Abstract
A liposomal vesicular structure is composed of one or more phospholipid bilayers in an aqueous environment. Liposomes are the most popular carriers used to design a delivery system for different compounds in the pharmacological, biochemical, biological, food, and agricultural sectors. Enzymes are commonly used ingredients in food industries, such as bakery, dairy, and beverages. Nevertheless, they are very sensitive to environmental stresses and harsh processing conditions and it is not possible to have a controlled release profile for their free forms. Fortunately, these limitations can be overcome by loading the enzymes in an efficient encapsulation system such as liposomal carriers. Many studies have indicated that the liposomes can be a proper candidate for encapsulation and delivery of enzymes. In this study, preparation and application of food-grade liposomes for encapsulation of a variety of enzymes have been reviewed and discussed. Particularly, application of enzyme-loaded liposomes for acceleration of ripening in different cheeses and various catalyzed reactions by liposomal enzymes have been covered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enzymes as unique natural biocatalysts have multifunctional roles in food products such as improving flavor, texture, and nutritional properties. Many food companies are looking for a way to improve the capability of enzymes for producing high acceptable food products with a top quality. Short time stability of enzymes has been known as a major challenge, which can be overcome by immobilization technique. Immobilization of enzymes is an efficient tool for biochemical and biotechnological processes which have been studied for both environmental and clinical fields. The goal of using immobilized enzymes includes their application in a wide range of temperatures and pH values to intensify their catalytic activity and to have higher production yields (Dos Santos and Dias-Souza 2016).
Separation of enzymes from environmental stresses, retaining the original biological activity of enzymes, and high loading efficiency of enzymes on non-catalytic materials are the main factors that have to be considered for applying a fine immobilization method. According to many studies, these factors can be achieved by using encapsulation techniques. The immobilization of two or more enzymes, protection of enzymes against environmental stresses and during process operations (such as protection of enzymes against denaturation), improving their stability, and easy handling of enzymes are the main advantages of encapsulation. Briefly, an enzyme is covered by wall materials through the encapsulation process. The diversity of food grade wall materials such as proteins, polysaccharides, and lipids is another advantage of encapsulation techniques (Assadpour and Jafari 2019a, b). For example, Deng et al. (2019) encapsulated pectinase into calcium alginate microparticles for fruit juice application (Dos Santos and Dias-Souza 2016); Ozaltin et al. (2019) encapsulated protease into produced microparticles by chitosan and alginic acid; and Haghighat-Kharazi et al. (2018) encapsulated α-amylase in beeswax for bread applications.
Recently, nanotechnology has been successfully used in the food sector and nanoencapsulation of bioactive compounds is one of the interesting branches of nanotechnology which has been used wieldy in the food industry (Koshani and Jafari 2019; Taheri and Jafari 2019). During nanoencapsulation, sensitive compounds are incorporated into various nanocarriers (nanoparticles). In the food sector, the European Food Safety Authority (EFSA) defined nanoparticles as engineered nanomaterials (ENM) smaller than 100 nm. There is a diversity of nanoencapsulation systems including bipolymeric nanoparticles, lipid-based nanoparticles, and natural nanoparticles (Jafari 2017). When the size of encapsulated particles is decreased to submicron, their surface area is increased, and in consequence, their solubility, adsorption, stability, and bioavailability are improved compared with microencapsulated particles (Garavand et al. 2019; Jafari and McClements 2017).
Liposomal entrapment is one of the lipid-based encapsulation systems that can be produced in micro and nano range. This method has been widely used for entrapping different compounds such as peptides (Mohan et al. 2016), enzymes (Jahadi et al. 2015), antioxidants (Cui et al. 2017; Tavakoli et al. 2018), antimicrobials (Cui et al. 2016), essential fatty acids (Ghorbanzade et al. 2017), and vitamins (Marsanasco et al. 2016) due to their advantages such as lipophilic/hydrophilic properties, ability to compartmentalize space, and flexible colloidal sizes (Faridi Esfanjani et al. 2018; Katouzian and Jafari 2016; Rafiee et al. 2018).
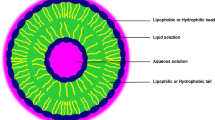
Liposome has a spherical shell structure which is assembled by the amphiphilic molecules. A phospholipid is a major component in liposome structure that has both hydrophilic (polar head) and hydrophobic (the fatty acid chain) groups. The detailed mechanism of liposome formation is not yet well known. When a phospholipid is added into water, one or more phospholipid bilayers surround the water as the core which results in the formation of liposomes (Ghorbanzade et al. 2017; Tavakoli et al. 2018; Shukla et al. 2017) as shown in Fig. 1; this magnificent carrier can provide an encapsulating medium for both hydrosoluble (hydrophilic) and liposoluble (hydrophobic) compounds (Liu et al. 2015; Mirafzali et al. 2014). The liposomal systems provide higher surface zone in food processing, which increases product solubility and bioavailability (Subramani and Ganapathyswamy 2020).
The capability of liposomal carriers to co-encapsulate both lipophilic and hydrophilic compounds, protection of encapsulated materials against environmental stresses, their high stability, and being food grade has made them good candidates for encapsulation of different enzymes. The encapsulation efficiency is one of the most important factors which need to be considered in the liposomal loading of enzymes; it depends on the formulation and preparation methods of the liposomes (Barragán et al. 2016). On the other hand, the protection and targeted delivery of enzymes are the main steps for their optimal application in the food sector which can be fulfilled by entrapping them efficiently within liposomes. Encapsulated enzymes have been used for the enrichment/fortification of foods, and producing of pharmaceutical products, biocatalysts or biosensors, hygienic products, detergents, enzyme-linked immunosorbent assay (ELISA) kits, etc. Enzymes can be entrapped into core or membranes of liposomes based on their hydrophilic/hydrophobic nature so that they can be protected against environmental stresses by a layer of the liposome. Some enzymes anchor on the surfaces of liposomes (Fig. 1); also, the surface modification of liposomes ensures the conjugation of enzymes onto their surface (Liu and Boyd 2013). For instance, Wichmann et al. (2003) anchored the α-amylase enzyme from Escherichia coli and guanylate kinase from Saccharomyces cerevisiae by a histidine-tag or indirectly via strep-tag and streptavidin or streptactin linker onto the liposomal membrane (Wichmann et al. 2003).
As mentioned, liposomal and nanoliposomal carriers are good alternatives to protect enzymes, control their release, and improve their techno-functional roles, which are the recent demand of the food industry. The main goal of this article is to provide a concise review of liposomal/nanoliposomal entrapment of enzymes and some of their application in the food sector.
An Overview of Liposomal/Nanoliposomal Formulation and Structure
Protection of sensitive bioactive components (e.g., enzymes, antioxidants, phenolic compounds, flavors, vitamin, and minerals) and increasing their efficacy by limiting the influence of undesirable environmental and process factors as well as preventing the interaction of the encapsulated ingredients with the outside conditions are potential applications of liposomes in different sectors (Singh et al. 2012). These advantages can be improved by applying nanotechnology in liposomal entrapment through increasing their surface area in nano scale carriers.
Before the review of different studies dealing with liposomal encapsulation of food-related enzymes, it is necessary to give a brief introduction of liposome and nanoliposome and to explain the most common methods for the production of these carriers.
