Abstract
The influence of two different edible coatings on air-drying kinetics and characteristics of pineapple slices was evaluated. Samples were osmotically dehydrated in aqueous solution containing 50 % of sucrose, 4 % of calcium lactate, and 2 % of ascorbic acid for 1 h. Osmo-treated pineapple slices coated with pectin, uncoated, and coated with a mix of whey protein isolate (WPI) + locust bean gum (LBG) + glycerol were hot-air-dried at 60 and 70 °C. Moisture and vitamin C content were evaluated before and after each treatment. Water activity and color were evaluated before and after drying. A simplified model based on Fick’s Law was used to estimate the effective diffusion coefficients during air-drying. The results showed that the impregnation pretreatment resulted in high vitamin C levels in dried pineapples. Pectin and WPI-LBG coatings did not affect the drying behavior of the samples and both coatings were effective in vitamin C retention during hot-air-drying. Water diffusion coefficients were strongly affected by temperature, whereas they were slightly affected by coatings. The coatings differently affected the lightness of the samples during air dehydration, and pectin coating showed the slightest change at this color parameter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drying is an important method of preservation of fruits and vegetables because it removes moisture from the food and reduces water activity, preventing the growth of spoilage microorganisms, slowing down the action of enzymes, and minimizing water-mediated deteriorative reactions during storage (Vega-Mercado et al. 2001; Mandala et al. 2005). This method can result in vitamin loss, browning, and undesirable texture changes in dried products (Ramallo and Mascheroni 2004, 2012; Chutintrasri and Noomhorm 2007; Kaya et al. 2010; Cortellino et al 2011). Ascorbic acid is frequently used as an indicator of the quality of food process because it is one of the most sensitive nutrients to heat, light, and oxygen, being easily degraded during convective drying (Uddin et al. 2001; Santos and Silva 2008). Enhancing vitamin C retention during drying would improve the nutritional quality of the food. The use of pretreatments to prevent oxidation during hot-air-drying can represent an alternative to reduce nutritional loss and to improve the quality of dried products.
Osmotic dehydration (OD) applied prior to drying can minimize negative impacts due to physical, sensorial, and nutritional changes resulting from the drying process (Riva et al. 2005; Sanjinez-Argandoña et al. 2005). Moreover, the enrichment of vitamins and mineral salts can be performed in osmotic dehydration (Castelló et al. 2009; Silva et al. 2014a, b) promoting the preservation of nutritional quality and reducing the losses during drying process.
Edible coatings application to the food prior to air-drying is another potential pretreatment to drying. Edible coatings are thin layers of an edible material applied to the surface of the food creating a selective barrier to the gas transport (Vargas et al. 2008). Edible coatings have been widely studied aiming to increase shelf life of minimally processed products (Oms-Oliu et al. 2008; Ansorena et al. 2011; Benítez et al. 2013) and reduce the solids uptake during OD (Matuska et al. 2006; García et al. 2010). A scarce number of researchers have investigated the use of edible coatings as pretreatment to convective drying (Garcia et al. 2012, 2014; Lago-Vanzela et al. 2013).
Polysaccharide and protein edible coatings present low water vapor barrier; however, they present good gas barrier properties, such as oxygen barrier (Fakhouri et al. 2007), and could be used to minimize oxidative reactions in food during hot-air-drying, pointing out the potential of using edible coatings prior to convective drying, since it could reduce undesirable changes due to large time of exposure of the food to oxygen. Lago-Vanzela et al. (2013) verified that pumpkin coated with starch solution presented higher trans-α-carotene and trans-β-carotene contents and better color after convective drying than the uncoated samples. Garcia et al. (2014) verified that pectin coating reduced vitamin C losses during convective drying of papaya slices, when compared to the uncoated samples, showing that the coating protected the samples against the oxidation of this biologically active compound.
The use of whey protein to manufacture edible films has also received attention due to its transparency, flexibility, biodegradable property, and oxygen barrier property (Perez-Gago et al. 2005, 2006; Hong and Krochta 2006). Ramos et al. (2013) characterized whey protein films made from two different protein products and verified that their oxygen barrier properties were better than polysaccharide-based film or other protein-based film (e.g., corn zein, wheat gluten, and soy protein isolate). Perez-Gago et al. (2005) verified that whey protein-based coatings reduced more enzymatic browning of apples slices than hydroxypropyl methylcellulose-based coating.
Locust bean gum (LBG) is a kind of galactomannan, found in the endosperm of Leguminosae, compatible with others gums, thickening agents, and proteins, usually used to increase the elasticity and strength of the gel (Gonçalves et al. 2004; Rocha et al. 2009). Many studies reported positive effects on functional properties of the mixtures of whey protein and anionic polysaccharides (Ibanoglu 2005; Neirynck et al. 2007; Rocha et al. 2009). To take advantage of these synergies, blends of proteins and polysaccharides as edible film forming agents have been studied by several authors in order to increase the barrier properties or increase mechanical properties (Arvanitoyannis and Biliaderis 1998; Lee et al. 2003; Osés et al. 2009). No study about employing whey protein–polysaccharide-based coating as pretreatment to drying was found in the literature.
This work aimed to investigate the influence of two different edible coatings on water activity, color changes, and vitamin C retention during hot-air-drying of osmotic dehydrated pineapple in sucrose–calcium lactate–ascorbic acid solution. Also, the effects of edible coatings on the kinetics of convective drying and the effective moisture diffusivities of coated and uncoated osmotically dehydrated pineapple were evaluated.
