Abstract
Development of environment-friendly films using natural biopolymers such as carbohydrates has increased because of environmental problems caused by synthetic plastics. This study examined the physical and antioxidative properties of biodegradable films from sword bean starch (SBS) containing goji berry extract (GBE) to develop a novel antioxidant film. As the GBE concentration increased, the tensile strength of the SBS films decreased from 26.32 to 12.42 MPa; however, the elongation at break increased from 7.39 to 12.36%. Although water barrier properties and thermal stability of SBS films were not enhanced by the addition of GBE, ultraviolet/visible light barrier and antioxidant properties were remarkably improved due to its bioactive compounds, specifically zeaxanthin and various polyphenols. In particular, 2,2-diphenyl-1-picrylhydrazyl and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging activities of the SBS film with 1.0% GBE were the highest among the films, which were consistent with the total phenolic content results. Therefore, the developed SBS films with GBE are applicable as new antioxidant films in the food packaging industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Synthetic plastics used in the food industry are nonbiodegradable and nonrenewable, leading to severe environmental pollution (Jaramillo et al. 2016). Thus, several studies have focused on developing biodegradable films from natural biopolymers such as polysaccharides, lipids, and proteins (Hassan et al. 2018).
Starch is considered as an appropriate source for biodegradable films due to its nontoxic, renewable, and inexpensive properties, and its abundance in nature (Jiménez et al. 2012; Versino et al. 2016). Accordingly, characterization of starch films prepared from various sources such as cassava, potato, wheat, and corn has been studied (Cruz-Gálvez et al. 2018; Song et al. 2018; Valencia-Sullca et al. 2018); however, these starches are inappropriate for a biodegradable film material because they are mainly used as a food resource for humans. Therefore, several studies characterizing the films developed from starches with low consumption and industrial utilization were recently conducted (Chanjarujit et al. 2018; Kim et al. 2018; Nouri and Nafchi 2014; Yang et al. 2018).
Legume starches are appropriate sources for biodegradable films with good physical properties as they are composed of high amylose content (Corrales et al. 2009). Sword bean (Canavalia gladiata) is one of the leguminous crops that belong to the Pea family (Fabaceae), and is mainly grown in South Asia or Africa (Adebowale et al. 2006; Ekanayake et al. 2006). In addition, sword bean has high nutrition value as it contains approximately 30% protein and 40% starch on a dry basis, and it can grow well under barren soil condition such as acidic pH (ranging from 4.3 to 6.8) and high salinity, compared with the other legumes (Adebowale et al. 2006). Despite these desirable properties, the usage of sword bean as a food source is limited due to its toxicity caused by canavalin and concanavalin A differently from other beans (Olu-Owolabi et al. 2011). Thus, it would be appropriate to use sword bean as a biodegradable film source due to its abundant starch content rather than as a food source; however, to our knowledge, no study is available on the film-forming ability of sword bean starch (SBS) yet.
Although starch films are colorless and odorless (Luchese et al. 2018a, 2018b), application of starch alone in making films is limited due to its high hydrophilicity, leading to poor mechanical properties of the films (Cruz-Gálvez et al. 2018). Therefore, several studies have been carried out to overcome this issue (Cruz-Gálvez et al. 2018; Nouri and Nafchi 2014). In particular, development of starch films along with natural plant extracts has recently gained immense attention (Cruz-Gálvez et al. 2018; Kim et al. 2018; Nouri and Nafchi 2014), and these extracts could improve the extensibility of biodegradable films.
Goji berry (Lycium barbarum L.) is an orange–red color fruit produced in Asian countries including Korea, Japan, and China (Kulczyński and Gramza-Michałowska 2016; Wojdyło et al. 2018). Goji berry possesses various bioactive properties such as anticarcinogenic, antidiabetic, and antioxidant (Wojdyło et al. 2018) due to its high content of polysaccharides (5–8% of dry weight), carotenoids (0.03–0.5% of dry weight) (Kulczyński and Gramza-Michałowska 2016), and polyphenols (0.025–0.19% of dry weight) (Wojdyło et al. 2018). In addition, zeaxanthin, which is the most abundant carotenoid (maximum 56% of total carotenoids) in goji berry, blocks the ultraviolet (UV) light (280–400 nm, specifically UV-B regions: 290–320 nm) (Roberts 2013; Sandmann et al. 1998), and goji berry extract (GBE) presents a high UV barrier property to the biodegradable films. Although several studies report starch films incorporated with various natural plant extracts such as betel leaves (Nouri and Nafchi 2014), rosemary (Piñeros-Hernandez et al. 2017), grape seed (Bof et al. 2016), blueberry fruit, macadamia peel, banana peel (Saberi et al. 2017a), and yerba mate extract (Jaramillo et al. 2016), no study is available on starch films blended with GBE that is miscible with the starch molecules as it has abundant polysaccharide content (Amagase and Farnsworth 2011; Kulczyński and Gramza-Michałowska 2016). Therefore, the objective of this study was to develop a novel antioxidant starch film containing GBE using SBS extracted from underutilized sword bean, investigate the physicochemical properties of the developed SBS film, and examine the intermolecular interactions between SBS and GBE.