Liposome General Composition
Generally, the fundamental components for production of liposomes are phospholipids which can have different types in terms of surface charges including neutral phospholipids, positively charged phospholipids, and negatively charged phospholipids (Moghimipour and Handali 2013) or can be of different origins such as phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylinositol (PI), and sphingomyelin (SM). The phospholipid type influences the dimensions and the physicochemical properties of the final obtained liposomes (Singh et al. 2012). The hydrophilic polymers which conjugate lipids and water are available in the liposomal structure. Also, cholesterol can be used in the liposome formulation since it improves the bilayer stability and fluidity of membrane, and decreases the permeability of hydrosoluble molecules through the membrane (Laouini et al. 2012), although there have been some debates about the unfavorable properties of cholesterol particularly from the nutritional point of view.
In order to classify different liposomes, composition and mechanism of intracellular delivery can be used; the following categories are available (Sharma and Sharma 1997):
-
pH-sensitive liposomes;
-
Conventional liposomes;
-
Immuno-liposomes;
-
Cationic liposomes;
-
Long-circulating liposomes.
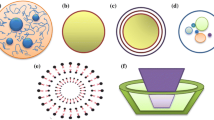
Number of bilayers and size of liposome structures are two main parameters which can effect on the encapsulation efficiency of various compounds into liposomes. Moreover, the circulation half-life of liposomes can be determined by the vehicle size (Laouini et al. 2012). Thus, another common approach to classify the liposomes is based on the size and membrane structures (Fig. 2) as follows (Akhavan et al. 2018; Demirci et al. 2017; Gómez-Hens and Fernández-Romero 2005):
-
(i)
Multivesicular liposomes (MVLs, < 1 μm);
-
(ii)
Multilamellar liposomes (MLLs, ≥ 400 nm);
-
(iii)
Unilamellar liposomes (ULLs, < 20 nm).
Several parameters can influence the liposomal encapsulation yield including phospholipid type and concentration, liposome size (diameter), encapsulant concentration, pH and ionic strength plus buffer salt, and its concentration (Demirci et al. 2017; Hwang et al. 2012). Protection of sensitive bioactive components (like enzymes, antioxidants, phenolic compounds, flavors, vitamin, and minerals) and increasing their efficacy by limiting the influence of undesirable environmental and process factors as well as preventing the interaction of the encapsulated ingredients with the outside conditions are potential applications of liposomes in different sectors (Singh et al. 2012).
Method of Liposome Production
There are many different methods available for the production of liposomal carriers which can be classified into the following (Laouini et al. 2012):
-
(i)
Conventional techniques including Bangham method or thin-film hydration, solvent (ether or ethanol) injection technique, reverse-phase evaporation (REV) technique, and detergent removal (Fig. 3).
-
(ii)
Novel upscalable techniques such as spray drying, heating method, supercritical reverse-phase evaporation (SCRPE), freeze drying, and modified ethanol injection method (including the crossflow injection technique, microfluidization, and membrane contactor).
The pros and cons of the most common methods for production of liposomes are presented in Table 1 (Liu et al. 2015; Mohammadi et al. 2015). The thin-film hydration method is proper for the encapsulation of hydrophilic and hydrophobic molecules, and this assay of liposome preparation was used by Bangham, the liposomal founder (Liu et al. 2015). A microfluidizer produces relatively stable liposome without rapid aggregation or fusion, liposome manufacture without using organic solvents in the microfluidization technique (Liu et al. 2015). In the reverse-phase evaporation method (REV), an emulsion (water-in-oil) organized by magnetic stirring or sonication of an instance containing phospholipids in an organic solvent such as diethylether, isopropyl ether, or mixtures of these ethers with chloroform or methanol and aqueous buffer (Mohammadi et al. 2015). The organic solvent is subsequently evaporated under reduced pressure, and the system is converted to an aqueous dispersion of liposomes. The removal of the organic solvent is a major step in this method, which may damage the encapsulated material (Walde and Ichikawa 2001). Dehydration–rehydration method is a relatively mild assay for production of liposome. It often has been used for the encapsulation of enzymes for applications in food technology in the past (Walde and Ichikawa 2001). A simple procedure for preparation of liposome is pro-liposome method that is defined as the mixing of phospholipid, ethanol, and water. This assay avoids the use of energy-expensive processes required and it is possible to achieve the high entrapment efficiencies (Perrett et al. 1991).
The sonication method produces small, unilamellar vesicles (SUV) with diameters in the range of 15–50 nm. The bath and probe tip sonicators are common instrumentation for preparation of sonicated particles (Dua et al. 2012). In the detergent removal assay, detergents such as bile salts or alkylglycosides can be removed by dialysis, gel chromatography, and dilution. The detergents such as bile salts and alkylglycosides with high solubility in organic and aqueous systems are employed. The chemical character and quantity of detergents as well as the nature of lipids are effective on the physical properties of vesicles such as size and shape (Khanniri et al. 2015). The heating method was defined to produce liposomes which involves hydration of the phospholipids in an aqueous solution containing 3% (vol) glycerol and depending on the absence or presence of cholesterol, increasing the temperature to 60 °C or 120 °C, respectively. The mozafari method is an improved version of the heating assay, and this assay authorizes wide-ranging production of the carrier systems just in one step without employing toxic solvents or detergents and without the requirement for the pre-hydration of the components (Colas et al. 2007).
Finally, in order to have the most appropriate liposomal production method, it is important to consider the following parameters (Dua et al. 2012; Rafiee and Jafari 2018): (1) the physicochemical properties of the liposomal ingredients and the components which need to be entrapped within liposomes; (2) the nature of the medium; (3) the most efficient concentration of the entrapped material; (4) additional procedures required during the delivery or application of the liposomes; (5) the optimum size of liposomal vesicles; and (6) the optimum required shelf-life.
Encapsulation of Various Food-Relevant Enzymes Within Liposomes
Enzymes are biological macromolecules which can accelerate different biological/biochemical reactions. These biocatalysts play a key role in the establishment and development of industrial bioprocesses (Barragán et al. 2016). Since enzymes are mostly proteins, they are sensitive to various physiological changes which could be initiated by some factors like heat, pH, ionic strength, and other denaturation agents (Barragán et al. 2016). The first investigation on encapsulation of enzymes via liposomes was carried out by Sessa and Weissmann in 1970. The purposes of using immobilized enzymes include their application in a wide range of temperatures and pH values to intensify their catalytic activity and to have higher production yields (Dos Santos and Dias-Souza 2016). Enzymes can be entrapped into core or membranes of liposomes based on their hydrophilic/hydrophobic nature, so that they can be protected against environmental stresses by a layer of the liposome. Some enzymes anchor on the surfaces of liposomes, as shown in Fig. 1; also, the surface modification of liposomes ensures the conjugation of enzymes onto their surface (Liu and Boyd 2013). Many studies have shown the capability of liposomal carriers for loading different enzymes. Most of the encapsulated food-relevant enzymes by liposomal carriers belong to the oxidoreductase and hydrolase classes, summarized in Tables 2 and 3, respectively.