Material
Pineapples (Ananas comosus L. Merril) of commercial ripeness degree, weighing approximately 1.2 kg, were purchased at São José do Rio Preto Supplying Center (CEAGESP; São José do Rio Preto, São Paulo, Brazil) to be used in the experiments. The pineapples were kept under refrigeration in a cold chamber (Consul—São José dos Pinhais, SP) at 5 ± 2 °C for no longer than 96 h before their use in the experiments.
Commercial sucrose was purchased at a local market. Whey protein isolate (WPI) by Arla Foods Ingredients (Viby, Denmark) was used as the protein source. This isolate contains a minimum of 93.5 % total protein content (74 % of β-lactoglobulin, 18 % of α-lactalbumin, and 6 % of bovine serum albumin), maximum content of 0.2 % lactose and fat, approximately 0.5 % of sodium, 1 % of potassium, and 0.1 % of calcium, as specified by Arla Foods Ingredients. Locust bean gum (LBG) (>75 % galactomannan content) was kindly supplied by Danisco (Cotia, Brazil). Low methoxylated amidated pectin (GRINDSTED® LA 210) with degree of methoxylation of 0.34 and degree of amidation of 0.17 was kindly supplied by Danisco (Cotia, Brazil). Food-grade calcium lactate pentahydrate in power was kindly supplied by PURAC Synthesis (São Paulo, Brazil), glycerol by Sigma-Aldrich (São Paulo, Brazil), and food-grade ascorbic acid in powder by Prozyn® (São Paulo, Brazil).
Methodologies
Sample Preparation
Pineapples were washed, manually peeled, and both ends parts were disposed of to reduce the variability of the tissue since these parts were less mature than the central portion of the fruits. The central pieces were sliced (1.0 ± 0.1 cm thickness) using an electrical cutter (Eco—São Paulo, Brazil), and each slice was cut in truncated cone format using a metal die. The samples were kept in a plastic bag (polyethylene plastic 40 × 60 × 0.02 mm; Olimplastic—Olimpia, Brazil) during the cutting procedure in order to avoid oxygen exposure. After that, the slices were randomly removed from the bag and used in the experiments. Approximately 100 slices were used in each experiment.
After samples were osmotically dehydrated in aqueous solution, a portion of the samples was coated with pectin or WPI + LBG and a portion was not coated (control). After coating, the samples were kept at room temperature (27 °C) for a holding time of 15 h, aiming to minimize concentration profiles as well as to promote partial drying of the coating. Convective drying was carried out at 60 °C for 11 h and at 70 °C for 7 h.
Osmotic Dehydration
Osmotic solutions were prepared with commercial sucrose (refined sugar), food-grade pentahydrate calcium lactate in powder, food-grade ascorbic acid in powder, and distilled water.
Pineapple slices were dehydrated in 50 % (w/w) sucrose aqueous solutions supplemented with 4 % (w/w) of calcium lactate and 2 % (w/w) of vitamin C for 1 h, conditions selected to reach a good calcium and vitamin impregnation according to previous research carried out by Silva et al. (2014a). The fruit slices were arranged in four nylon mesh baskets, with approximately 350 g of samples in each basket. The baskets were immersed into 20 kg of osmotic solution in a jacketed stainless steel vessel (0.30 × 0.30 × 0.30 m3). The syrup-to-fruit ratio was approximately 1:14. The solution temperature was maintained at 27 °C with an external circulation of thermostatically controlled water. A central propeller (10 cm diameter) continuously agitated the solution by using a 1.6-kW mechanical stirrer (Marconi, model MA-261—Piracicaba, Brazil). A rotation of 1850 rpm provided constant and vigorous agitation. Thus, a negligible liquid phase mass transfer resistance was considered and the solution concentration was assumed constant at the fruit surface during the whole osmotic dehydration.
After 1 h, the baskets were removed from the osmotic bath and the samples were immersed in distilled water at room temperature for 10 s in order to remove the excess of osmotic solution from the surface. They were then blotted with absorbing paper and weighed.
Edible Coating Application
Solutions of low methoxylated amidated pectin and LGB + WPI were used to coat the osmotically dehydrated pineapple slices. Non-coated samples were used as control during the drying procedure.
Pectin aqueous solution (2 %, w/w) was prepared at 70 °C with constant stirring and then cooled to a fixed temperature of 40 °C in a water bath. When the solution reached 40 °C, the samples were immersed for 1 min using a perforated basket designed for this purpose. Gelling was activated with subsequent immersion of the samples in a 1.0 % (w/w) aqueous solution of food-grade calcium lactate pentahydrate (PURAC Synthesis—São Paulo, Brazil) for 30 s. The samples were then washed by immersion in distilled water for 30 s (Shigematsu et al. 2005; Garcia et al. 2014).
Stock solution of 1 % (w/w) LBG were prepared by stirring dry LBG powder in distilled water for 1 h. Then the solution was heated and kept for 30 min at 80 °C and cooled. The coatings were prepared mixing 5 % (w/w) WPI with aqueous solution at 0.05 % concentration of LBG stock solution and 2 % (w/w) of glycerol as plasticizer. A NaCl solution (20 %, w/w) was added to a final salt concentration of approximately 50 mM to ensure constant ionic strength. The solution was stirred for 2 h at room temperature. The solution pH was then adjusted to 7.0 and stirred for 2 h more. After that, the solution was heated and kept at 75 °C for 10 min to denaturalize protein fraction and cooled to room temperature. Osmotically dehydrated pineapple slices were weighed and immersed into WPI + LGB coating solutions for 1 min.
After coating, the samples were weighed and kept at room temperature for 15 h before drying to reduce concentration profiles and promote partial drying of the coatings. The average mass of the edible coating applied to the slices surface was measured through a mass balance.
