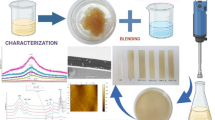
Abstract
Edible coatings attract interest today as efficient and safe techniques for controlling the deterioration and extending the shelf-life of food products. In the present study, a layer-by-layer (LbL) electrostatic deposition of oppositely charged natural polysaccharides, a polyanion alginate and a polycation chitosan, was implemented for coating a model food: fresh-cut melon. The performance of the alginate–chitosan coating was compared with single-layer coatings and with non-coated control. The LbL coating was found to possess the beneficial properties of both ingredients, combining good adhesion to melon matrix of the inner alginate layer with antimicrobial activity of the outer chitosan layer, thereby reducing the bacteria, yeast, and fungi counts by 1–2 log CFU. The bilayer coating slowed down tissue texture degradation, so that after 14 days of storage only LbL samples maintained an appreciable firmness. An unexpected benefit of the LbL coating was that its enhanced gas-exchange properties exceeded those of both monolayer coatings and even of the non-coated control. As a result, the LbL coating prevented an increase in headspace CO2 and ethanol concentrations, which are the signs of hypoxic stress and off-flavor development observed in other samples, especially in alginate-coated melons. The phenomenon was presumably related to swelling behavior of the chitosan layer in the humid atmosphere of the fresh-cut melon package, giving the melon pieces an attractive succulent appearance. At the same time, the LbL coating resulted in somewhat increased produce weight loss due to the reduced surface water vapor resistance. The method is cheap, simple, and can improve the quality and safety of food products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Edible coatings protect food products from mechanical, physical, chemical, and microbial damage and can extend their shelf life (Huber and Embuscado 2009; Baldwin et al. 2011). They attract much interest and practical research since they are based on natural, biodegradable, and edible components that satisfy environmental concerns and respond to customer demands for safe and healthy food (Han and Gennadios 2005). However, matching an appropriate coating material with properties and requirements of a specific food product is the major challenge for successful implementation of this approach. To be of practical application, edible coating needs to have perfect adhesion abilities, highly effective microbial protection, appropriate gas and moisture exchange properties, a good esthetic appearance, and to be totally tasteless, all with a reasonable cost.
Ready-to-eat fresh-cut fruit are one of the most promising areas where edible coatings can be applied because this technique can compensate the damage inflicted to fruit integrity in the course of physical processing (e.g., peeling and cutting) and thus control deterioration and extend market life of the value-added products (Dhall 2013; Corbo et al. 2010; Valencia-Chamorro et al. 2011; Pizato et al. 2013). Fresh-cut melons are among the most commercially important fresh-cut fruit products representing about 22 % of the market (Cook 2011). Melons are popular with consumers because of their unique flavor and nutritional value. They are naturally low in fat and sodium, have no cholesterol, and provide many essential nutrients such as potassium, vitamin A, and vitamin C. Melons were recommended as essential diet ingredient to ensure adequate nutrition, promote individual health, and reduce one’s risk of chronic diseases (Lester 1997). Fresh-cut melons are important in order to facilitate the consumption of this healthy commodity. However, they are prone to fast deterioration due to the range of quality problems (softening, juice leakage, flavor degradation, weight loss, microbial spoilage, and food safety risks) that can be solved or alleviated by successful coating but aggravated by inappropriate one (e.g., off-flavor generation). Therefore, fresh-cut melons can serve a model to accentuate coating efficacy.
In previous reports, coating with anionic polysaccharides gellan, pectin, and alginate reduced wounding stress in fresh-cut ‘Piel de Sapo’ melon, increased its water vapor resistance, and prevented dehydration (Ferrari et al. 2011). Calcium chloride used as a cross-linking agent helped to maintain fruit firmness (Oms-Oliu et al. 2008). Polysaccharides of this type adhere well to fresh-cut fruit, forming a smooth and uniform coating, supposedly due to chemical similarity with fruit carbohydrate structure and via cross-linking through Ca2+ bridges with pectin naturally occurring on the cut surface (Olivas et al. 2007; Tapia et al. 2008). At the same time, these coatings possess no antimicrobial activity. They did not improve microbiological stability of fresh-cut melon and required inclusion of exogenous non-polymer antimicrobial materials such as essential oils in order to control the decay of the food products and to ensure their microbiological safety (Oms-Oliu et al. 2008; Raybaudi-Massilia et al. 2008). However, such antimicrobial additives may affect the product’s flavor and impair the performance of the coating (Vargas et al. 2008).