Materials and Methods
Materials
Sword bean (Canavalia gladiata) and goji berry (Lycium barbarum) powder used in this study were obtained from Boryeong Co. (Gyeonggi, Korea) and Foodsense Co. (Gyeonggi, Korea), respectively. NaOH (40%, w/w), HCl (36%, v/v), sorbitol, sodium carbonate, 2 N Folin–Ciocalteu reagent, gallic acid, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Iodine (I2) and potassium iodide (KI) were purchased from Samchun Pure Chemical Co. (Gyeonggi, Korea), and ethanol (95%, v/v) was purchased from Daejung Chemical & Metals Co. (Gyeonggi, Korea).
Extraction of SBS
Prior to grinding, sword bean samples were washed with running tap water to remove impurities such as dust, and the washed samples were dried on a clean bench with a fan running until removal of remaining water. Sword beans were ground with a blender (Sanplatec Co., Osaka, Japan) and sieved with a 200-mesh sieve to obtain fine sword bean powder (SBP), which was then submerged in NaOH (0.3%) solution at a 1:5 ratio (w/v) and stirred with a stirring plate (Corning Inc., New York, NY, USA) at 4 °C for 24 h. The SBP–NaOH mixture was then filtered using a 200-mesh sieve and centrifuged at 3000×g for 10 min. Afterward, the pellets were resuspended and washed with distilled water (DW) and this process was repeated until the brown layer on white starch pellet (WSP) was completely removed. The obtained WSP was mixed with DW and neutralized to pH 7.0 using 1 N HCl. Neutralized WSP was preserved at 4 °C for 1 h and dried at 25 °C for 24 h. The dried starch (30% yield) was finely ground and sieved through a 200-mesh sieve. Amylose content of the obtained SBS was measured according to the method described in a previous study (Kang and Song 2019). Briefly, iodine colorimetric method using starch and I2-KI solution was applied, and the absorbance was measured at 620 nm. The amylose content of SBS was determined to be 21.27 ± 0.07%. In addition, SBS prepared in this study had 11.10 ± 0.18% moisture content, 0.37 ± 0.18% crude fat, 0.15 ± 0.06% crude protein, and 0.14 ± 0.08% crude ash and this chemical composition was similar to that of the previous report (Adebowale et al. 2006).
Preparation of GBE
Goji berry powder (GBP, 10 g) was extracted in 50% ethanol (50 mL) by stirring at 25 °C for 5 h. The extracted solution was filtered with Whatman No. 2 filter paper (8 μm, GE Healthcare UK Ltd., Little Chalfont, UK), and the filtrate was concentrated using a vacuum evaporator to completely remove ethanol and lyophilized to obtain GBE with various bioactive compounds, such as polysaccharides, polyphenols, and carotenoids which are the major compounds in goji berry.
Preparation of SBS Films
The optimal concentrations of SBS and sorbitol were set based on the preliminary experiments. The physical properties of SBS films prepared with different concentrations (2.5%, 3%, and 3.5%) of SBS and sorbitol as a plasticizer were compared and 3% SBS was selected as the optimal concentration. In addition, to fix the optimal concentration of sorbitol, the physical properties of SBS films with different concentrations (20%, 30%, and 40% of SBS, w/w) of sorbitol were compared and the optimal concentration of sorbitol was determined to be 30%. SBS (3%, w/v) and sorbitol (30% of SBS, w/w) were dissolved in DW and stirred at 80 °C for 20 min using a stirring plate (Corning Inc.). Gelatinization temperature of SBS applied in this study was set according to the report of Adebowale et al. (2006). After gelatinization, various amounts (0.5%, 0.75%, and 1.0%) of GBE were added to the gelatinized starch solution, and the mixtures were homogenized with a homogenizer (IKA, Staufan, Germany) at 13,000 rpm for 3 min. Prior to casting, the mixtures were degassed for 5 min and filtered with four layers of gauze. Approximately 25 mL of the filtered film-forming solution was then casted on a glass plate (10 cm × 13 cm) and dried at 25 °C for 12 h. The SBS film without GBE was used as a control film for subsequent experiments.
Physical Properties of SBS Films
After film conditioning in a constant temperature and humidity chamber (Daewon Science, Gyeonggi, Korea) at 25 °C and 50% relative humidity (RH) for 24 h, the SBS films with or without GBE were cut into 2.54 cm × 10 cm of pieces for measuring their physical properties. Film thickness was determined using a micrometer (Mitutoyo, Tokyo, Japan). The SBS films’ tensile strength (TS, MPa) and elongation at break (EB, %) were measured with an Instron test machine (Testometric Co., Lancashire, UK) under the condition: grip distance, 5 cm; stretching speed, 50 cm/min.
The SBS films were prepared as 2 cm × 2 cm of pieces to measure the moisture content (MC), water solubility (WS), and water vapor permeability (WVP). These were dried in a drying oven at 105 °C for 24 h to determine the MC of each film, which was calculated by subtracting the film weight after drying for 24 h from the initial film weight and then dividing this value by the initial weight. Five measurements were conducted to obtain MC data. To determine the WS of SBS films, each film was immersed in DW and dissolved at 25 °C for 24 h. The film pieces undissolved in DW were dried and the WS of the films was determined by calculating the relative percentage of the film’s weight loss. The film piece-sealed polymethyl-acrylate cup containing 15 mL of DW was used to measure the WVP of the SBS films (Baek and Song 2019). The cup was kept at 25 °C and 50% RH, and the weight of cup was measured every 1 h.