Convective Drying
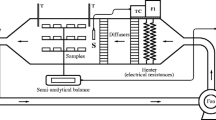
After osmotic dehydration, coating addition, and holding time at room temperature, the samples were placed into fixed bed pilot dryers at 60 and 70 °C for 11 and 7 h, respectively. A digital microprocessor unit with a J-type thermocouple was used to control the air temperature (Novus, model N440, São Paulo, Brazil). The air velocity inside the dryer was 1.0 m s−1 controlled by a centrifugal fan connected to a frequency inverter (WEG, CWF10, Jaraguá do Sul, Brazil). Each drying was carried out in duplicate. Two dryers were used for the experiments, allowing the drying of the coated and non-coated (control) pineapple slices in parallel, in order to dry the same raw material at the same temperature. The relative humidity inside the dryers’ chambers (67 × 38 × 33.5 cm) was registered at approximately 10 % during the drying of the samples by a humidity sensor DO9861T-R1 (Delta Ohm, Caselle di Selvazzano, Italy). The air was heated using electrical resistance elements and flowed parallel to the bed, which consisted of three wire nets (1 × 31 × 31 cm). The samples were weighed every 20 min during the first 90 min of drying, every 30 min during the next hour, and every 60 min for the remaining time. Eight samples weighing nearly 85 g were placed in each wire net. Every time the samples were weighed, the trays inside the dryers were rotated to standardize the moisture inside the dryers. To determine equilibrium water content, samples were kept into the dryers until a constant weight was achieved.
Analytical Methodologies
The total solids content of fresh, osmo-dehydrated, held at room temperature (coated or uncoated), and dried at 60 and 70 °C (osmo-dehydrated, coated, uncoated, and dried pineapple slices) were gravimetrically determined in triplicate in a vacuum oven at 60 °C and 10 kPa until a constant weight was achieved (AOAC 1995).
The water activity of the samples (coated or not) before and after drying was measured in triplicate at 25 °C in a water activity meter (Aw Sprint; Novasina, Switzerland). Color measurement, carried out before and after drying the samples (coated or not), was evaluated in eight replicates using a Colorflex spectrophotometer (HunterLab; Hunter Associates Laboratory, Inc., model MiniScan XE Plus, VA, USA), version 4.10 of the Universal software with the following settings: illuminant D65, observer at 10°, and reading the absolute values of L* (lightness or darkness), a* (redness or greenness), and b* (yellowness or blueness) before and after drying. In addition, chroma (Eq. 1), which indicates the purity or saturation of the color, and hue angle (Eq. 2), which expresses the color change (an angle of 0 or 360° represents red hue, while angles of 90, 180, and 270° indicate yellow, green, and blue hue, respectively), were calculated as follows:
The vitamin C content of the fresh, osmotically dehydrated, coated, uncoated, and dried samples was determined in duplicate using the modified method described by Benassi and Antunes (1988). In order to avoid vitamin C losses because of oxygen exposure, samples were analyzed immediately after processing. Samples (25 g) were homogenized in 50 mL of extractor solution (oxalic acid at 2 %; w/w) using a Turratec equipment (Tecnal, TE-102 model—Piracicaba, Brazil) for 1 min. An aliquot (20 g) was volumetrically diluted with the extractor solution to 50 mL. Ten milliliters of the diluted solution was titrated with 0.01 % 2.6-dichlorophenolindophenol solution, which was standardized with a standard ascorbic acid (Labsynth, Synth—São Paulo, Brazil) solution after each titration procedure. Due to the technical difficulty of the homogenization procedure of the dried samples, approximately 4 g of dried pineapple slices was rehydrated with approximately 20 g of distilled water for 20 min before homogenization in 50 mL of extractor solution using the Turratec equipment.
Calculations
Vitamin C
The vitamin C content was determined in milligrams of ascorbic acid in 100 g of fresh product, and the retentions of vitamin C were determined according to Murphy et al. (1975) as described by Eq. 3:
where Ret is the retention of vitamin C after a process time, C f is the amount of vitamin C in the samples at the end (after drying), and C i is the amount of vitamin C in the samples (coated and uncoated) before convective drying at 60 and 70 °C, in milligrams of ascorbic acid/100 g of sample; M f is the mass of samples at the end (after convective drying) and M i is mass of samples at the beginning (before convective drying), in grams.
Effective Diffusion Coefficients
The effective diffusion coefficients of moisture (D eff ) were determined according to Fick’s Law applied to an infinite slab. The diffusion model has been applied to the drying of biological materials by changing the fractional contents to express the moisture on a dry basis (db) (Garcia et al. 2007). The analytical solution of the following diffusion equation (Eq. 4) has been previously described by Crank (1975):
where X 0 indicates the initial water content (at t = 0, dry basis); X is the fractional or residual moisture content, dry basis (dimensionless); \( \overline{X} \) is the average moisture (dry basis) at time t (s); X eq is the fraction of the moisture at equilibrium (dry basis); D eff is the effective diffusion coefficient of moisture (m2 s−1); z is the thickness of the fresh samples (m); and n is the number of terms of the series.
Since OD and edible coatings did not considerably change the size of the samples, z was considered as the initial value. So the thickness z in Eq. (4) was assumed to be 1.0 cm.
Twelve terms were used in the calculations of moisture diffusivity, i.e., n = 12 in Eq. 4.