Enzymes have been used widely in the food industry because of their ability to improve functional, sensory, and nutritional properties of products such as bread quality improvement through increased bread volume and improved crumb structure, reduction of acrylamide formation during baking, amylase dough softening, starch liquefaction, clarification of fruit juices, enhancement of flavor and fragrance in fruit juices, production of high fructose corn syrups (beverage sweeteners), fruit liquefaction in juice production, cheddar cheese production, cheese flavor development, production of lipolyzed milk fat, sweetening milk and whey, shelf-life improvement of food products, and food flavor improvement (Raveendran et al. 2018; Whitehurst and Law 2002). These are just some examples of rudimental roles of enzymes in food industrials, which have been improved by encapsulation of enzymes. Liposomal/nanoliposomal enzymes can be easily used in food formulations due to their food compatibility, and also higher efficiency. Most of the applications of liposomal/nanoliposomal enzymes in the food industry have been in dairy sector, particularly cheese production. In the following sections, a brief overview of the related studies based on the applied enzymes will be discussed.
Oxidoreductase Enzymes Loaded Within Liposomes
Catalase (EC 1.11.1.6)
Application in food packaging, food preservation, and cheese production are major uses of catalase in the food industry (Kaushal et al. 2018). Some studies have shown the improving capability and stability of catalase by encapsulation into liposome and nanoliposome. For instance, Yoshimoto et al. (2007) encapsulated bovine liver catalase into the aqueous core of nanoliposomes for improving its stability and activity. The particle size of liposomes containing catalase was in the range of 30–100 nm and the stability of catalase within these nanoliposomal cargos was significantly enhanced compared with un-encapsulated catalase during storage at 4 °C in the pH = 7.4 (Yoshimoto et al. 2007). Abuin et al. (2012) investigated the disintegration of hydrogen peroxide by catalase encapsulated inside dipalmitoylphosphatidyl choline unilamellar liposomes. The diffusion of hydrogen peroxide across the liposome bilayer controlled the rate of oxygen production and the results revealed that hydrogen peroxide decomposition rate by catalase-loaded liposomes depends on the fluidity of bilayer and the enzyme reaction inside liposomes (Abuin et al. 2012; Yoshimoto and Higa 2014).The maximum rates of the process were observed at the membrane main transition temperatures (Abuin et al. 2012). Zhang et al. (2017b) encapsulated catalase within liposomes formed by cisplatin (IV)-prodrug-conjugated phospholipids. They reported that the liposomal system can be used for clinical treatments, such as chemo-radiotherapy treatment of cancers (Zhang et al. 2017b).
Glucose Oxidase (EC 1.1.2.3.4)
Glucose oxidase is the enzyme responsible for the oxidation of β-D-glucose to gluconic acid and hydrogen peroxide (Bankar et al. 2009). The glucose oxidase contains two identical subunits which bind noncovalently to flavin adenine dinucleotide (FAD) (Yoshimoto et al. 2006). In general, microbial-originated glucose oxidase has major applications in food, beverage, pharmaceutical, clinical, chemistry, biotechnology, and other industries (Bankar et al. 2009). Improving food shelf-life and improving flavor are two important applications of glucose oxidase in the food industry (Raveendran et al. 2018).
The immobilization of biomolecules like enzymes is an important step in many bioanalytical applications. Kaszuba and Jones (1999) encapsulated glucose oxidase combined with horseradish peroxidase (HRP) and/or lactoperoxidase within the anionic and cationic liposomes fabricated by the vesicle extrusion method. Their results indicated that the production of hydrogen peroxide increased approximately linearly at higher substrate levels and elevated with the concentration of encapsulated glucose oxidase (Kaszuba and Jones 1999). In another work, glucose oxidase from Aspergillus niger was loaded into liposomes by Olea and Faure (2003). They found that the onion-type multilamellar vesicles provided many advantages in comparison with unilamellar vesicles for encapsulation of glucose oxidase, such as higher encapsulation yields (80%) (Olea and Faure 2003). Wang et al. (2003) observed that glucose oxidase encapsulated in liposomal carriers had higher stability in catalyzing H2O2 compared with the un-encapsulated enzyme (Wang et al. 2003). Also, the hydrogen peroxide (H2O2) produced during the glucose oxidation was able to inactivate the glucose oxidase (Bao et al. 2003). Yoshimoto et al. (2006) encapsulated glucose oxidase (GO) into liposomes produced by the extrusion technique to increase the stability of GO against H2O2. They investigated the influence of glucose level on the fluorescence properties of the glucose oxidase to show the high stability of liposomal glucose oxidase against H2O2. These researchers concluded that glucose oxidase was entrapped within the liposomes because the glucose concentration into liposomes was low regardless of its concentration in the bulk liquid. This observation can be related to the impermeability of the liposomal membrane to glucose (Yoshimoto et al. 2006).
Yoshimoto et al. (2010) encapsulated glucose oxidase into the catalase-conjugated liposomes, a novel liposomal system in which catalase molecules were conjugated to the liposome surface. They reported that this liposomal carrier was appropriate to control the rate of reactions catalyzed via glucose oxidase (Yoshimoto et al. 2010). Yanan et al. (2013) immobilized glucose oxidase in biomimetic silica particles by coupling liposomes, and found that the pH and operational and thermal stabilities of the encapsulated glucose oxidase were significantly improved in comparison with the free glucose oxidases (Yanan et al. 2013). Graça et al. (2014) used a single phospholipid together with a mixture of two phospholipids (1,2-dipalmitoyl-phosphatidylglycerol (DPPG) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG)) for the loading of glucose oxidase into a liposome in order to design a new amperometric glucose biosensor. The specific mixture of POPG and DPPG liposomes leads to an improvement in biosensor response and tuning of the liposomal structures because of their distinctive phase transition temperatures caused this improvement (Graça et al. 2014).
Laccases (EC 1.10.3.2)
Laccases are very useful enzymes in biotechnology due to their ability to oxidize phenolic and non-phenolic lignin-related substrates and other environmental pollutants (Martí et al. 2012). Also, laccase has been used in bakery products to reduce dough time-to-peak and to accelerate dough breakdown (Labat et al. 2000).
The liposomal/nanoliposomal entrapment can be used as a fine method for encapsulation of laccase. For example, Martí et al. (2012) investigated the encapsulation capacity of laccases entrapped in liposomes. These researchers found high encapsulation efficiency at higher lipid/laccase ratios. The loss of laccase activity loaded in the liposomes was lower than 10% compared with 40–60% loss for free laccases after heating the samples for 3 days (Martí et al. 2012). Trametes versicolor laccase was encapsulated at pH = 4.5 in onion-type multilamellar liposomes and the encapsulation efficiency, laccase activity, and stability in various conditions were determined by Prévoteau and Faure (2012). They reported that the encapsulation efficiency was more than 65% at 25 °C and 37 °C in citrate buffer solutions which was reduced to 54% by addition of NaCl. The multilamellar vesicles substantially improved both the stability and activity of laccase (Prévoteau and Faure 2012).
Hydrolase Enzymes Loaded Within Liposomes
α-Amylase (EC 3.2.1.1)
α-Amylase as a significant industrial endo amylase can hydrolyze the internal 1,4-glycosidic linkages to maltose, dextrin, and glucose (Zhang et al. 2017a). α-Amylases are commonly applied enzymes in many industrial processes like the starch liquefaction, baking, designing of textiles, detergents, and bioethanol production (Sindhu et al. 2017). Galzigna et al. (1979) encapsulated α-amylase from hog pancreas in charged and uncharged liposomes and compared its behavior with the free α-amylase. They found that α-amylase loaded into negatively charged liposomes had a sigmoidal relationship with substrate concentration and the reaction velocity (Galzigna et al. 1979). Type of phosphatidylcholine vesicles (GUV and MLV), lipid concentration, and the unloaded or loaded enzyme conditions can influence the kinetic parameters of the enzyme (Sanchez and Perillo 2000) so that using of phosphatidylcholine in MLVs led to an enhancement of the KM value of α-amylase for starch as the substrate. Also, the high salt concentrations or the pH values of the media can adjust the binding of the enzyme.