Fourier Transformation Infrared Spectroscopy
To examine the effect of GBE on the SBS film structure, Fourier transformation infrared (FTIR) spectra of the prepared SBS films were analyzed according to the method of Kang and Song (2019). A vacuum infrared spectrometer (Vertex 80v, Billerica, MA, USA) equipped with an ATR/DTGS detector was used to obtain FTIR spectra of SBS films in the wavenumber range from 4000 to 400 cm−1. FTIR analysis was performed at 2 cm−1 resolution with 16 scans.
Thermal Properties of SBS Films
To investigate the thermal stability of SBS films containing GBE, thermogravimetric analysis (TGA) was conducted using a TGA-DSC machine (Mettler Toledo, Columbus, OH, USA) according to the method of Baek and Song (2019). Moreover, the derivative thermogravimetric analysis (DTGA) was carried out to identify the maximum decomposition temperature (Tmax) of SBS films. In general, 5 mg of each SBS film was heated at a speed of 10 °C/min from 25 to 700 °C.
Scanning Electron Microscopy
The micromorphology of SBS films was analyzed according to the method described by Kang and Song (2019). A low-voltage scanning electron microscope (Carl Zeiss Microscopy, Jena, Germany) was used to visualize the surface and cross-section of the SBS films. All films were prepared as 0.5-cm2 pieces, and the SEM images of platinum coated films were obtained at 5 kV accelerating voltage with ×3000 magnification.
Atomic Force Microscopy
The surface topology of SBS films was analyzed via atomic force microscopy (AFM) according to the method described by Ju et al. (2019). The AFM images of SBS films with or without GBE were obtained using an AFM spectrometer (Horiba Jobin-Yvon Inc., New Jersey, NJ, USA). The surface roughness (Ra, average surface roughness) of each film was calculated based on the three-dimensional images (20 μm × 20 μm).
Optical Properties of SBS Films
Changes in the CIELAB color (L*, a*, and b*) values of SBS films with GBE were measured using a colorimeter (Minolta, Tokyo, Japan). The total color difference (△E) of SBS films with GBE against the control film was calculated using the following equation.
where Lc*, ac*, and bc* are the colors of the control film and Ls*, as*, and bs* are the colors of the SBS films containing GBE.
All films were cut into 1.5 cm × 4 cm pieces. A spectrophotometer (Shimadzu Co., Kyoto, Japan) was used to measure the opacity of the SBS films, and the absorbance of each film was measured at 600 nm. The following equation was used for calculating the opacity of SBS films with or without GBE.
where A and T are the absorbance and thickness of each film, respectively.
With increasing GBE concentration, changes in the light transmittance of SBS films were analyzed in the wavelength range of 200–800 nm using a spectrophotometer (Shimadzu Co.).
Total Phenolic Content of SBS Films
To measure the total phenolic content (TPC) of SBS films with GBE, the method described by Piñeros-Hernandez et al. (2017) was used with minor modifications. Ten milligrams of each film was added to 10 mL of DW and extracted in an incubator at 37 °C for 1 h. After the extraction, 150 μL of the extracted solution, 1 mL of DW, and 200 μL of 2 N Folin–Ciocalteu reagent were mixed and reacted at 25 °C for 30 s. Thereafter, the solution was mixed with 2 mL of 7.5% sodium carbonate solution and incubated at 37 °C for 1 h by shaking at 150 rpm. The absorbance at 765 nm of the solution was measured, and TPC of each film was calculated using a gallic acid standard curve. All TPC values were presented as milligram gallic acid equivalents (GAE)/gram of dried film. In addition, the TPC of GBE was also measured and calculated to be 38.80 ± 0.58 mg GAE/g.
Antioxidant Activity of SBS Films
The antioxidant activities of SBS films containing GBE were analyzed with DPPH and ABTS assays according to the method described by Baek and Song (2019). All films were prepared to be 0.1 g in weight and were immersed and extracted in 10 mL of DW at 37 °C for 30 min. The film extracted solution was used for subsequent experiments.
The aforementioned film solution (0.1 mL) was mixed with 3.9 mL of DPPH solution and was reacted in the dark for 1 h. The DPPH radical scavenging activity of SBS films was determined according to the following equation.
where Db and Ds are the absorbances of blank and each film extracted solution at 517 nm, respectively.
ABTS solution (2.45 mM potassium persulfate and 7 mM ABTS, 1:2, v/v) was diluted with ethanol to be 0.7 of its absorbance at 734 nm after 16 h reaction in the dark. The film extract solution (0.1 mL) and ABTS solution (1.9 mL) were then mixed and incubated at 37 °C for 10 min. The absorbance of the mixture was then measured at 734 nm. The ABTS radical scavenging activity of SBS films was determined by the following equation.
where Ab and As are the absorbances of blank and each film extracted solution at 734 nm, respectively.
Statistical Analysis
The SAS software (version 9.4, SAS Institute Inc., Cary, NC, USA) with one-way analysis of variance (ANOVA) and Duncan’s multiple range tests (p < 0.05) was used to statistically analyze the experimental data. All data were obtained by repeating at least five times and were presented as mean ± standard deviation.
Results and Discussion
Physical Properties of SBS Films
Physical properties of starch films are mainly dependent on the amylose content of starch (Basiak et al. 2017; Cazón et al. 2017). It has been reported that high amylose content (> 20%) of starch can produce biodegradable films with better physical properties than those with low amylose content (Corrales et al. 2009). Sword bean starch (SBS) used in this study comprises approximately 21% of amylose (data not shown) and is similar to those of other starches studied previously (Hoover et al. 2010). Thus, the physical properties, such as TS (26.32 ± 2.30 MPa) and EB (7.39 ± 1.18%), of the SBS film developed in this study (Table 1) were similar to those of other starch films (Versino et al. 2016); however, the starch films are usually fragile and less elastic, resulting in poor application in food packaging (Cruz-Gálvez et al. 2018).