Drying Rate
The drying rate of the coated and non-coated pineapple samples was calculated using Eq. (5):
where M s is the mass of dried solids, A is the superficial area exposed to the drying air, M t+dt is the moisture content at t + dt (kg water/kg dry matter), M t is the moisture content at t (kg water/kg dry matter), and t is the time (s). The drying times were 11 h when the drying temperature was 60 °C and 7 h when the drying temperature was 70 °C.
Statistical Analysis and Fitting
The data were statistically analyzed by an analysis of variance (ANOVA) and Tukey’s test at a 5 % significance level, using Statistica 7.0 software.
Effective diffusion coefficients were calculated from the experimental data according to Eq. 4 using Statistica 7.0 software, which uses the least square estimation method to do the calculations. The least square estimation method aimed at minimizing the sum of the squared deviations of the values observed for the dependent variable from those predicted by the model. A convergence criterion of 1 × 10−6 was used and the estimated parameters (D eff ) and standard errors were displayed on a sheet.
The fitting efficiency was evaluated by the determination coefficient (R 2) and the relative root mean squared error (RMSE). The RMSE was calculated using Eq. 6 (Daniel and Wood 1980) as follows:
where x calc represents water content on a dry basis and was calculated according to Eq. 4, x exp is the experimental value, and N represents the number of observations or residuals.
Results and Discussion
Water Content, Water Activity, and Drying Kinetics
Table 1 presents the percentage of edible coatings added to the fruits’ surface, the moisture content (wet basis), and the water activity of fresh, osmotically dehydrated, and coated fruits before and after convective drying at 60 and 70 °C.
Table 1 shows that a higher mass was added to the sample’s surface when coated with pectin comparing with WPI + LBG. This fact is related to the composition of the coating. Increasing the coating thickness could result in higher protection to oxidative damages during air-drying. However, the structural arrangement of the macromolecules also could contribute to protect the fruit.
Water content of fresh pineapple was reduced during the osmotic treatment due to the chemical potential gradient existing between the product and the osmotic solution. In 1 h of osmotic dehydration, the initial moisture of pineapple slices decreased approximately 15 % (Table 1).
After 15 h of holding time at room temperature (27 °C), it was verified that the water content of the control samples (osmo-dehydrated uncoated pineapple slices, before drying) decreased, although the moisture of the coated fruits presented slightly higher than after OD (Table 1). This fact indicates that the holding time at room temperature was not enough to evaporate the water incorporated during coating appliance.
Edible coatings did not influence the final water activity of samples after drying at 60 and 70 °C (Table 1). However, the drying times employed in this work decreased the water activity of the samples to security levels against microorganism growth. According to Yan et al. (2008), water activity of nearly 0.6 reduces or inactivates the growth of microorganisms.
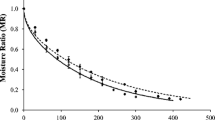
Figures 1 and 2 show experimental and calculated values of the moisture content (dimensionless) versus drying time. The moisture decreased continuously with drying time. It was verified that for a certain value of drying time, the moisture of 70 °C drying curves were lower than the moisture of the 60 °C drying curves. Therefore, the results showed that the air temperature had a significant effect on the water content of the pineapple slices (Figs. 1 and 2 and Table 2).
Pectin and WPI + LBG coatings did not increase the resistance to mass transfer during drying since water content of coated and uncoated samples did not present a statistically significant difference, according to Tukey test, after drying at 60 and 70 °C (Table 1). Furthermore, the drying curves of coated and uncoated pineapple slices, at the same temperature (Figs. 1 and 2), were overlapped.
This result can be related to the chemical nature of proteins and polysaccharide that present low moisture barrier properties because of their high polarity. Moreover, these polymers present high solubility coefficients of water, and for this reason, they present high rates of water vapor permeation (Krochta et al. 1994) and, consequently, low resistance to mass transfer during drying.
Effective Diffusion Coefficient and Drying Rate
Table 2 shows the effective diffusion coefficients of water calculated according to Eq. 4. The data showed a good fit; the R 2 values were above 0.976 and the values for RMSE were below 5 %.
The water diffusivities of pineapple slices were significantly higher at the 70 °C drying temperature (Table 2). This is in agreement with the results presented in Figs. 1 and 2 which show that drying time decreased greatly when drying temperature increased. Similar results were reported by Nicoleti et al. (2001) for drying of osmotic dehydrated pineapple and by Garcia et al. (2007) for drying of osmo-treated pumpkins.
Water diffusivities of coated samples were higher than non-coated slices due to the strong hydrophilic nature of coatings (McHugh and Krochta 1994). This fact is related to the water diffusivity in coatings which was greater than in the pineapple slices, considering that both the coating and the fruit tissue contributed to the effective diffusivity value. So, the water diffusivity in the coated samples includes both the diffusivity in the fruit tissue and in the coating.
Figures 3 and 4 present the changes in the drying rates as a function of moisture at 60 and 70 °C drying temperatures. A constant drying rate period was not detected. Therefore, the drying process of the pre-treated pineapple slices occurs in the range of the falling rate period, where diffusion is the dominant physical mechanism governing moisture movement in the samples. Since plant materials usually contain mostly bound water, a constant rate period, in general, is not detected. Similar results were found by other authors that studied the drying of osmotic dehydrated pineapples (Nicoleti et al. 2001) and apples (Doymaz 2010).
Vitamin C
The effects of the osmotic treatment, coating, and drying temperatures on vitamin C content of the samples are shown in Table 3.
Determination of vitamin C was carried out immediately after processing without any prior storage to avoid vitamin C degradation. The vitamin C content of the dehydrated fruits was expressed as milligrams per 100 g of dried sample, so high vitamin C contents were verified. The retention of vitamin C was calculated according to Eq. 3.
