have been pursued in some studies [16–18]. To our knowledge, there has been no study on how aeration (agitation) affects the improvements in fermentation observed from adaptation. This study looks at how aeration (agitation) affects fermentation speed in adapted and unadapted strains of P. stipitis.
Materials and Methods
Microorganism and Medium
Stock culture of P. stipitis CBS 6054 was grown on yeast extract, peptone, and xylose agar plates at 30 °C for 3 days. The agar plates contain 10 g/L yeast extract, 20 g/L peptone, 20 g/L xylose, and 20 g/L agar. Colonies from the plates were grown overnight in a filter-sterilized fermentation medium containing 1.7 g/L yeast nitrogen base (without amino acid or ammonium sulfate), 2.27 g/L urea, 6.56 g/L peptone, and 20 g/L xylose. Nutrient solution (50× the concentration used) was prepared by dissolving 1.7 g of yeast nitrogen base, 2.27 g of urea, and 6.56 g of peptone in 20 mL of water.
Hydrolysis of Dilute Acid-pretreated Corn Stover
The pretreatment conditions for the dilute H2SO4 acid-pretreated corn stover used in this study have been reported elsewhere [19]. Acid-pretreated corn stover (12.5% total solids) was neutralized with NH4OH and hydrolyzed with Celluclast 1.5 L (15 filter paper units/g total solids [TS]) and Novozyme 188 (16.8 cellobiase units/g TS) at 50 °C and pH 5.0 for 48 h. The hydrolyzate was neutralized to pH 6.0 and filter sterilized.
Adaptation
Liquid adaptation was performed by growing cells overnight in the filter-sterilized corn stover hydrolyzate. Solid agar adaptation was performed by growing cells on agar plates containing 10 g/L yeast extract, 20 g/L peptone, 20 g/L agar, and corn stover hydrolyzate.
Fermentation
Fermentations were performed in sterile 125-mL Erlenmeyer flasks (with 0.2 μm vent cap) in an air-shaker incubator at 30 °C at the speed of 100 rpm [A] and 150 rpm [B]. Each Erlenmeyer flask contained 50 mL of corn stover hydrolyzate, 1 mL of nutrient solution, and 2 mL of inoculum (initial cell concentration 2 g/L), and 1.5 ml of phosphate buffer. Samples were taken from the Erlenmeyer flasks at different time intervals, centrifuged, and put in high-performance liquid chromatography (HPLC) vials. Separation and quantification of sugars and ethanol were performed using HPLC analysis with a Biorad® HPX-87H column at 60 °C and RI detection at 40 °C. The mobile phase was 5 mM H2SO4 with a flow rate of 0.5 mL/min.
Results and Discussion
Comparison of Wild Strains and Liquid-adapted Strains of Pichia stipitis
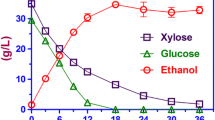
The performance of the wild-type (unadapted) and liquid hydrolyzate-adapted strains of P. stipitis was compared on 12.5% corn stover hydrolyzate at 100 rpm (Fig. 1). The liquid-adapted strains used glucose at a faster rate compared to the unadapted strains. After 96 h of fermentation, the liquid-adapted strains were beginning to use xylose, while the wild strains did not consume any of the xylose. Because xylose consumption begins after glucose is consumed [12], a faster glucose consumption rate with the liquid-adapted strains allowed P. stipitis to start consuming xylose earlier than the wild strain. Therefore, a longer fermentation time will be required to use all the sugars in the wild strains compared to the liquid-adapted strains. A complete consumption of xylose will require fermentation past the 96 h used in this study. The ethanol production rate in the liquid-adapted strain was also higher than the unadapted strains. Our observation is similar to results obtained from previous studies on hydrolyzates [16–18].
Comparison of Wild Strains and Solid Agar-adapted Strains of Pichia stipitis
Fermentation results for the unadapted and solid agar-adapted strains of P. stipitis on 12.5% corn stover hydrolyzate at 100 rpm are shown in Fig. 2. The solid agar adaptation improved both the sugar consumption rate as well as the rate of ethanol production. The solid agar-adapted strains started using xylose after 96 h of fermentation, while the wild strains did not consume xylose. In this study, fermentation was stopped at 96 h, which is not sufficient time for complete sugar utilization at 100 rpm. The conversion of all the sugars to ethanol will require less time with the solid agar-adapted strains compared to the wild strains. The observed increased rate of substrate utilization and ethanol production using solid agar adaptation was higher than the results for liquid-adapted strains.
Comparison of Wild Strains and Liquid-adapted Strains at Optimized Conditions
The fermentation results for the wild stains and liquid-adapted strains of P. stipitis on 12.5% hydrolyzate at 150 rpm rotation speed are shown in Fig. 3. The difference in fermentation speed between the wild and liquid-adapted strains were minimal at 150 rpm. The presence of oxygen enhances the ability of P. stipitis to cope with inhibitory compounds in undetoxified corn stover. A summary of the fermentation results are shown in Table 1. From Table 1, liquid and solid agar adaptation increased the sugar consumed from 64 to 72% at 100 rpm after 96 h of fermentation. However, when rotation speed in the flask was increased to 150 rpm, 92% of the total sugar was consumed within 72 h of fermentation.