With an increasing amount of GBE from 0.5% to 1.0%, the thickness and EB of SBS films increased (p < 0.05) from 7.39 ± 1.18% to 12.36 ± 2.62% and the TS decreased (p < 0.05) from 26.32 ± 2.30 MPa to 12.42 ± 0.68 MPa (Table 1), which was in accordance with the result of other starch films containing natural plant extracts (Baek and Song 2019; Corrales et al. 2009; Ju et al. 2019; Kim et al. 2018; Nouri and Nafchi 2014). The increase in flexibility and decrease in rigidity of the starch films are mainly due to the weakening of intermolecular interactions between starch polymers caused by the addition of active materials, leading to disruption of starch chain entanglement (Baek and Song 2019; Bof et al. 2016; Homayouni et al. 2017). In addition, the natural plant extracts comprising polyphenols make starch films more flexible by the plasticizing effect (Jaramillo et al. 2016). As GBE contains numerous bioactive compounds such as carotenoids and flavonoids, the EB of SBS films increased with elevated GBE concentration.
The physical properties of starch films are also associated with moisture content (MC) of the films (Versino et al. 2016). MC of SBS films was significantly (p < 0.05) increased by the addition of GBE (Table 1). As the concentration of GBE in the SBS films increased from 0.5 to 1.0%, MC of SBS films changed from 8.91 ± 0.44% to 12.02 ± 1.06%. These results are in good agreement with the changes in the physical properties (TS and EB) of the SBS films. Water molecules can act as a plasticizer in the film matrix, leading to increase in the flexibility of films (Shaili et al. 2015). In addition, GBE has polysaccharides as one of major components, which consist of six different monosaccharides, such as glucose, mannose, xylose, rhamnose, arabinose, and galactose (Amagase and Farnsworth 2011; Kulczyński and Gramza-Michałowska 2016). These saccharides can also be used as a plasticizer in the preparation of films because of their hydroxyl groups (Saberi et al. 2017b). Therefore, these results indicate that GBE is considered to overcome the brittleness of starch films by increasing the MC value and by acting as a plasticizer (Ju et al. 2019; Kim et al. 2018; Nouri and Nafchi 2014; Piñeros-Hernandez et al. 2017).
Water Barrier Properties of SBS Films
With increasing GBE concentration from 0.5 to 1.0%, MC (from 8.91 ± 0.44 to 12.02 ± 1.06%), WS (from 23.41 ± 1.42 to 36.88 ± 1.34%), and WVP (from 2.13 ± 0.23 × 10−9 to 3.53 ± 0.25 × 10−9 g m−1 s−1 Pa−1) of SBS films significantly (p < 0.05) increased (Table 1). These increases are mainly due to high hydrophilicity of GBE, which occurs in the hydroxyl groups present in polysaccharide complexes and various polyphenols in GBE (Kulczyński and Gramza-Michałowska 2016; Nouri and Nafchi 2014). Similarly, increases in MC, WS, and WVP of the starch films have been reported by adding several plant extracts such as moringa leaf extract, cocoa nib extract, and betel leaf extract (Ju et al. 2019; Kim et al. 2018; Nouri and Nafchi 2014). As MC in the starch films is more than 10%, the hydrogen bonds between starch and water molecules can be decreased and new hydrogen bonds are formed among the water molecules (Lourdin et al. 1997). As a result, the intermolecular interactions in starch films are weakened. In addition, GBE can facilitate the decrease in the intermolecular interactions in the film network due to its various compounds that can act as plasticizer (Souza et al. 2012). These changes in the internal structure of starch films increase the movement of water molecules, leading to elevated WVP (Rodríguez et al. 2006).
High WS of starch films indicates poor water resistance (Woggum et al. 2015). WS of the SBS film was 23.41% (Table 1), which is less than that of other starch films (cassava starch film 27.5%, wheat starch film 30%, pea starch film 32%, high amylose corn starch film 38%, foxtail millet starch film 42%, khorasan wheat starch film 42%, rice starch film 44%, and proso millet starch film: 50%) (Basiak et al. 2017; Baek and Song 2019; Ju et al. 2019; Yang et al. 2018). These results indicate that the films prepared using SBS have relatively high water resistance compared with other starch films and may be more suitable for the packaging of foods with high water content.
FTIR Spectra of SBS Films
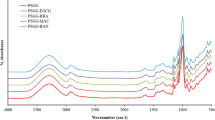
Hydrogen bonds among the starch molecules play a pivotal role in the internal structure of starch films (Chanjarujit et al. 2018). In addition, natural plant extracts such as GBE can produce new hydrogen bonds when they are incorporated into starch films, resulting in changes in the internal structure (Feng et al. 2018; Teixeira et al. 2018). The analysis of FTIR spectra can be used to identify the changes in hydrogen bonds and intermolecular interactions in the starch films by the addition of natural plant extracts (Woggum et al. 2015). As a result of FTIR analysis (Fig. 1), all FTIR peaks of SBS films were in accordance with those reported in other starch films (Chanjarujit et al. 2018; Feng et al. 2018; Saberi et al. 2017b; Woggum et al. 2015), and the addition of GBE did not cause notable changes in most peaks in the spectra as the FTIR spectrum of GBE was similar to that of the SBS film without GBE (Fig. 1).