Significant differences between ascorbic acid content of fresh pineapple were verified which can be attributed to the heterogeneity among the raw fruits. Factors such as climate, fertilizer practice, or high intensity of sunlight can result in large variations in ascorbic acid content of pineapple (Hamner and Nightingale 1945; Singleton and Gortner 1965).
Silva et al. (2014a), working with pineapple, verified that 1 h of osmotic dehydration in quaternary solution (sucrose + calcium lactate + ascorbic acid + water) resulted in appreciable vitamin C impregnation in the samples (more than 19 times of the initial value) as found in the present work.
The application of coatings at the fruit’s surface resulted in additional mass of 12.83 to 16.67 % related to the initial mass (Table 1) changing the vitamin C concentration, as shown in Table 3. After 15 h at room temperature (control samples), it was verified that there was a decrease in the vitamin C content of all the samples as a result of oxygen exposure even though some samples were coated. Indeed, a significant difference between the vitamin C content of uncoated and coated pineapple slices was verified (Table 3). The highest water and vitamin C losses during the holding time were verified in control (OD) and WPI + LBG coated (OD + WPI-LBG) samples. It is possible to estimate the water and vitamin C losses based on the added mass and the composition of the edible coatings (Table 1). The water loss was 12.8 ± 1.1 % (OD control), 7.3 ± 0.7 % (OD + pectin), and 10.1 ± 5.9 % (OD + WPI-LBG) while the vitamin C retention was 79.4 ± 1.8 % (control), 79.2 ± 1.7 % (pectin), and 83.3 ± 1.4 % (OD + WPI-LBG).
According to Bonilla et al. (2012), wet systems increase oxygen permeability because the network structure is not packed as in dry condition. Gontard et al. (1996) verified an increase in oxygen and carbon dioxide permeability due to the increase of relative humidity of several films based on polysaccharides and proteins. Hong and Krochta (2006) verified great influence of relative humidity on oxygen permeability of WPI coating. Another important parameter to be considered is the high water activity of the samples after OD (Table 1) since high water content in the samples reduces the aqueous phase viscosity, increasing diffusion and facilitating reactions of oxidation (Lee and Labuza 1975; Santos and Silva 2008).
High vitamin C content was found in dried samples (Table 3) due to the moisture loss which concentrates the vitamin C in the pieces of fruit and also as a result of the low losses of ascorbic acid during hot-air-drying. Control samples had the lowest vitamin C retention during drying (Table 3) even though good retention levels have been found. The protective role of osmotic dehydration on volatiles and sensitive compounds to hot-air-drying is already known (Shi et al. 1999; Ramesh et al. 2001). The high retention of vitamin C verified for the control fruits can be attributed to the protective effect provided by osmotic dehydration and by vitamin C incorporation in the fruit slices. Riva et al. (2005), working with apricot cubes pretreated or not for 30 and 60 min in 60 % (w/w) sucrose solution, verified higher vitamin C retention after air dehydration of osmo-treated compared to non-treated samples. Guavas pretreated for 2 h in sucrose solution (60 °Bx) at 40 °C presented ascorbic acid retention around 30–35 % after 120 min of air dehydration at 60 °C. Vitamin C was not detected in dried guava without osmotic treatment (Sanjinez-Argandoña et al. 2005). Vega-Gálves et al. (2008) studied the effect of calcium salts on quality of red pepper during drying and verified that the samples pretreated for 10 min at 25 °C in aqueous solution of 20 % (w/w) NaCl + 1 % (w/w) CaCl2 + 0.3 % (w/w) Na2S2O5 showed higher vitamin C retention during air dehydration at 70 °C than non-treated samples dried at 50, 60, and 70 °C. The authors related the result to the protective effect of CaCl2 on ascorbic acid oxidation. In the present work, it is possible that the effect of both sucrose and calcium contributed to the great protection against vitamin C oxidation.
Control samples lost more vitamin C during drying at 70 °C/7 h than during drying at 60 °C/11 h, indicating that temperature had a higher effect on vitamin degradation than drying time (Table 3). This behavior is in agreement with the results found by other authors (Orikasa et al. 2008; Kaya et al. 2010; Ramallo and Mascheroni 2012).
The highest retentions obtained for coated samples show that pectin and WPI + LBG coatings were efficient barriers to oxygen, avoiding the oxidation of vitamin C. This result is related to the strong polymer chain interactions due to large degrees of hydrogen bonding in the proteins and polysaccharides matrix that restricts chain motion, resulting in low oxygen permeability values at low relative humidity (Krochta et al. 1994).
Low losses of vitamin C observed in dried coated samples could be attributed to the experimental errors, as retention values around 100 % were obtained. Probably the error sources for the calculated retention were due to accumulated deviations in successive measurements and calculus operations since several processing steps were involved, i.e., osmotic dehydration, coating applying, holding time, and hot-air-drying. However, comparison of vitamin C content found in dried samples showed higher values in the coated samples than in the control samples, all with similar water content.
At 60 °C drying temperature, the retention of vitamin C in the samples coated with pectin and WPI-LBG was very close; however, at 70 °C drying temperature, pectin was more efficient in avoiding vitamin C oxidation than WPI + LBG coating (Table 3). According to Silva et al. (2012), films of WPI + LBG are formed by a network strongly crosslinked via non-covalent and covalent bonds that provide a compact matrix that makes gas molecule diffusion difficult. This property of WPI + LBG coating may have contributed to the highest vitamin C retention during drying at 60 °C. However, the drying temperature of 70 °C could have modified the oxygen permeability of WPI + LBG coating, resulting in lower retention of vitamin C when compared to the pectin-coated samples dried at 70 °C (Table 3).