An alternative coating material, the cationic polysaccharide chitosan, is composed of randomly distributed d-glucosamine and N-acetyl-d-glucosamine units and possesses intrinsic antimicrobial activity towards bacteria, yeast, and molds (Dutta et al. 2009; Rhoades and Roller 2000). Using chitosan for coating fresh-cut melon controlled the growth of spoilage and pathogenic microorganisms on the product and improved its microbiological quality (Krasaekoopt and Mabumrung 2008; Sangsuwan et al. 2008). However, the affinity of chitosan to cut fruit or vegetable surfaces is limited, resulting in uneven distribution of the coating and hindering its performance (Vargas et al. 2009).
Since single coating materials often cannot satisfy the diverse practical requirements, the situation calls for greater interest in multicomponent coatings. Rationally designed multicomponent edible coatings are currently sought after to provide new materials with well-controlled, beneficial properties (Falguera et al. 2011). The layer-by-layer (LbL) electrostatic deposition technique originated in materials science, and its applications range from optical devices to biomaterial coatings (Decher 1997; Vázquez et al. 2002). This approach is based on the alternate deposition of oppositely charged polyelectrolytes, and may result in the efficient control of coating properties and functionality. A foreseen trend in minimally processed fruit coating is wider implementation of the LbL technique using cationic (chitosan, poly-l-lysine) and anionic (pectin, alginate) biopolymers as promising ingredients (Vargas et al. 2008).
Furthermore, a few studies that have been published very recently using this approach with fresh-cut fruits, have demonstrated effective protection and shelf-life extension of food products. A multilayered edible coating made of chitosan and pectin was reported to significantly extend the shelf life of fresh-cut papaya (Brasil et al. 2012). The control of microbial growth in that work relied on inclusion of cyclodextrin-encapsulated cinnamaldehyde as an exogenous antimicrobial agent. Nanolayered coatings of a negatively charged polysaccharide κ-carrageenan, and a positively charged protein lysozyme were reported to effectively reduce the weight loss and maintain acidity of fresh-cut pears (Medeiros et al. 2012). The antimicrobial effect of that coating was not reported. The antimicrobial efficacy of LbL coatings in fresh-cut fruit based on natural antimicrobial properties of the coating components requires additional investigation, as well as their implications of using LbLs for overall product quality (Sipahi et al. 2012).
In the present work, we have utilized electrostatic interactions between negatively charged carboxylic groups of well-adhesive polyelectrolyte alginate and positively charged ammonium groups of antimicrobial polyelectrolyte chitosan for the formation of LbL coatings (Fig. 1). Alginate and chitosan cannot be mixed and then applied as a multicomponent coating since a homogeneous solution is not formed upon mixing. Combining superimposed alginate and chitosan layers seems promising for achieving coating characterized by both good adhesion to cut fruit and uniformly distributed antimicrobial protection. To the best of our knowledge, this combination has not been utilized so far for the preservation of fresh-cut fruits or vegetables. However, its efficacy has been proven in other areas, e.g., encapsulation systems for drug delivery (Ribeiro et al. 2005) or the imparting antimicrobial properties to textiles (Gomes et al. 2012).
The research objective was to test the performance of the LbL alginate–chitosan coating on a food model: fresh-cut melons. In addition, we aimed to check if the natural antimicrobial properties of the chitosan layer may be used to circumvent the incorporation of an exogenous antimicrobial agent.
Materials and Methods
Plant Material and Experimental Design
The work was performed with orange-flesh cantaloupe melons of ananas type, Cucumis melo L. subsp. melo var. cantalupensis Naudin (Cucurbitaceae family), cv. Yaniv (Hazera Genetics Ltd., Brurim, Israel). The melons were purchased at commercial maturity from the grower, the agricultural association Ein Yahav (North Arava, Israel). Whole melons were washed with water, decontaminated with 200 ppm solution of sodium hypochlorite, then rinsed with potable water and dried in air.