Protection of α-amylase against pepsin and acid can be achieved by replacement of cholesterol via stearic acid in liposomal formulations which in turn reduces the risk of cholesterol consumption through liposomes. Hsieh et al. (2002) used several lipids (including tristearin or linoleic acid, stearic acid) as an alternative to cholesterol in the fabrication of liposomes and studied the encapsulation efficiency as well as stability against temperature and pH. Furthermore, they investigated the activity of liposomal encapsulated α-amylase in pepsin solutions for up to 3 h. It was revealed that the stability of encapsulated α-amylases against pH and temperature and encapsulation efficiency of liposomes produced by lecithin:stearic acid with the ratio of 1:0.25 were almost close to the liposomes obtained by cholesterol and lecithin at the same ratio. Albeit, stearic acid in the liposomal system was impressive in protection against acidic treatments and pepsin (Hsieh et al. 2002).
Amyloglucosidase (EC 3.2.1.3)
Amyloglucosidase is an economical and significant enzyme which is applied in several industries, such as effluent treatment, detergents, baking, and natural sweeteners. Li et al. (2007) entrapped amyloglucosidase from Aspergillus niger into dipalmitoylphosphatidylcholine (DPPC) multilamellar and unilamellar liposomal vesicles. Their results showed that loaded enzymes were extremely stable compared with the free form and the loaded enzyme in liposomes was reused with the maintenance of 60% activity after 3 cycles (Li et al. 2007).
β-Galactosidase (EC 3.2.1.23)
β-D-galactosidase or lactase is an important enzyme which hydrolyzes the β-1,4-D-galactosidic linkages. It is applied in numerous processing practices, such as food, dairy, and fermentation industries and environmental processes (Anisha 2017). β-Galactosidase has been entrapped into liposomes using reverse-phase evaporation and dehydration–rehydration methods. Acid resistant and enzymatic activity were found to be retained after 1-month storage at 5 °C under nitrogen (Matsuzaki et al. 1989). Rao et al. (1994) compared the dehydration-rehydration vesicle (DRV) and reverse-phase evaporation vesicle (REV) for encapsulation of β-galactosidase from Aspergillus flavus into liposomes. They reported that the encapsulation efficiencies in DRV and REV liposomes were 18.3 and 27.6%, respectively, and these liposomal carriers digested up to 50% of lactose in milk under simulated gastrointestinal conditions (Rao et al. 1994). Kim et al. (1999) studied fortification of whole milk with encapsulated β-galactosidase into dried liposomes fabricated by DRV in the presence of trehalose. According to their results, 87% of β-galactosidase was retained in dried liposomes at 17 °C after 60 days; also these liposomes were more stable compared with the multilamellar vesicle suspensions produced without trehalose. The β-galactosidase-loaded liposome had high stability and showed a high ability to digest lactose in milk after lysis of liposomes (Kim et al. 1999).
Rodríguez-Nogales and López (2006) reported that β-galactosidase encapsulated in liposomes demonstrated higher thermal stability at storage temperatures (30–80 °C for 60 min) and the stability of β-galactosidase against proteolytic activity was increased by the entrapment process. It was observed that the ratio of cholesterol, phosphatidylcholine, type of sonicator, and enzyme:lipid ratio were the most important factors for encapsulation of β-galactosidase into liposomes (Rodríguez-Nogales and López 2006). These researchers showed that the optimum formulation of liposomes with an encapsulation efficiency of 28% was achieved by cholesterol:phosphatidylcholine and enzyme:lipid ratios of 0.53 and 13.76, respectively, at pH = 6 and utilizing a probe-type sonicator. Rao et al. (1994) investigated the activity of encapsulated β-galactosidase by different liposomal carriers including dehydration-rehydration vesicles (DRV) and reverse-phase evaporation vesicles (REV) in milk and reported that DRV and REV liposomes hydrolyzed 65% and 20% of the lactose, respectively at the refrigeration temperature for 20 days (Rao et al. 1994). Picon et al. (1995) used DRV liposomes for encapsulation of cyprosins (the proteinases present in cardoon rennet) to accelerate the ripening of Manchego cheese and found that addition of liposomal cyprosins to milk speeds up the development of flavor intensity in cheese through 15 days storage without enhancing bitterness (Picon et al. 1995). Kim et al. (1999) prepared dried liposomes containing β-galactosidase for the digestion of lactose in milk and mentioned that liposomal β-galactosidase was stable and reconstituted mostly upon rehydration; also it was able to digest lactose in milk after the efficacious lysis of liposomes in the presence of bile salts (Kim et al. 1999).
Flavourzyme (EC 3.4.11.1)
Flavourzyme is a commercial protease with both endo and exopeptidase activities which is mostly used in the production of cheese and acceleration of cheese ripening particularly for flavor enhancing (Feng et al. 2014; Jahadi et al. 2015). Vafabakhsh et al. (2013) investigated the encapsulation of flavourzyme through liposomes prepared by the thin-layer method for possible application in the ripening of cheese as a flavor-enhancing enzyme. Investigation of the thermal stability (at 35, 45, and 55 °C) showed that enzyme-loaded liposomes were stable at 35 °C for 6 h. The results revealed a gradual proteolysis of casein by liposomal flavourzyme compared with un-encapsulated enzymes (Vafabakhsh et al. 2013). Also, flavourzyme has been encapsulated in liposomes using a heating method without employing any chemical solvents or detergents by Jahadi et al. (2015). Their results indicated that the highest encapsulation efficiency and activity of liposomal enzyme were acquired in 4.5% lecithin concentration, 5% flavourzyme/lecithin, the temperature of 45 °C, stirring time of 30 min, and pH = 6 (Jahadi et al. 2015). Jahadi et al. (2016) in another study loaded flavorsome into liposomes by the heating method to speed up the ripening of Iranian white cheese. The optimum proteolysis indices and the most scores of sensory properties were achieved by applying 0.3% w/w enzyme, ripening for 30 days and 8 h curd maintenance in saturated brine (Jahadi et al. 2016). Jahadi et al. (2015) used heating method for the preparation of nanoliposomal flavourzyme for the ripening acceleration of Iranian brined cheese. The encapsulation process inhibited pre-maturation of protein curd and protected casein from early proteolysis. Liposomal flavourzyme did not affect curd and whey composition significantly, as well as cheese efficiency (Jahadi et al. 2015). In another study done by Jahadi et al. (2020), the entrapment efficiency and diameter of the liposomal flavourzyme were 26.5% and 189 nm, respectively. These researchers also reported that use of liposome-encapsulated flavourzyme was able to accelerate the ripening process in Iranian white brined cheeses and increased proteolysis rates and moisture contents.
Generally, encapsulation of cheese-ripening enzymes (protease, peptidase, or lipase) in liposomal systems can increase the rate of cheese ripening, which is due to proteolysis and lipolysis reactions facilitated by these enzymes and results in a favorable texture, smell, and taste of cheese along with reduction of the cost of product storage (Balbaa and Awad 2018).