Color Measurement
The color parameters of the pineapple samples with and without coating, before and after drying, are presented in Table 4.
The lightness (L*) of the samples decreased approximately 15–19 % during drying, being the higher reduction verified at 70 °C (Table 4). This result can be related to oxidative reactions that occur during air dehydration which is potentiated by drying temperature. Thus, this behavior is in agreement with the results found by other authors for pineapple juice (Rattanathanalerk et al. 2005), pineapple puree (Chutintrasri and Noomhorm 2007), and pineapple rings (Cortellino et al. 2011).
Coatings acted distinctly over the lightness of the samples during air dehydration. Pineapple coated with pectin presented the slightest change in L* value during drying, while control samples and WPI + LBG coated samples were darker. The use of pectin coating must have minimized the contact between the surface of the pineapple and oxygen, decreasing oxidative browning of pineapple during drying. WPI coating has been used on fresh-cut fruits and vegetables to prevent browning by action of oxygen (Le Tien et al. 2001; Perez-Gago et al. 2005, 2006). However, as pretreatment to drying, it is possible that the temperature provided non-enzymatic reactions in coating due to reactions among reducing sugars (such as fructose and glucose present in fruit) and WPI.
Before drying, all the samples presented a yellow predominant color (hue value around 90°). Convective drying significantly reduced the hue angle value of the samples, decreasing the predominance of yellow color (represented by angle of 90°) and increasing the presence of red color (represented by angle of 0°). Chroma values significantly increased after drying, indicating that the pineapple color became more intense with drying. However, significant differences among dried samples were not verified (Table 4).
Conclusion
Edible coating of pectin and WPI + LBG did not affect the efficiency of drying and did not influence the reduction of water activity of samples during drying. The majority of coated pineapples presented the highest water diffusion coefficients during convective drying.
Impregnation of ascorbic acid during osmotic dehydration yields dried pineapple with high vitamin C content. Drying temperature had a greater effect on vitamin C degradation than the time of process.
Pectin and WPI + LBG coatings were effective barriers to oxygen during convective drying at 60 °C. However, the samples coated with pectin presented higher vitamin C retention than with WPI + LBG during drying at 70 °C. Coating efficiency at different temperatures was attributed to the permeability properties to oxygen.
Convective drying reduced lightness of the pineapple, changed hue from yellowness to slightly orange, and provided more intense color products. The slightest reduction in lightness samples was observed in samples coated with pectin.
These results highlighted the potential of using edible coatings as an alternative to improve the protection of biologically active nutrients during hot-air-drying.
References
A.O.A.C. (1995). Official methods of analysis of the Association of Official Analytical Chemists. In W. Horwitz (Ed.), Method 926.12 (chapter 33, pp. 5). Arlington: A.O.A.C.
Ansorena, M. R., Marcovich, N. E., & Roura, S. I. (2011). Impact of edible coatings and mild heat shocks on quality of minimally processed broccoli (Brassica oleracea L.) during refrigerated storage. Postharvest Biology and Technology, 59, 53–63.
Arvanitoyannis, I., & Biliaderis, C. G. (1998). Physical properties of polyol-plasticized edible films made from sodium caseinate and soluble starch blends. Food Chemistry, 62(3), 333–342.
Benassi, M. T., & Antunes, A. J. (1988). A comparison of meta-phosphoric and oxalic acids as extractant solutions for the determination of vitamin C in selected vegetables. Arquivos de Biologia e Tecnologia, 31(4), 507–513.
Benítez, S., Achaerandio, I., Sepulcre, F., & Pujolà, M. (2013). Aloe vera based edible coatings improve the quality of minimally processed ‘Hayward’ kiwifruit. Postharvest Biology and Technology, 81, 29–36.
Bonilla, J., Atarés, L., Vargas, M., & Chiralt, A. (2012). Edible films and coatings to prevent the detrimental effect of oxygen on food quality: possibilities and limitations. Journal of Food Engineering, 110, 208–213.
Castelló, M. L., Igual, M., Fito, P. J., & Chiralt, A. (2009). Influence of osmotic dehydration on texture, respiration and microbial stability of apple slices (var. granny smith). Journal of Food Engineering, 91(1), 1–9.
Chutintrasri, B., & Noomhorm, A. (2007). Color degradation kinetics of pineapple puree during thermal processing. Lebensmittel-Wissenschaft und-Technologie, 40, 300–306.
Cortellino, G., Pani, P., & Torreggiani, D. (2011). Crispy air-dried pineapple rings: optimization of processing parameters. 11th International Congress on Engineering and Food (ICEF11). Procedia Food Science, 1, 1324–1330.
Crank, J. (1975). The mathematics of diffusion (2nd ed.). Oxford: Clarendon.
Daniel, C., & Wood, F. (1980). Fitting equations to data, revised edition. New York: Wiley.
Doymaz, I. (2010). Effect of citric acid and blanching pre-treatments on drying and rehydration of Amasya red apples. Food and Bioproducts Processing, 88, 124–132.
Fakhouri, F. M., et al. (2007). Filmes e coberturas comestíveis compostas à base de amidos nativos e gelatina na conservação e aceitação sensorial de uvas Crimson. Ciência e Tecnologia de Alimentos, 27(02), 369–375.
Garcia, C. C., Mauro, M. A., & Kimura, M. (2007). Kinetics of osmotic dehydration and air-drying of pumpkins (Cucurbita moschata). Journal of Food Engineering, 82(3), 284–291.