A typical trial included 28–30 melons; up to 20 pulp plugs were excised from the “equator” region of each melon using a sterile cork borer of 1.7 cm in diameter and a scalpel. The plugs were of 4 cm long and 9.0 ± 0.5 g mass. The plugs from different melons were mixed to receive a random distribution of fruit samples. The total number of 560 plugs was distributed between four treatments: (a) alginate only, (b) chitosan only, (c) LbL alginate–chitosan, and (d) uncoated control, 140 plugs per treatment. After performing the treatments as described below, the plugs were packed in non-perforated PETE clam-shell containers commonly used for fresh-cut fruit packaging, five plugs (45 ± 2 g) per container. Each treatment group comprised 28 containers, including 12 containers assigned for microbiological tests, 12 containers for weight loss and texture evaluation, three containers for headspace atmosphere analyses, and one container for determining the water vapor resistance (WVR) of the plug surfaces. The content of the containers assigned to weight loss and texture evaluation was aseptically weighed in a sterile laminar-flow cabinet in order to determine their initial mass.
The containers were stored at 6 °C as a simulation of cooled retail display conditions for up to 14 days with four quality evaluation samplings during this period. In course of each sampling, three containers were withdrawn for microbiological tests; three random plugs (ca. 27 g) were taken from each of these containers and served as one replicate. Three other containers were withdrawn for weight loss and firmness evaluation. Their content was weighed, and afterwards three random plugs were taken from each container for texture analysis, each of the nine plugs serving a replicate. Headspace samples for the atmosphere analysis were taken from the same containers throughout the storage period, three containers per treatment, each container serving a replicate. The WVR measurement was performed on the day of coating (the first day of the trial) as described below. The trials were repeated twice demonstrating similar trends. The results of a typical trial are presented in this paper.
Coating Solutions and Treatment
Alginate Coating
Sterile aqueous solution was prepared by dissolving the sodium alginate powder in double distilled water (DDW) sterilized in autoclave. Sodium alginate (Sigma Aldrich) was dissolved in 200 ml of water upon stirring at 70 °C during 2 h to obtain a 1.5 % (w/v) solution. The solution was cooled to room temperature and fresh-cut melons were immersed in the alginate solution for 2 min and then immersed for 2 min in 5 % aqueous solution of CaCl2 (Sigma Aldrich) to perform gelation of alginate molecules by cross-linking of alginate COO− groups with Ca+2. The coated melons were air dried at room temperature for 30 min.
Chitosan Coating
Sterile aqueous solution was prepared by dissolving the chitosan powder in DDW sterilized in autoclave. Chitosan (Sigma Aldrich) was dissolved in 200 ml of water acidified with acetic acid to pH 5 to obtain 1.5 % solution. The solution was stirred at room temperature for 12 h. Fresh-cut melons were immersed in the chitosan solution for 2 min and air dried at room temperature for 30 min.
Alginate–Chitosan LbL Coating
Alginate (1.5 %), chitosan (1.5 %), and CaCl2 (5 %) solutions were prepared as described above. Fresh-cut melons were immersed for 2 min in alginate and CaCl2 solutions as described above, rinsed with water, immersed for 2 min in chitosan solution, and air dried at room temperature for 30 min.
Microbiological Analyses
The microbiological tests were performed in triplicate, each replicate comprising three pulp plugs (∼27 g) randomly taken from the same container. The plugs were weighed and transferred into sterile Stomacher bags that each contained 180 ml of sterile saline solution (0.9 % NaCl) and homogenized for 2 min at high speed in a Stomacher 400 circulator (Seward, Worthing, UK). Test samples were serially diluted in saline solution and aerobic plate counts were determined by surface inoculation of plate count agar (PCA) (Oxoid, Basingstoke, UK). Mold and yeast counts were determined by surface inoculation of potato dextrose agar supplemented with 100 ppm chloramphenicol for controlling bacterial growth (PDA + A). The plates were incubated at 30 °C for 48 h (PCA) and at 25 °C for 5 days. (PDA + A) and the number of colony-forming units (CFU) per gram of plant material was calculated. A total value of 0.9 CFU was assigned to all Petri dishes in a sample showing no colonies at the least dilution. For statistical analysis, the data were transformed into logarithmic form as decimal logarithms of the CFU per gram.