It has been reported that starch films have some typical peaks in the FTIR spectra (Chanjarujit et al. 2018; Saberi et al. 2017b; Woggum et al. 2015). The broad peak at 3300–3200 cm−1 represents OH stretching of glucose and water molecules (Woggum et al. 2015). The peaks at 2930 and 1648 cm−1 are related to CH stretching of CH2 and OH vibration of water present in the starch film matrix, respectively (Saberi et al. 2017b; Woggum et al. 2015). In addition, the peaks at 1450–1400 cm−1, 1149 cm−1, and 930 cm−1 correspond to CH bending of CH2, CO stretching, and glycosidic linkages, respectively (Chanjarujit et al. 2018; Saberi et al. 2017b). Among the FTIR peaks of SBS films, however, a slight shift of 1648 cm−1 peak to a lower wavenumber (1630 cm−1) by the addition of GBE was observed with the increase in its intensity (Fig. 1). These shifts are due to the interactions between the SBS molecules and polyphenols of GBE (Luchese et al. 2018b). Feng et al. (2018) also found similar results in the hydroxypropyl starch films containing tea polyphenol. In addition, the peak at 1648 cm−1 is associated with water adsorption in the amorphous region of starch films and the formation of new hydrogen bonds among the water molecules (Feng et al. 2018; Teixeira et al. 2018). It is known that the major limitation of starch films is their brittleness owing to the amorphous regions formed by amylose (Cazón et al. 2017). Based on our FTIR results, therefore, the addition of GBE could reduce the brittleness of starch films by replenishing water molecules in the regions. These results are also in good accordance with the decrease in TS and the increases in MC and EB of SBS films (Table 1).
Thermal Properties of SBS Films
The changes in thermal stability of SBS films with increasing amount of GBE were investigated (Fig. 2). Regardless of GBE addition, the TGA curves (Fig. 2a) of SBS films had a typical pattern of three stages in thermal decomposition, similar to those of other starch films (Baek and Song 2019; Jaramillo et al. 2016). The first stage from 50 to 150 °C is related to water evaporation in the films, having less than 10% of weight loss. The second stage from 150 to 280 °C corresponds to the decomposition of low molecular weight compounds, such as plasticizers (Jaramillo et al. 2016; Kim et al. 2018). The final stage from 280 to 350 °C, which represents the main decomposition stage, is related to the degradation of starch molecules.
In the DTGA curves, Tmax values of SBS films with 0%, 0.5%, 0.75%, and 1.0% GBE were 321.46 °C, 298.48 °C, 294.89 °C, and 292.77 °C, respectively (Fig. 2b). These results indicate that the thermal stability of SBS films decreased as the amounts of GBE increased. This is a common phenomenon caused by the addition of natural plant extracts, which could weaken the internal film network, leading to decrease in thermal stability, and has been observed in other studies (Jaramillo et al. 2016; Kim et al. 2018; Piñeros-Hernandez et al. 2017). The hydroxyl groups in GBE components, such as monosaccharides and polyphenols, decreased the thermal stability because the crystalline structure in SBS films was weakened by their plasticizing effect (Jaramillo et al. 2016; Souza et al. 2012). After thermal decomposition, the char residue content of the SBS film without GBE was 13.22%, presumably owing to impurities or inorganic substances present in SBS (Caetano et al. 2018). In addition, with increasing GBE concentration from 0.5 to 1.0%, the char residues of SBS films increased from 20.91 to 27.23% because the polyphenolic compounds such as flavonoids with aromatic ring in GBE are stable up to 700 °C (Caetano et al. 2018; Jaramillo et al. 2016).
SEM and AFM Analysis of SBS Films
The SEM images of SBS films with or without GBE were analyzed to determine the homogeneity of films and the compatibility between SBS and GBE in the film (Fig. 3). Although SEM image of all SBS films presented fairly homogeneous surface, the addition of GBE caused a rougher surface than that of the control film. Additionally, some holes were observed in the SBS films containing GBE, whereas the film without GBE did not have holes in the cross-sectional SEM image (Fig. 3). The difference in the interior structure of SBS films containing GBE from the control film is related to carotenoids and polyphenols in GBE, such as zeaxanthin, quercetin, and kaempferol (Kulczyński and Gramza-Michałowska 2016). In particular, the holes in the SBS films with GBE could be explained by weakened internal structure by the addition of GBE. These holes might be the reason for increased WVP. Similarly, internal holes or cracks in the starch films incorporated with natural plant extracts, such as carvacrol, lemon essential oils, and rosemary extracts, were reported in previous studies, and those extracts increased the heterogeneity of the film matrices (Homayouni et al. 2017; Piñeros-Hernandez et al. 2017; Song et al. 2018); however, notably, the SBS films revealed a more homogenous and compatible structure than other starch films (Fig. 3), because polysaccharide complexes in GBE were more miscible with the starch molecules than essential oils used in other studies.
The increase in roughness on the surface of SBS films containing GBE was also observed by AFM analysis (Fig. 4). The Ra values (average of height deviations from a mean surface) increased from 7.2 to 40.4 nm with increasing GBE concentration. Similar to our results, the increase in the surface roughness of starch films by the addition of natural plant extracts was reported in other studies (Jaramillo et al. 2015; Ju et al. 2019). The surface roughness of biodegradable films may depend on the amount of a plasticizer added (Monteiro et al. 2018). As the concentration of a plasticizer increases, new hydrogen bonds in the film network are formed and the internal structure becomes more disordered, resulting in increased surface roughness. In this study, the changes in the surface roughness of SBS films were affected by the content of GBE, which may work as a plasticizer.