García, M., Díaz, R., Martínez, Y., & Casariego, A. (2010). Effects of chitosan coating on mass transfer during osmotic dehydration of papaya. Food Research International, 43, 1656–1660.
Garcia, C. C., Canizares, D., Silva, K. S., Darros-Barbosa, R., & Mauro, M. A. (2012). Utilização de métodos combinados para obtenção de mamão formosa (Carica papaya) seco. Boletim do CEPPA, 30(2), 185–196.
Garcia, C. C., Caetano, C. L., Silva, K. S., & Mauro, M. A. (2014). Influence of edible coating on the drying and quality of papaya (Carica papaya). Food and Bioprocess Technology. doi:10.1007/s11947-014-1350-6. Preprint online.
Gonçalves, M. P., Torres, D., Andrade, C. T., Azero, E. G., & Lefebvre, J. (2004). Rheological study of the effect of Cassia javanica galactomannans on the heat-set gelation of a whey protein isolate at pH 7. Food Hydrocolloids, 18, 181–189.
Gontard, N., Thibault, R., Cuq, B., & Guilbert, S. (1996). Influence of relative humidity and film composition on oxygen and carbon dioxide permeabilities of edible films. Journal of Agricultural and Food Chemistry, 44, 1064–1069.
Hamner, K. C., & Nightingale, G. T. (1945). Ascorbic acid content of pineapples as correlated with environmental factors and plant composition. Journal of Food Science, 11(6), 535–541.
Hong, S. I., & Krochta, J. M. (2006). Oxygen barrier performance of whey-protein-coated plastic films as affected by temperature, relative humidity, base film and protein type. Journal of Food Engineering, 77, 739–745.
Ibanoglu, E. (2005). Effect of hydrocolloids on the thermal denaturation of proteins. Food Chemistry, 90, 621–626.
Kaya, A., Ayadin, O., & Kolayli, S. (2010). Effect of different drying conditions on the vitamin C (ascorbic acid) content of Hayward kiwifruits (Actinidia deliciosa Planch). Food and Bioproducts Processing, 88(2–3), 165–173.
Krochta, J. M., Baldwin, E. A., & Nisperos-Carriedo, M. (1994). Edible coatings and films to improve foods quality. Lancaster: Technomic Publishing Company.
Lago-Vanzela, E. S., Nascimento, P., Fontes, E. A. F., Mauro, M. A., & Kimura, M. (2013). Edible coatings from native and modified starches retain carotenoids in pumpkin during drying. LWT - Food Science and Technology, 50, 420–425.
Le Tien, C., Vachon, C., Mateescu, M. A., & Lacroix, M. (2001). Milk protein coating prevent oxidative browning of apples and potatoes. Journal of Food Science, 66(4), 512–516.
Lee, S. H., & Labuza, T. P. (1975). Destruction of ascorbic acid as a function of water activity. Journal of Food Science, 40, 370–373.
Lee, J. Y., Park, H. J., Lee, C. Y., & Choi, W. Y. (2003). Extending shelf-life of minimally processed apples with edible coatings and antibrowning agents. Lebensmittel-Wissenschaft und-Technologie, 36, 323–329.
Mandala, I. G., Anagnostaras, E. F., & Oikonomou, C. K. (2005). Influence of osmotic dehydration conditions on apple air-drying kinetics and their quality characteristics. Journal of Food Engineering, 69, 307–316.
Matuska, M., Lenart, A., & Lazarides, H. N. (2006). On the use of edible coatings to monitor osmotic dehydration kinetics for minimal solids uptake. Journal of Food Engineering, 72, 85–91.
McHugh, T. H., & Krochta, J. M. (1994). Permeability properties of edible films. In: J. M. Krochta, E. A. Baldwin, M. O. Nisperos-Carriedo (Eds.), Edible coatings and films to improve food quality (pp. 139–187). Lancaster: Technomic Pub. Co.
Murphy, E. W., Criner, P. E., & Gray, B. C. (1975). Comparisons of methods for calculating retentions of nutrients in cooked foods. Journal of Agricultural and Food Chemistry, 23, 1153–1157.
Neirynck, N., Van der Meeren, P., Lukaszewicz-Lausecker, M., Cocquyt, J., Verbeken, D., & Dewettinck, K. (2007). Influence of pH and biopolymer ratio on whey protein–pectin interactions in aqueous solutions and in O/W emulsions. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 298, 99–107.
Nicoleti, J. F., Telis-Romero, J., & Telis, V. R. N. (2001). Air-drying of fresh and osmotically pre-treated pineapple slices: fixed air temperature versus fixed slice temperature drying kinetics. Drying Technology, 19(9), 2175–2191.
Oms-Oliu, G., Soliva-Fortuny, R., & Martín-Belloso, O. (2008). Using polysaccharide-based edible coatings to enhance quality and antioxidant properties of fresh-cut melon. LWT- Food Science and Technology, 41, 1862–1870.
Orikasa, T., Wu, L., Shiina, T., & Tagawa, A. (2008). Drying characteristics of kiwifruit during hot air drying. Journal of Food Engineering, 85, 303–308.
Osés, J., Fabregat-Vázquez, M., Pedroza-Islas, R., Tomás, S. A., Cruz-Orea, A., & Maté, J. I. (2009). Development and characterization of composite edible film based on whey protein isolate and mesquite gum. Journal of Food Engineering, 92, 56–62.
Perez-Gago, M. B., Serra, M., Alonso, M., Mateos, M., & del Río, M. A. (2005). Effect of whey protein and hydroxypropyl methylcellulose-based edible composite coatings on color change of fresh-cut apples. Postharvest Biology and Technology, 36, 77–85.