Lipase (EC 3.1.1.3)
Lipases catalyze the esteric bonds in triglycerides and produce diglycerides, monoglycerides, and glycerol (Sanromán and Deive 2017). Lipases are widely used enzymes in various processing sectors, such as the oil industry, bakery, dairy, and other areas. It has been reported that encapsulation of this enzyme into liposomes yields a cheese with a desirable flavor and texture in half of the normal time, whereas the required level of enzyme is decreased (Kirby et al. 1987). In a study by Kheadr et al. (2002), Palatase M and Lipase 50 were loaded into liposomes with an encapsulation efficiency of 35.9% and 40.3%, respectively. The addition of encapsulated lipase into cheese formulation provided accelerated cheese lipolysis without undesirable effects on its flavor or texture (Kheadr et al. 2002). Also, Kheadr et al. (2003) studied cheddar cheese lipolysis and proteolysis acceleration using liposomal enzymatic cocktails. Enzyme cocktails were included lipase and fungal protease (FP cheeses), lipase and bacterial protease (BP cheeses), or lipase and flavourzyme (ZP cheeses). The encapsulated enzymes (BP and ZP) resulted in cheeses via a more developed surface and a higher flavor power in a shorter time and no bitter defect was determined except in a 90-day-old FP cheese compared with control cheese (Kheadr et al. 2003). Macario et al. (2013) studied the application of pure silica nanoparticles for encapsulation of lipase in a liposomal system. In their study, the lipase from Rhizomucor michel was encapsulated in a liposome and coated by a porous silica shell. The results indicated that silica coverage can stabilize the internal phase of liposomal carriers and protect the encapsulated enzyme. Silica:liposome ratio of 2 had the best results during the heterogeneous biocatalyst preparation (Macario et al. 2013).
Trypsin (EC 3.4.21.4)
Trypsin is a serine protease which detaches mainly the carboxyl side of arginine or lysine of peptide chains (Gombos et al. 2008). Trypsin has been encapsulated in multilamellar vesicles (MLVs), multilamellar vesicles produced by a microfluidizer (MLVs-MF), and dehydrated-rehydrated vesicles (DRVs) of liposomal carriers (Larivière et al. 1991). The encapsulated trypsin was more efficient in comparison with the un-encapsulated enzyme for the treatment of cheese. The encapsulation efficiencies were 10, 14, 12, and 13% for MLVs, MLVs-MF, MF, and DRVs, respectively. It was reported that the large-scale production of trypsin-loaded liposomes can be successfully achieved by applying a microfluidizer resulting in the acceleration of cheese ripening (Larivière et al. 1991). Hwang et al. (2012) studied the effect of buffer pH and ionic strength and phospholipid concentration on the trypsin encapsulation within liposomes. They found that the trypsin encapsulation yield (1.5–17.5%) was linearly correlated with the dipalmitoylphosphatidylcholine concentration and it is important to optimize the pH and ionic strength of the encapsulation buffer for each enzyme based on the phospholipid of the lipid bilayer and the pH of the enzyme to recover the activity of the loaded enzyme into liposomes. To sum up, it was concluded that pH had more influence on the trypsin activity compared with ionic strength (Hwang et al. 2012).
Other Proteolytic Enzymes
Neutrase (EC 3.4.24.28) as a commercial bacterial protease is commonly applied in the acceleration of Saint-Paulin cheese. Alkhalaf et al. (1989) fabricated neutrase-loaded neutral, positive, and negative charged liposomes by reversed phase evaporation. They reported that the low pH, high temperature, and high NaCl lead to a decrease in liposomal stability. Besides, it was found that positive and neutral liposomes were more stable than the negative ones (Alkhalaf et al. 1989). The proteinase from Bacillus subtilis has also been entrapped in liposomes using the dehydration–rehydration method, which was added to pasteurized ewe milk to accelerate Manchego cheese ripening. In another study, development of flavor intensity was accelerated by encapsulation of proteinase in a liposome and no sign of bitter flavor was noticed (Picon et al. 1995). Alkhalaf et al. (1989) studied proteolytic enzyme (neutrase) loaded in liposomes prepared by reversed phase evaporation for acceleration of Saint-Paulin cheese ripening and found that negatively charged, positively charged, and then neutral liposomes showed the highest to lowest retention rates, respectively (Alkhalaf et al. 1989). Larivière et al. (1991) prepared different types of liposomes (multilamellar, multilamellar-microfluidized, microfluidized and dehydrated-rehydrated) containing trypsin applied them in cheddar cheese parison with free enzymes (Larivière et al. 1991).
Kheadr et al. (2000) accelerated cheddar cheese ripping with liposomal proteinases (bacterial and fungal). The samples showed a slightly higher moisture content, lower protein content, less firmness, and more brittleness compared with control cheeses (containing no liposomal system); also, proteolysis and texture development during the ripening of cheddar cheese were faster than the control sample (Kheadr et al. 2000). Kheadr et al. (2003) investigated the proteolysis and lipolysis process of Cheddar cheese using liposome-encapsulated enzymatic cocktails (flavourzyme, neutral bacterial protease, acid fungal protease and lipase) during 3 months of ripening at 8 °C and their data revealed that addition of a mixture of bacterial protease and lipase in liposomal system had an acceptable performance (Kheadr et al. 2003). Vafabakhsh et al. (2013) studied the proteolysis reaction in re-combined milk containing 0.03% free or encapsulated flavourzyme. According to their results, a higher rate of proteolysis was observed in samples with encapsulated during 4 h incubation at 30 °C. Also these samples showed a gradual proteolysis of casein in com addition of liposomal enzymes could lead to the acceleration of cheese ripening as mentioned before, so it is more efficient compared with the use of a free enzyme (Vafabakhsh et al. 2013). The selection of the appropriate liposome composition relevant to the type of enzyme is a significant factor in the encapsulation of enzymes. Piard et al. (1986) produced several types of liposomes to entrap rulactine (proteinase) for the acceleration of Saint-Paulin cheese ripening. Their results indicated that the maturation of the cheese was depending on the entrapment level of the enzyme within the liposomal carriers, the stability of the vesicles in the cheese matrix and during the ripening stage (Piard et al. 1986).
Conclusion and Future Trends
There are many different enzymes which can play a key role in the food and medical industries. For instance, in the food sector, enzymes are major additive compounds in the bakery, dairy, and beverage industries. The employment of free form of enzymes has many restrictions, such as sensitivity to environmental circumstances and processing stresses. Hence, an optimal encapsulation system is necessary to overcome the aforementioned limitations. Liposomal systems have attracted the attention of researchers for loading of enzymes in foods, cosmetics, and pharmaceutical industries due to fast and straightforward methods for preparing the liposomes. Applying nanotechnology in liposomal encapsulation has improved the functional properties of these carriers due to increased surface area. So far, based on our knowledge, there are no studies which discuss the liposomal and nanoliposomal encapsulation of various enzymes for food application. Thus, in this study, the formulation and preparation of liposomes and nanoliposomes plus the studies on encapsulation of various enzymes by liposomes were discussed for food applications.