Optical Properties of SBS Films
The changes in the surface color and opacity of SBS films containing GBE are presented in Table 2. With increasing amount of GBE, L* and a* values of SBS films decreased and b* value increased significantly (p < 0.05), resulting in increased △E value from 18.45 to 35.24 (Table 2). Zeaxanthin, which is a main carotenoid in GBE, is a yellow compound (Amagase and Farnsworth 2011), and the color of SBS films became more yellow with increasing GBE content. Similarly, the addition of betel leave and moringa leaf extract altered the color of starch films to yellow (Ju et al. 2019; Nouri and Nafchi 2014).
The opacity of SBS films increased from 1.12 to 1.66 by the addition of GBE (Table 2). These results are in accordance with our previous studies on the starch films incorporated with natural plant extracts (Baek and Song 2019; Kim et al. 2018; Ju et al. 2019). The increase in the opacity of starch films containing natural plant extracts is mainly due to their own color, especially yellow and red. In addition, it has also been reported that the thicker the films, the greater their opacity (Basiak et al. 2017). In the present study, the thickness of SBS films increased from 0.077 to 0.095 mm with the addition of GBE (Table 1). This could somewhat affect the changes in the opacity of SBS films; however, the transparency of SBS films was maintained when GBE was incorporated up to 1% (Table 2). In contrast, the opacities of other starch films containing 1% curcumin, cocoa nibs extract, and moringa leaf extract increased from 0.24 to 1.39, 1.34 to 5.63, and 0.43 to 1.41, respectively (Baek and Song 2019; Kim et al. 2018; Ju et al. 2019), indicating that GBE is more compatible with the starch films than the other natural plant extracts in previous studies.
The SBS films with GBE had lower UV/visible light transmittance than that of the control film without GBE (Fig. 5). In addition, the transmittance of SBS films against UV light region (200–400 nm) decreased significantly (p < 0.05) with increasing amount of GBE, similar to other studies (Ju et al. 2019; Nouri and Nafchi 2014; Yang et al. 2018). In particular, SBS film with 1% GBE effectively blocked the UV light because zeaxanthin in GBE can absorb UV light, particularly UV-B region (Amagase and Farnsworth 2011; Sandmann et al. 1998). Therefore, these results suggest that GBE addition increases UV light barrier property of SBS films, and the developed film can be applied to the foods that are vulnerable to UV light oxidation.
Antioxidant Activity of SBS Films
The changes in TPC and the antioxidant activities of SBS films containing GBE were determined (Table 3). As the concentration of GBE increased, TPC and the antioxidant activities against DPPH and ABTS radicals of SBS films increased. The increased antioxidant activities of SBS films with the addition of GBE can be explained by phenolic compounds present in the goji berry extract, which cause increase in TPC as well as DPPH and ABTS radical scavenging activities. In particular, goji berries used in this study have been known to have various types of phenolic compounds, such as quercetin, caffeic acid, chlorogenic acid, and p-coumaric acid, that possess antioxidant activities (Wojdyło et al. 2018). This tendency is consistent with the previous results regarding the biocomposite films using pea starch or guar gum containing various natural plant extracts, such as blueberry ash fruit, macadamia peel, and banana peel extracts (Saberi et al. 2017a). In addition, TPC of SBS films with GBE (4.83–9.06 mg GAE/g dried film) was similar to that of other starch films containing moringa leaf extract (4.91–10.96 mg GAE/g dried film) and curcumin (approximately 3–6 mg GAE/g dried film) at the same concentration range (Baek and Song 2019; Ju et al. 2019); however, the radical scavenging activities against DPPH and ABTS of the SBS film with 1% GBE (48.58% and 95.66%) were higher than those of the other films with 1% moringa leaf extract (37.89% and 59.45%) or curcumin (15.48% and 52.40%), indicating that GBE contains more antioxidants such as polysaccharide complexes, carotenoids, and polyphenols (Kulczyński and Gramza-Michałowska 2016; Wojdyło et al. 2018). On the contrary, it was reported that the blended film of corn starch and chitosan containing grape seed extract (GSE) or lemon essential oil (LEO) had no antioxidant activity (Bof et al. 2016), although the concentration of GSE and LEO was higher than that of GBE used in this study. Furthermore, the SBS films developed in this study had higher TPC and DPPH radical scavenging activity than those of cassava starch films containing more than 5% rosemary extracts (Piñeros-Hernandez et al. 2017). Therefore, our results suggest that GBE is a better active material to confer the antioxidant activity to starch films than other active compounds reported in the literature.
Conclusions
Sword bean starch and goji berry extract, which were initially applied in the preparation of biodegradable films, were used for development of an antioxidant-biodegradable film. With increasing amount of GBE from 0.5 to 1.0%, TS of SBS films decreased, but EB, MS, WS, and WVP increased. Addition of GBE affected the thermal properties of SBS films, leading to decreased thermal stability. In addition, the color and opacity of SBS films were altered by the addition of GBE, and UV/visible light barrier properties were improved considerably due to zeaxanthin, which is a major carotenoid in GBE. In particular, the antioxidant activities of SBS films increased significantly with elevated concentration of GBE, and the TPC of SBS films was also enhanced. These results suggest that SBS films containing GBE are applicable as a novel antioxidant film to improve shelf life of foods.