Texture Analysis
Texture studies were performed using TA-XT2 Texture Analyzer (Stable Micro Systems Ltd., Godalming, UK). A TA-52 2-mm stainless cylinder probe was used at speed of 1.0 mm s−1 and travel distance of 20 mm to puncture the plugs horizontally positioned over the 8-mm hole. The maximum force required to puncture the plugs was recorded. Nine replicates were analyzed for each treatment.
Atmosphere Composition
The headspace atmosphere was sampled from three replicate containers of each treatment. Oxygen and carbon dioxide concentrations were determined using an OXYBABY 6.0 gas analyzer (WITT-GASETECHNIK GmbH & Co KG, Witten, Germany) comprising an electro-chemical cell for oxygen analysis and an IR-absorption cell for CO2 analysis. Concentrations of ethanol and acetaldehyde vapors in the headspace were determined by gas chromatography using external standards for quantification. Samples of headspace atmosphere analysis were withdrawn from packages by gas-tight syringes and analyzed with a Varian 3300 gas chromatograph equipped with a flame ionization detector and a 20 % Carbowax 20M packed column using helium as the carrier gas; column, injector, and detector temperatures were 80, 110, and 180 °C, respectively.
Water Vapor Resistance
Water vapor resistance (WVR) of coated and non-coated fresh-cut fruit surfaces was determined as described by Rojas-Graü et al. (2007) with minor modifications. Five plugs from each treatment were taken for the analysis, each plug serving a replicate. Each plug was put on a separate polypropylene weighing boat and kept at 25 °C in a desiccator maintained at 93 % relative humidity (RH) with saturated solution of KNO3 (Serdyuk et al. 2007). The RH level was selected for the trial in order to simulate the in-package conditions, keeping in mind that barrier properties of polysaccharide coatings and films depend on humidity conditions (Guilbert et al. 1995; Kurek et al. 2012). The weight of melon plugs was measured on analytical balance during 28 h at 4-h intervals. The slope of the curve of weight loss vs. time (weight loss rate) was estimated by linear regression analysis using the Excel spreadsheet. WVR was calculated according to a modified Fick’s first law equation (Ben-Yehoshua et al. 1985) as
where A w is water activity of melon pulp, %RH is relative humidity in the chamber, P sat is water vapor pressure in saturated air at 25 °C, R is specific gas constant for water vapor, T is temperature in Kelvin, A is plug surface area, and J is water vapor flux assumed equal weight loss rate (g/s). The value of water activity of melon pulp was taken as 0.988 according to Fernandez-Salguero et al. (1993).
Statistical Analysis
The number of replicates in each test is specified above in appropriate sections. Microsoft Office Excel spreadsheets were used to calculate means, standard deviations, 95 % t-based confidence intervals, and linear regression coefficients. The statistical analyses were carried out using JMP version 5.0.1 software (SAS Institute 2003) including a one-way analysis of variance (ANOVA) followed by the Tukey–Kramer honestly significant difference (HSD) post hoc test.
Results and Discussion
Antimicrobial Effect of the Applied Coatings
Total aerobic counts on the surface of non-coated melons steadily increased during storage reaching 9.7 log CFU g−1 by day 11 (Table 1). Alginate coating demonstrated no antimicrobial activity in that test. In contrast, chitosan significantly inhibited the microbial growth reducing the aerobic counts by 2–2.8 log CFU as compared with the non-coated control. Antimicrobial properties of chitosan are well characterized (Dutta et al. 2009); therefore, this observation was anticipated in good agreement with previous studies. Chitosan manifested its antimicrobial potential also when it was added as an external layer in an LbL coating, reducing total aerobic counts by approximately 1.5 log CFU.
The effects of coatings on mold and yeast growth (Table 2) were similar to the pattern described above for total aerobic counts, except for certain inhibitory activity demonstrated by alginate during the first 5 days of storage. In contrast, antifungal effects of chitosan and alginate–chitosan coatings were sustained until day 11, with higher efficacy shown by just the chitosan monolayer.
Antimicrobial activity is one of the most required and desired contributions of edible coating to food safety and quality (Campos et al. 2011). It is noteworthy that outer chitosan layer could be an alternative for the adding of external antimicrobial agent. Combining chitosan with additional means such as lactoperoxidase system may further enhance its antimicrobial activity (Cissé et al. 2013).