Perez-Gago, M. B., Serra, M., & del Río, M. A. (2006). Color change of fresh-cut apples coated with whey protein concentrate-based edible coatings. Postharvest Biology and Technology, 39, 84–92.
Ramallo, L. A., & Mascheroni, R. H. (2004). Prediction and determination of ascorbic acid content during pineapple drying. Drying—Proceedings of the 14th International Drying Symposium (IDS 2004), C, 1984–1991.
Ramallo, L. A., & Mascheroni, R. H. (2012). Quality evaluation of pineapple fruit during drying process. Food and Bioproducts Processing, 90, 275–283.
Ramesh, M. N., Wolf, W., Tevini, D., & Jung, G. (2001). Influence of processing parameters on the drying spice paprika. Journal of Food Engineering, 49, 63–72.
Ramos, O. L., Reinas, I., Silva, S. I., Fernandes, J. C., Cerqueira, M. A., Pereira, R. N., Vicente, A. A., Fatima Pocas, M., Pintado, M. E., & Xavier Malcata, F. (2013). Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocolloids, 30(1), 110–122.
Rattanathanalerk, M., Chiewchan, N., & Srichumpoung, W. (2005). Effect of thermal processing on the quality loss of pineapple juice. Journal of Food Engineering, 66, 259–265.
Riva, M., Campolongo, S., Leva, A. A., Maestrelli, A., & Torreggiani, D. (2005). Structure-property relationships in osmo-air-dehydrated apricots cubes. Food Research International, 38, 533–542.
Rocha, C., Teixeira, J. A., Hilliou, L., Sampaio, P., & Gonçalves, M. P. (2009). Rheological and structural characterization of gels from whey protein hydrolysates/locust bean gum mixed systems. Food Hydrocolloids, 23, 1734–1745.
Sanjinez-Argandoña, E. J., Cunha, R. L., Menegalli, F. C., & Hubinger, M. D. (2005). Evaluation of total carotenoids and ascorbic acid in osmotic pretreated guavas during convective drying. Italian Journal of Food Science, 17(3), 305–314.
Santos, P. H. S., & Silva, M. A. (2008). Retention of vitamin C in drying processes of fruits and vegetables—a review. Drying Technology, 26(12), 1421–1437.
Shi, J., Le Maguer, M., Kakuda, Y., Liptay, A., & Niekamp, F. (1999). Lycopene degradation and isomerization in tomato dehydration. Food Research International, 32, 15–21.
Shigematsu, E., Eik, N. M., Kimura, M., & Mauro, M. A. (2005). Influência de pré-tratamentos sobre a desidratação osmótica de carambolas. Ciência e Tecnologia de Alimentos, 25(3), 536–545.
Silva, K. S., Mauro, M. A., Costa, M. J., Gonçalves, M. P., & Rocha, C. M. R. (2012). Caracterização de filmes de misturas de proteínas do soro de queijo com goma de alfarroba. In J. S. Amaral, Barreira, J. C. M., Barros, L., Ferreira, I. C. R., Mafra, I., Oliveira, M. B. P. P. (Eds.), 11° Encontro de Química dos Alimentos – Qualidade dos alimentos: novos desafios (p. 40). Bragança, 16-19 Setembro de 2012, (comunicação oral); ISBN 978-972-745-132-6.
Silva, K. S., Fernandes, M. A., & Mauro, M. A. (2014a). Osmotic dehydration of pineapple with impregnation of sucrose, calcium and ascorbic acid. Food and Bioprocess Technology, 7, 385–397.
Silva, K. S., Fernandes, M. A., & Mauro, M. A. (2014b). Effect of calcium on the osmotic dehydration kinetics and quality of pineapple. Journal of Food Engineering, 134, 37–44.
Singleton, V. L., & Gortner, W. A. (1965). Chemical and physical development of the pineapple fruit II. Carbohydrate and acid constituents. Journal of Food Science, 30(1), 19–23.
Uddin, M. S., Hawlader, M. N. A., & Zhou, L. (2001). Kinetics of ascorbic acid degradation in dried kiwifruits during storage. Drying Technology, 19, 437–446.
Vargas, M., Pastor, C., Chiralt, A., McClements, D. J., & González Martínez, C. (2008). Recent advances in edible coatings for fresh and minimally processed fruits. Critical Reviews in Food Science and Nutrition, 48, 496–511.
Vega-Gálvez, A., Lemus-Mondaca, R., Bilbao-Sáinz, C., Fito, P., & Andre´s, A. (2008) Effect of air drying temperature on the quality of dehydrated dried red bell pepper (var. Lamuyo). Journal of Food Engineering, 85, 42–50.
Vega-Mercado, H., Gongora-Nieto, M. M., & Barbosa-Canovas, G. V. (2001). Advances in dehydration of foods. Journal of Food Engineering, 49, 271–289.
Yan, Z., Sousa-Gallagher, M. J., & Oliveira, F. A. R. (2008). Sorption isotherms and moisture sorption hysteresis of intermediate moisture content banana. Journal of Food Engineering, 86, 342–348.
Acknowledgments
The authors would like to thank CAPES for the scholarship, PURAC Synthesis (Brazil), Danisco (Brazil), and Prozyn (Brazil).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva, K.S., Garcia, C.C., Amado, L.R. et al. Effects of Edible Coatings on Convective Drying and Characteristics of the Dried Pineapple. Food Bioprocess Technol 8, 1465–1475 (2015). https://doi.org/10.1007/s11947-015-1495-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1495-y