References
Adebowale, K. O., Afolabi, T. A., & Olu-Owolabi, B. I. (2006). Functional, physicochemical and retrogradation properties of sword bean (Canavalia gladiata) acetylated and oxidized starches. Carbohydrate Polymers, 65(1), 93–101.
Amagase, H., & Farnsworth, N. R. (2011). A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Research International, 44(7), 1702–1717.
Baek, S. K., & Song, K. B. (2019). Characterization of active biodegradable films based on proso millet starch and curcumin. Starch-Stärke, 71, 1800174.
Basiak, E., Lenart, A., & Debeaufort, F. (2017). Effect of starch type on the physico-chemical properties of edible films. International Journal of Biological Macromolecules, 98, 348–356.
Bof, M. J., Jimenez, A., Locaso, D. E., Garcia, M. A., & Chiralt, A. (2016). Grapefruit seed extract and lemon essential oil as active agents in corn starch-chitosan blend films. Food and Bioprocess Technology, 9(12), 2033–2045.
Caetano, K. S., Lopes, N. A., Costa, T. M. H., Brandelli, A., Rodrigues, E., Flôres, S. H., et al. (2018). Characterization of active biodegradable films based on cassava starch and natural compounds. Food Packaging and Shelf Life, 16, 138–147.
Cazón, P., Velazquez, G., Ramírez, J. A., & Vázquez, M. (2017). Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocolloids, 68, 136–148.
Chanjarujit, W., Hongsprabhas, P., & Chaiseri, S. (2018). Physicochemical properties and flavor retention ability of alkaline calcium hydroxide-mungbean starch films. Carbohydrate Polymers, 198, 473–480.
Corrales, M., Han, J. H., & Tauscher, B. (2009). Antimicrobial properties of grape seed extracts and their effectiveness after incorporation into pea starch films. International Journal of Food Science and Technology, 44(2), 425–433.
Cruz-Gálvez, A. M., Castro-Rosas, J., Rodríguez-Marín, M. L., Cadena-Ramírez, A., Tellez-Jurado, A., Tovar-Jiménez, X., Chavez-Urbiola, E. A., Abreu-Corona, A., & Gómez-Aldapa, C. A. (2018). Antimicrobial activity and physicochemical characterization of a potato starch-based film containing acetonic and methanolic extracts of Hibiscus sabdariffa for use in sausage. LWT – Food Science and Technology, 93, 300–305.
Ekanayake, S., Nair, B. M., Asp, N. G., & Jansz, E. R. (2006). Effect of processing of sword bean (Canavalia gladiata) on physicochemical properties of starch. Starch-Stärke, 58(5), 215–222.
Feng, M., Yu, L., Zhu, P., Zhou, X., Liu, H., Yang, Y., Zhou, J., Gao, C., Bao, X., & Chen, P. (2018). Development and preparation of active starch films carrying tea polyphenol. Carbohydrate Polymers, 196, 162–167.
Hassan, B., Chatha, S. A. S., Hussain, A. I., Zia, K. M., & Akhtar, N. (2018). Recent advances on polysaccharides, lipids, and protein based edible films and coatings: A review. International Journal of Biological Macromolecules, 109, 1095–1107.
Homayouni, H., Kavoosi, G., & Nassiri, S. M. (2017). Physicochemical, antioxidant and antibacterial properties of dispersion made from tapioca and gelatinized tapioca starch incorporated with carvacrol. LWT - Food Science and Technology, 77, 503–509.
Hoover, R., Hughes, T., Chung, H. J., & Liu, Q. (2010). Composition, molecular structure, properties, and modification of pulse starches: A review. Food Research International, 43(2), 399–413.
Jaramillo, C. M., Seligra, P. G., Goyanes, S., Bernal, C., & Famá, L. (2015). Biofilms based on cassava starch containing extract of yerba mate as antioxidant and plasticizer. Starch-Stärke, 67(9-10), 780–789.
Jaramillo, C. M., Gutiérrez, T. J., Goyanes, S., Bernal, C., & Famá, L. (2016). Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydrate Polymers, 151, 150–159.
Jiménez, A., Fabra, M. J., Talens, P., & Chiralt, A. (2012). Edible and biodegradable starch films: A review. Food and Bioprocess Technology, 5(6), 2058–2076.
Ju, A., Baek, S. K., Kim, S., & Song, K. B. (2019). Development of an antioxidative packaging film based on Khorasan wheat starch containing moringa leaf extract. Food Science and Biotechnology, 28(4), 1057–1063.
Kang, J. H., & Song, K. B. (2019). Characterization of Job’s tears (Coix lachryma-jobi L.) starch films incorporated with clove bud essential oil and their antioxidant effects on pork belly during storage. LWT - Food Science and Technology, 111, 711–718.
Kim, S., Baek, S. K., Go, E., & Song, K. B. (2018). Application of adzuki bean starch in antioxidant films containing cocoa nibs extract. Polymers, 10(11), 1210.
Kulczyński, B., & Gramza-Michałowska, A. (2016). Goji berry (Lycium barbarum): Composition and health effects – A review. Polish Journal of Food and Nutrition Sciences, 66(2), 67–76.