The sugar consumption rates and ethanol production for both the wild and liquid-adapted strains of P. stipitis were higher at 150 rpm rotation speed compared to 100 rpm. The high sugar consumption and ethanol production rate at 150 rpm is attributed to higher aeration and good mixing. Because xylose conversion starts after glucose is consumed, a faster glucose consumption reduces the time it takes for xylose consumption to begin and therefore reduces the total time it takes to complete fermentation. At rotation speeds of 100 rpm where aeration was low, it took a longer time to complete glucose consumption, and therefore xylose consumption could not start in wild strains.
In a recent study, it was found that a high initial cell concentration increased the rate of xylose utilization and ethanol formation in P. stipitis [20]. Results from our previous work [20] and this work suggest that there are two options available for using P. stipitis for biomass fermentation. The first option is to operate at suboptimal conditions of low aeration where adaptation or a high initial cell concentration improves the speed of the fermentation. The second option is to operate at optimal fermentation conditions where microaerophilic conditions need to be maintained. Choosing between these two options under industrial conditions will require cost benefit analysis to determine the most viable option.
Conclusion
Adaptation of P. stipitis to both liquid hydrolyzate and agar resulted in an increase in sugar consumption and ethanol production in undetoxified acid-pretreated corn stover. However, the improvements observed in the fermentation rate were less pronounced at rotation speeds of 150 rpm compared to 100 rpm in shake flask experiments. At less optimal conditions of aeration (100 rpm), adaptation significantly improved fermentation rate. However, at increased aeration rates (150 rpm), the improvements in fermentation because of liquid adaptation were less pronounced. In using P. stipitis for the fermentation of biomass hydrolyzates, adaptation could be used to improve fermentation rate at less aeration rates, but at optimal aeration rates, the benefits of adaptation seems to be less pronounced.
References
Palmqvist, E., & Hahn-Hägerdal, B. (2000). Bioresource Technology, 74, 17–24.
Persson, P., Andersson, J., Gorton, L., Larsson, S., Nilvebrant, N. O., & Jonsson, L. J. (2002). Journal of Agricultural and Food Chemistry, 50, 5318–5325.
Van Zyl, C., Prior, B. A., & du Preez, J. C. (1988). Applied Biochemistry and Biotechnology, 17, 357–369.
Clark, T. A., & Mackie, K. L. (1984). Journal of Chemical Technology and Biotechnology, 34B, 101–110.
Tran, A. V., & Chambers, R. P. (1986). Enzyme and Microbial Technology, 8, 439–444.
Chandrakant, P., & Bisaria, V. S. (1998). Critical Reviews In Biotechnology, 18, 295–331.
Hahn-Hagerdal, B., Karhumaa, K., Fonseca, C., Spencer-Martins, I., & Gorwa-Grauslund, M. F. (2007). Applied Microbiology and Biotechnology, 74, 937–953.
Lee, J. (1997). Journal of Biotechnology, 56, 1–24.
Amartey, S., & Jeffries, T. (1996). World Journal of Microbiology & Biotechnology, 12, 281–283.
Delgenes, J. P., Moletta, R., & Navarro, J. M. (1996). Enzyme and Microbial Technology, 19, 220–225.
Fenske, J. J., Hashimoto, A., & Penner, M. H. (1998). Applied Biochemistry and Biotechnology, 73, 145–157.
Agbogbo, F. K., Coward-Kelly, G., Torry-Smith, M., & Wenger, K. S. (2006). Process Biochemistry, 41, 2333–2336.
Laplace, J. M., Delgenes, J. P., Moletta, R., & Navarro, J. M. (1991). Applied Microbiology and Biotechnology, 36, 158–162.
Roberto, I. C., Demancilha, I. M., Felipe, M. G. A., Silva, S. S., & Sato, S. (1994). Arquivos de Biologia e Tecnologia, 37, 55–63.
Skoog, K., Jeppsson, H., & Hahnhagerdal, B. (1992). Applied Biochemistry and Biotechnology, 34‑5, 369–375.
Amartey, S., & Jeffries, T. (1996). World Journal of Microbiology & Biotechnology, 12, 281–283.
Nigam, J. N. (2001a). Journal of Applied Microbiology, 90, 208–215.
Nigam, J. N. (2001b). Journal of Biotechnology, 87, 17–27.
Schell, D. J., Farmer, J., Newman, M., & McMillan, J. D. (2003). Applied Biochemistry and Biotechnology, 105−108, 69−85.
Agbogbo, F. K., Coward-Kelly, G., Torry-Smith, M., Wenger, K. S., & Jeffries, T. W. (2007). Applied Biochemistry and Biotechnology, 136–140, 653−662.
Acknowledgment
This investigation was funded by the US DOE project managed by Abengoa Bioenergy R&D. The authors thank the sponsors for their support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Agbogbo, F.K., Haagensen, F.D., Milam, D. et al. Fermentation of Acid-pretreated Corn Stover to Ethanol Without Detoxification Using Pichia stipitis . Appl Biochem Biotechnol 145, 53–58 (2008). https://doi.org/10.1007/s12010-007-8056-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-007-8056-4