As mentioned in this article, most of the encapsulated enzymes within liposome/nanoliposome systems have been used in dairy (especially cheese production) sector. Therefore, future studies could focus on the application of liposomal/nanoliposomal enzymes in other food areas such as beverages and baking products. Also, future researches can focus on modified lipids in the formulation of liposomes and production of multilayer liposomes by coating biopolymers on the lipid layer. Besides, food formulations comprising liposomal encapsulated enzymes should be further studied in future researches considering their release behavior, solubility, and potential toxicity.
References
Abuin, E., Lissi, E., & Ahumada, M. (2012). Diffusion of hydrogen peroxide across DPPC large unilamellar liposomes. Chemistry and Physics of Lipids, 165(6), 656–661.
Akhavan, S., Assadpour, E., Katouzian, I., & Jafari, S. M. (2018). Lipid nano scale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trends in Food Science & Technology, 74, 132–146.
Alkhalaf, W., El Soda, M., Gripon, J.-C., & Vassal, L. (1989). Acceleration of cheese ripening with liposomes-entrapped proteinase: influence of liposomes net charge. Journal of Dairy Science, 72(9), 2233–2238.
Anisha, G. (2017). β-Galactosidases, Current Developments in Biotechnology and Bioengineering (pp. 395–421). Elsevier.
Assadpour, E., & Jafari, S. M. (2019a). An overview of biopolymer nanostructures for encapsulation of food ingredients, Biopolymer Nanostructures for Food Encapsulation Purposes (pp. 1–35). Elsevier.
Assadpour, E., & Jafari, S. M. (2019b). An overview of lipid-based nanostructures for encapsulation of food ingredients, Lipid-Based Nanostructures for Food Encapsulation Purposes (pp. 1–34). Elsevier.
Balbaa, M., & Awad, D. (2018). The use of liposomes in enzymes and drug design: liposomes drug delivery system, Research Advancements in Pharmaceutical, Nutritional, and Industrial Enzymology (pp. 128–140). IGI Global.
Bankar, S. B., Bule, M. V., Singhal, R. S., & Ananthanarayan, L. (2009). Glucose oxidase—an overview. Biotechnology Advances, 27(4), 489–501.
Bao, J., Furumoto, K., Yoshimoto, M., Fukunaga, K., & Nakao, K. (2003). Competitive inhibition by hydrogen peroxide produced in glucose oxidation catalyzed by glucose oxidase. Biochemical Engineering Journal, 13(1), 69–72.
Barragán, L. P., Buenrostro-Figueroa, J., González, C. A., & Marañon, I. (2016). Production, stabilization, and uses of enzymes from fruit and vegetable byproducts, Biotransformation of Agricultural Waste and By-Products (pp. 271–286). Elsevier.
Colas, J. C., Shi, W., Rao, V. S. N. M., Omri, A., Mozafari, M. R., & Singh, H. (2007). Microscopical investigations of nisin-loaded nanoliposomes prepared by Mozafari method and their bacterial targeting. Micron, 38(8), 841–847.
Cui, H., Wu, J., & Lin, L. (2016). Inhibitory effect of liposome-entrapped lemongrass oil on the growth of Listeria monocytogenes in cheese. Journal of Dairy Science, 99(8), 6097–6104.
Cui, H., Yuan, L., Li, W., & Lin, L. (2017). Antioxidant property of SiO2-eugenol liposome loaded nanofibrous membranes on beef. Food Packaging and Shelf Life, 11, 49–57.
Demirci, M., Caglar, M. Y., Cakir, B., & Gülseren, İ. (2017). 3 - Encapsulation by nanoliposomes A2 - Jafari, Seid Mahdi, Nanoencapsulation Technologies for the Food and Nutraceutical Industries (pp. 74–113). Academic Press.
Deng, Z., Wang, F., Zhou, B., Li, J., Li, B., & Liang, H. (2019). Immobilization of pectinases into calcium alginate microspheres for fruit juice application. Food Hydrocolloids, 89, 691–699.
Dos Santos VL, Dias-Souza MV (2016) Strategies based on microbial enzymes and surface-active compounds entrapped in liposomes for bacterial biofilm control, Nanobiomaterials in Antimicrobial Therapy. Elsevier, pp 385-418
Dua, J., Rana, A., & Bhandari, A. (2012). Liposome: methods of preparation and applications. Int J Pharm Stud Res, 3(2), 14–20.
Faridi Esfanjani, A., Assadpour, E., & Jafari, S. M. (2018). Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends in Food Science & Technology, 76, 56–66.
Feng, L., Qiao, Y., Zou, Y., Huang, M., Kang, Z., & Zhou, G. (2014). Effect of flavourzyme on proteolysis, antioxidant capacity and sensory attributes of Chinese sausage. Meat Science, 98(1), 34–40.
Galzigna, L., Garbin, L., & Burlina, A. (1979). Liposome-incorporated enzymes: studies on amylase. Clinical Biochemistry, 12(6), 267–269.
Garavand, F., Rahaee, S., Vahedikia, N., & Jafari, S. M. (2019). Different techniques for extraction and micro/nanoencapsulation of saffron bioactive ingredients. Trends in Food Science and Technology, 89, 26–44.
Ghorbanzade, T., Jafari, S. M., Akhavan, S., & Hadavi, R. (2017). Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chemistry, 216, 146–152.
Gombos, L., Kardos, J., Patthy, A., Medveczky, P., Szilágyi, L., Málnási-Csizmadia, A., & Gráf, L. (2008). Probing conformational plasticity of the activation domain of trypsin: the role of glycine hinges. Biochemistry, 47(6), 1675–1684.
Gómez-Hens, A., & Fernández-Romero, J. M. (2005). The role of liposomes in analytical processes. TrAC Trends in Analytical Chemistry, 24(1), 9–19.
Graça, J., De Oliveira, R., De Moraes, M., & Ferreira, M. (2014). Amperometric glucose biosensor based on layer-by-layer films of microperoxidase-11 and liposome-encapsulated glucose oxidase. Bioelectrochemistry, 96, 37–42.
Haghighat-Kharazi, S., Milani, J. M., Kasaai, M. R., & Khajeh, K. (2018). Microencapsulation of α-amylase in beeswax and its application in gluten-free bread as an anti-staling agent. LWT, 92, 73–79.
Hill, K. J., Kaszuba, M., Creeth, J. E., & Jones, M. N. (1997). Reactive liposomes encapsulating a glucose oxidase-peroxidase system with antibacterial activity. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1326(1), 37–46.
Hsieh, Y. F., Chen, T. L., Wang, Y. T., Chang, J. H., & Chang, H. M. (2002). Properties of liposomes prepared with various lipids. Journal of Food Science, 67(8), 2808–2813.
Hwang, S. Y., Kim, H. K., Choo, J., Seong, G. H., Hien, T. B. D., & Lee, E. (2012). Effects of operating parameters on the efficiency of liposomal encapsulation of enzymes. Colloids and Surfaces B: Biointerfaces, 94, 296–303.
Jafari, S. M. (2017). Nanoencapsulation technologies for the food and nutraceutical industries. Academic Press.
Jafari, S. M., & McClements, D. J. (2017). Nanotechnology approaches for increasing nutrient bioavailability, Advances in food and nutrition research (pp. 1–30). Elsevier.
Jahadi, M., Khosravi-Darani, K., Ehsani, M. R., Mozafari, M. R., Saboury, A. A., & Pourhosseini, P. S. (2015). The encapsulation of flavourzyme in nanoliposome by heating method. Journal of Food Science and Technology, 52(4), 2063–2072.