Lourdin, D., Coignard, L., Bizot, H., & Colonna, P. (1997). Influence of equilibrium relative humidity and plasticizer concentration on the water content and glass transition of starch materials. Polymer, 38(21), 5401–5406.
Luchese, C. L., Garrido, T., Spada, J. C., Tessaro, I. C., & Caba, K. I. (2018a). Development and characterization of cassava starch films incorporated with blueberry pomace. International Journal of Biological Macromolecules, 106, 834–839.
Luchese, C. L., Abdalla, V. F., Spada, J. C., & Tessaro, I. C. (2018b). Evaluation of blueberry residue incorporated cassava starch film as pH indicator in different simulants and foodstuffs. Food Hydrocolloids, 82, 209–218.
Monteiro, M. K. S., Oliveira, V. R. L., Santos, F. K. G., Barros Neto, E. L., Leite, R. H. L., Aroucha, E. M. M., Silva, R. R., & Silva, K. N. O. (2018). Incorporation of bentonite clay in cassava starch films for the reduction of water vapor permeability. Food Research International, 105, 637–644.
Nouri, L., & Nafchi, A. M. (2014). Antibacterial, mechanical, and barrier properties of sago starch film incorporated with betel leaves extract. International Journal of Biological Macromolecules, 66, 254–259.
Olu-Owolabi, B. I., Afolabi, T. A., & Adebowale, K. O. (2011). Pasting, thermal, hydration, and functional properties of annealed and heat-moisture treated starch of sword bean (Canavalia gladiata). International Journal of Food Properties, 14(1), 157–174.
Piñeros-Hernandez, D., Medina-Jaramillo, C., López-Córdoba, A., & Goyanes, S. (2017). Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocolloids, 63, 488–495.
Roberts, R. L. (2013). Lutein, zeaxanthin, and skin health. American Journal of Lifestyle Medicine, 7(3), 182–185.
Rodríguez, M., Osés, J., Ziani, K., & Maté, J. I. (2006). Combined effect of plasticizers and surfactants on the physical properties of starch based edible films. Food Research International, 39(8), 840–846.
Saberi, B., Vuong, Q. V., Chockchaisawasdee, S., Golding, J. B., Scarlett, C. J., & Stathopoulos, C. E. (2017a). Physical, barrier, and antioxidant properties of pea starch-guar gum biocomposite edible films by incorporation of natural plant extract. Food and Bioprocess Technology, 10(12), 2240–2250.
Saberi, B., Chockchaisawasdee, S., Golding, J. B., Scarlett, C. J., & Stathopoulos, C. E. (2017b). Physical and mechanical properties of a new edible film made of pea starch and guar gum as affected by glycols, sugars and polyols. International Journal of Biological Macromolecules, 104(Pt A), 345–359.
Sandmann, G., Kuhn, S., & Böger, P. (1998). Evaluation of structurally different carotenoids in Escherichia coli transformants as protectants against UV-B radiation. Applied and Environmental Microbiology, 64(5), 1972–1974.
Shaili, T., Abdorreza, M. N., & Fariborz, N. (2015). Functional, thermal, and antimicrobial properties of soluble soybean polysaccharide biocomposites reinforced by nano TiO 2. Carbohydrate Polymers, 134, 726–731.
Song, X., Zuo, G., & Chen, F. (2018). Effect of essential oil and surfactant on the physical and antimicrobial properties of corn and wheat starch films. International Journal of Biological Macromolecules, 107(Pt A), 1302–1309.
Souza, A. C., Benza, R., Ferrão, E. S., Ditchfield, C., Coelho, A. C. V., & Tadini, C. C. (2012). Cassava starch biodegradable films: Influence of glycerol and clay nanoparticles content on tensile and barrier properties and glass transition temperature. LWT – Food Science and Technology, 46(1), 110–117.
Teixeira, B. S., Garcia, R. H. L., Taknami, P. Y. I., & del Mastro, N. L. (2018). Comparison of gamma radiation effects on natural corn and potato starches and modified cassava starch. Radiation Physics and Chemistry, 142, 44–49.
Valencia-Sullca, C., Atarés, L., Vargas, M., & Chiralt, A. (2018). Physical and antimicrobial properties of compression-molded cassava starch-chitosan films for meat preservation. Food and Bioprocess Technology, 11(7), 1339–1349.
Versino, F., Lopez, O. V., Garcia, M. A., & Zaritzky, N. E. (2016). Starch-based films and food coatings: An overview. Starch-Stärke, 68(11-12), 1026–1037.
Woggum, T., Sirivongpaisal, P., & Wittaya, T. (2015). Characteristics and properties of hydroxypropylated rice starch based biodegradable films. Food Hydrocolloids, 50, 54–64.
Wojdyło, A., Nowicka, P., & Bąbelewski, P. (2018). Phenolic and carotenoid profile of new goji cultivars and their anti-hyperglycemic, anti-aging and antioxidant properties. Journal of Functional Food, 48, 632–642.
Yang, S. Y., Cao, L., Kim, H., Beak, S. E., & Song, K. B. (2018). Utilization of foxtail millet starch film incorporated with clove leaf oil for the packaging of Queso Blanco cheese as a model food. Starch-Stärke, 70(3-4), 1700171.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, S., Kang, JH. & Song, K.B. Development of a Sword Bean (Canavalia gladiata) Starch Film Containing Goji Berry Extract. Food Bioprocess Technol 13, 911–921 (2020). https://doi.org/10.1007/s11947-020-02447-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-020-02447-4