Jahadi, M., Khosravi-Darani, K., Ehsani, M. R., Mozafari, M. R., Saboury, A. A., Zoghi, A., & Mohammadi, M. (2016). Modelling of proteolysis in Iranian brined cheese using proteinase-loaded nanoliposome. International Journal of Dairy Technology, 69(1), 57–62.
Jahadi, M., Khosravi-Darani, K., Ehsani, M. R., Colombo Pimentel, T., Gomes da Cruz, A., & Mozafari.M.R. (2020). Accelerating ripening of Iranian white brined cheesesusing liposome-encapsulated and free proteinases. Biointerface Research in Applied Chemistry, 10(1), 4966–4971.
Jones, M. N., Hill, K. J., Kaszuba, M., & Creeth, J. E. (1998). Antibacterial reactive liposomes encapsulating coupled enzyme systems. International Journal of Pharmaceutics, 162(1-2), 107–117.
Kasinathan, N. (2014). Application of experimental design in preparation of nanoliposomes containing hyaluronidase. Journal of Drug Delivery 2014.
Kaszuba, M., & Jones, M. N. (1999). Hydrogen peroxide production from reactive liposomes encapsulating enzymes. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1419(2), 221–228.
Katouzian, I., & Jafari, S. M. (2016). Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends in Food Science & Technology, 53, 34–48.
Kaushal, J., Singh, S. G., Raina, A., & Arya, S. K. (2018). Catalase enzyme: application in bioremediation and food industry. Biocatalysis and agricultural biotechnology.
Khanniri, E., Bagheripoor-Fallah, N., Sohrabvandi, S., Mortazavian, A. M., Khosravi-Darani, K., & Mohammadi, R. (2015). Application of liposomes in some dairy products. Critical Reviews in Food Science and Nutrition, 56, 484–493.
Kheadr, E. E., Vuillemard, J. C., & El Deeb, S. A. (2000). Accelerated Cheddar cheese ripening with encapsulated proteinases. International Journal of Food Science & Technology, 35(5), 483–495.
Kheadr, E., Vuillemard, J. C., & El-Deeb, S. (2002). Acceleration of Cheddar cheese lipolysis by using liposome-entrapped lipases. Journal of Food Science, 67(2), 485–492.
Kheadr, E. E., Vuillemard, J., & El-Deeb, S. (2003). Impact of liposome-encapsulated enzyme cocktails on cheddar cheese ripening. Food Research International, 36(3), 241–252.
Kim, C.-K., Chung, H.-S., Lee, M.-K., Choi, L.-N., & Kim, M.-H. (1999). Development of dried liposomes containing β-galactosidase for the digestion of lactose in milk. International Journal of Pharmaceutics, 183(2), 185–193.
Kirby, C., Brooker, B., & Law, B. (1987). Accelerated ripening of cheese using liposome-encapsulated enzyme. International Journal of Food Science & Technology, 22(4), 355–375.
Koshani, R., & Jafari, S. M. (2019). Ultrasound-assisted preparation of different nanocarriers loaded with food bioactive ingredients. Advances in Colloid and Interface Science, 270, 123–146.
Labat, E., Morel, M., & Rouau, X. (2000). Effects of laccase and ferulic acid on wheat flour doughs. Cereal Chemistry, 77(6), 823–828.
Laouini, A., Jaafar-Maalej, C., Limayem-Blouza, I., Sfar, S., Charcosset, C., & Fessi, H. (2012). Preparation, characterization and applications of liposomes: state of the art. Journal of Colloid Science and Biotechnology, 1(2), 147–168.
Larivière, B., El Soda, M., Soucy, Y., Trépanier, G., Paquin, P., & Vuillemard, J. (1991). Microfluidized liposomes for the acceleration of cheese ripening. International Dairy Journal, 1(2), 111–124.
Li, M., Hanford, M. J., Kim, J.-W., & Peeples, T. L. (2007). Amyloglucosidase enzymatic reactivity inside lipid vesicles. Journal of Biological Engineering, 1(1), 4.
Liu, Q., & Boyd, B. J. (2013). Liposomes in biosensors. Analyst, 138(2), 391–409.
Liu, W., Ye, A., & Singh, H. (2015). Progress in applications of liposomes in food systems, Microencapsulation and microspheres for food applications (pp. 151–170). Elsevier.
Macario, A., Verri, F., Diaz, U., Corma, A., & Giordano, G. (2013). Pure silica nanoparticles for liposome/lipase system encapsulation: application in biodiesel production. Catalysis Today, 204, 148–155.
Marsanasco, M., Calabró, V., Piotrkowski, B., Chiaramoni, N. S., & del V. Alonso, S. (2016). Fortification of chocolate milk with omega-3, omega-6, and vitamins E and C by using liposomes. European Journal of Lipid Science and Technology, 118(9), 1271–1281.
Martí, M., Zille, A., Paulo, A. C., Parra, J. L., & Coderch, L. (2012). Laccases stabilization with phosphatidylcholine liposomes. Journal of Biophysical Chemistry, 3(1), 81–87.
Matsuzaki, M., McCafferty, F., & Karel, M. (1989). The effect of cholesterol content of phospholipid vesicles on the encapsulation and acid resistance of β-galactosidase from E. coli. International Journal of Food Science & Technology, 24(4), 451–460.
Mirafzali, Z., Thompson, C. S., & Tallua, K. (2014). Application of liposomes in the food industry, Microencapsulation in the Food Industry (pp. 139–150). Elsevier.
Moghimipour, E., & Handali, S. (2013). Liposomes as drug delivery systems: properties and applications. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 4(1), 169–185.
Mohammadi, R., Mahmoudzade, M., Atefi, M., Khosravi-Darani, K., & Mozafari, M. (2015). Applications of nanoliposomes in cheese technology. International Journal of Dairy Technology, 68(1), 11–23.
Mohan, A., McClements, D. J., & Udenigwe, C. C. (2016). Encapsulation of bioactive whey peptides in soy lecithin-derived nanoliposomes: influence of peptide molecular weight. Food Chemistry, 213, 143–148.
Olea, D., & Faure, C. (2003). Quantitative study of the encapsulation of glucose oxidase into multilamellar vesicles and its effect on enzyme activity. The Journal of Chemical Physics, 119(12), 6111–6118.
Ozaltin, K., Postnikov, P. S., Trusova, M. E., Sedlarik, V., & Di Martino, A. (2019). Polysaccharides based microspheres for multiple encapsulations and simultaneous release of proteases. International Journal of Biological Macromolecules, 132, 24–31.
Perrett, S., Golding, M., & Williams, W. (1991). A simple method for the preparation of liposomes for pharmaceutical applications: characterization of the liposomes. Journal of Pharmacy and Pharmacology, 43(3), 154–161.
Piard, J., El Soda, M., Alkhalaf, W., Rousseau, M., Desmazeaud, M., Vassal, L., & Gripon, J. (1986). Acceleration of cheese ripening with liposome-entrapped proteinase. Biotechnology Letters, 8(4), 241–246.
Picon, A., Gaya, P., Medina, M., & Nunez, M. (1995). The effect of liposome-encapsulated Bacillus subtilis neutral proteinase on Manchego cheese ripening. Journal of Dairy Science, 78(6), 1238–1247.
Prévoteau, A., & Faure, C. (2012). Effect of onion-type multilamellar liposomes on Trametes versicolor laccase activity and stability. Biochimie, 94(1), 59–65.
Rafiee, Z., & Jafari, S. M. (2018). Application of lipid nanocarriers for the food industry. In J.-M. Mérillon & K. G. Ramawat (Eds.), Bioactive molecules in food (pp. 1–43). Cham: Springer International Publishing.
Rafiee, Z., Nejatian, M., Daeihamed, M., & Jafari, S. M. (2018). Application of different nanocarriers for encapsulation of curcumin. Critical Reviews in Food Science and Nutrition, 1–30.
Rao, D., Chawan, C., & Veeramachaneni, R. (1994). Liposomal encapsulation of β-galactosidase: comparison of two methods of encapsulation and in vitro lactose digestibility. Journal of Food Biochemistry, 18(4), 239–251.
Raveendran, S., Parameswaran, B., Beevi Ummalyma, S., Abraham, A., Kuruvilla Mathew, A., Madhavan, A., Rebello, S., & Pandey, A. (2018). Applications of microbial enzymes in food industry. Food Technology and Biotechnology, 56(1), 16–30.
Rodríguez-Nogales, J. M., & López, A. D. (2006). A novel approach to develop β-galactosidase entrapped in liposomes in order to prevent an immediate hydrolysis of lactose in milk. International Dairy Journal, 16(4), 354–360.
Sanchez, J. M., & Perillo, M. A. (2000). α-Amylase kinetic parameters modulation by lecithin vesicles: binding versus entrapment. Colloids and Surfaces B: Biointerfaces, 18(1), 31–40.
Sanromán, M., & Deive, F. (2017). Food enzymes, Current Developments in Biotechnology and Bioengineering (pp. 119–142). Elsevier.
Sessa, G., & Weissmann, G. (1970). Incorporation of lysozyme into liposomes a model for structure-linked latency. Journal of Biological Chemistry, 245(13), 3295–3301.
Sharma, A., & Sharma, U. S. (1997). Liposomes in drug delivery: progress and limitations. International Journal of Pharmaceutics, 154(2), 123–140.
Shukla, S., Haldorai, Y., Hwang, S. K., Bajpai, V. K., Huh, Y. S., & Han, Y.-K. (2017). Current demands for food-approved liposome nanoparticles in food and safety sector. Frontiers in Microbiology, 8, 2398.
Sindhu, R., Binod, P., & Pandey, A. (2017). α-Amylases, Current Developments in Biotechnology and Bioengineering (pp. 3–24). Elsevier.
Singh, H., Thompson, A., Liu, W., & Corredig, M. (2012). Liposomes as food ingredients and nutraceutical delivery systems, Encapsulation technologies and delivery systems for food ingredients and nutraceuticals (pp. 287–318). Elsevier.
Subramani, T., & Ganapathyswamy, H. (2020). An overview of liposomal nano-encapsulation techniques and its applications in food and nutraceutical. Journal of Food Science and Technology.
Taheri, A., & Jafari, S. M. (2019). Gum-based nanocarriers for the protection and delivery of food bioactive compounds. Advances in Colloid and Interface Science, 269, 277–295.
Tavakoli, H., Hosseini, O., Jafari, S. M., & Katouzian, I. (2018). Evaluation of physicochemical and antioxidant properties of yogurt enriched by olive leaf phenolics within nanoliposomes. Journal of Agricultural and Food Chemistry, 66(35), 9231–9240.
Vafabakhsh, Z., Khosravi-Darani, K., Khajeh, K., Jahadi, M., Komeili, R., & Mortazavian, A. M. (2013). Stability and catalytic kinetics of protease loaded liposomes. Biochemical Engineering Journal, 72, 11–17.
Walde, P., & Ichikawa, S. (2001). Enzymes inside lipid vesicles, preparation, reactivity and applications. Biomolecular Engineering, 18(4), 143–177.
Wang, S., Yoshimoto, M., Fukunaga, K., & Nakao, K. (2003). Optimal covalent immobilization of glucose oxidase-containing liposomes for highly stable biocatalyst in bioreactor. Biotechnology and Bioengineering, 83(4), 444–453.
Whitehurst, R. J., & Law, B. A. (2002). Enzymes in food technology. Wiley Online Library.
Wichmann, C., Naumann, P., Spangenberg, O., Konrad, M., Mayer, F., & Hoppert, M. (2003). Liposomes for microcompartmentation of enzymes and their influence on catalytic activity. Biochemical and Biophysical Research Communications, 310(4), 1104–1110.
Yanan, Z., JIANG, Y., Jing, G., Liya, Z., Ying, H., & Fei, J. (2013). Immobilization of glucose oxidase in liposome-templated biomimetic silica particles. Chinese Journal of Catalysis, 34(4), 741–750.
Yoshimoto, M., & Higa, M. (2014). A kinetic analysis of catalytic production of oxygen in catalase-containing liposome dispersions for controlled transfer of oxygen in a bioreactor. Journal of Chemical Technology & Biotechnology, 89(9), 1388–1395.
Yoshimoto, M., Sato, M., Wang, S., Fukunaga, K., & Nakao, K. (2006). Structural stability of glucose oxidase encapsulated in liposomes to inhibition by hydrogen peroxide produced during glucose oxidation. Biochemical Engineering Journal, 30(2), 158–163.
Yoshimoto, M., Sakamoto, H., Yoshimoto, N., Kuboi, R., & Nakao, K. (2007). Stabilization of quaternary structure and activity of bovine liver catalase through encapsulation in liposomes. Enzyme and Microbial Technology, 41(6-7), 849–858.
Yoshimoto, M., Sato, M., Yoshimoto, N., & Nakao, K. (2008). Liposomal encapsulation of yeast alcohol dehydrogenase with cofactor for stabilization of the enzyme structure and activity. Biotechnology Progress, 24(3), 576–582.
Yoshimoto, M., Takaki, N., & Yamasaki, M. (2010). Catalase-conjugated liposomes encapsulating glucose oxidase for controlled oxidation of glucose with decomposition of hydrogen peroxide produced. Colloids and Surfaces B: Biointerfaces, 79(2), 403–408.
Yoshimoto, M., Yamashita, T., & Kinoshita, S. (2011). Thermal stabilization of formaldehyde dehydrogenase by encapsulation in liposomes with nicotinamide adenine dinucleotide. Enzyme and Microbial Technology, 49(2), 209–214.
Zhang, Q., Han, Y., & Xiao, H. (2017a). Microbial α-amylase: a biomolecular overview. Process Biochemistry, 53, 88–101.
Zhang, R., Song, X., Liang, C., Yi, X., Song, G., Chao, Y., Yang, Y., Yang, K., Feng, L., & Liu, Z. (2017b). Catalase-loaded cisplatin-prodrug-constructed liposomes to overcome tumor hypoxia for enhanced chemo-radiotherapy of cancer. Biomaterials, 138, 13–21.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohammadi, A., Jafari, S.M., Mahoonak, A.S. et al. Liposomal/Nanoliposomal Encapsulation of Food-Relevant Enzymes and Their Application in the Food Industry. Food Bioprocess Technol 14, 23–38 (2021). https://doi.org/10.1007/s11947-020-02513-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-020-02513-x