Firmness
Examination of firmness of coated and uncoated fresh-cut melons demonstrated a clear advantage and synergetic effect of combined LbL alginate–chitosan coating (Fig. 2). It is well established that edible coatings physically enhance the structure of fresh-cut produce and slow down their texture degradation (Huber and Embuscado 2009; Baldwin et al. 2011). This beneficial effect of edible coating was also observed in our studies. After 7 days of storage, all coated melons were firmer than the uncoated ones. Although all samples tended to soften during storage, the rate of softening varied depending on treatment. After 11 days of storage, alginate coating had no more beneficial effect on product texture, while chitosan and especially alginate–chitosan LbL coatings effectively reduced the texture degradation, probably due to their antimicrobial effect that could inhibit the production of microbial hydrolytic enzymes affecting the cell wall integrity of fresh-cut products (Chen 2002). Remarkably, after 14 days of storage, LbL alginate–chitosan-coated melons demonstrated the best texture preservation. One of the reasons for that might be that the combined LbL coating had advantages of both coating materials when internal alginate layer provided perfect adhesion and the external chitosan layer mitigated structure degradation caused by microbial enzymes. In addition, due to the alginate component, the LbL coating included Ca+2 ions, the effective texture enhancers responsible for texture maintenance by polysaccharide cross-linking.
Atmosphere Composition
Composition of the headspace atmosphere is determined by physiological activity of the product and in addition may be affected by microbial metabolism. One of the main problems faced upon application of edible coatings to fresh products is the normal gas exchange. If a coating is not permeable enough, the restriction of normal gas exchange results in the formation of hypoxic conditions inside fruit tissues indicated by generation of off-flavor volatiles and enhanced CO2 production (Baldwin et al. 1999; Han and Gennadios 2005; Fallik et al. 2005). Gas-exchange properties of various coatings can be quantified by measuring the evolution of CO2 content into the headspace atmosphere of melon-containing packages (Fig. 3).
No significant changes in CO2 concentrations occurred during the first 9 days of storage. Afterwards, alginate-coated and non-coated melons showed a sharp increase in CO2 concentration. Only slight atmosphere composition changes were observed in the packages containing samples coated with chitosan and alginate–chitosan. Taking into account the antimicrobial potential of chitosan and the timing of the CO2 concentration upsurge, one could assume that the enhanced CO2 accumulation at the end of storage could be affected by microbiological factors. Although often overlooked, the production of CO2, ethanol, organic acids, and volatile esters by spoilage bacteria and yeasts can affect headspace atmosphere composition and sensory quality of fresh-cut products (Jacxsens et al. 2003). In the work of Luna-Guzmán and Barrett (2000), the increase in respiration rates observed in fresh-cut cantaloupes after 1 week of storage was assigned to the onset of microbial spoilage, even though macroscopic decay was not yet clearly visible at that stage. Furthermore, packaging in antimicrobial chitosan-containing film prevented the increase in respiratory activity and in headspace ethanol content observed after extended storage of fresh-cut cantaloupes and pineapples (Sangsuwan et al. 2008).
On the other hand, it could be seen that in alginate-coated melons the change in atmosphere composition was significantly greater than in the non-coated fruits, in spite of their similar microbiological quality. This discrepancy might be a sign of certain hypoxic stress in alginate-coated melons due to restrictions in gas exchange. This hypothesis was supported by the measurement of headspace concentrations of ethanol vapors, the volatile associated with anaerobic fermentation. Previous studies demonstrated ethanol vapor accumulation as a reliable marker of off-flavor development in melons, with good correlation between headspace ethanol level and off-flavor severity (Fallik et al. 2005). The accumulation of ethanol followed a similar trend to that found in CO2 studies (Fig. 4).
Thus, the composition of in-package atmosphere reflected the interaction of microbiological (microbial spoilage) and physiological (hypoxic fermentation) factors. In both aspects, the alginate coating had weak points, providing no antimicrobial protection and obstructing the gas exchange. Unexpectedly, applying chitosan over the alginate layer improved the coating performance in both directions. While the antimicrobial effect of chitosan was anticipated, adding the chitosan layer also significantly improved the gas exchange properties of the coating and greatly alleviated the signs of hypoxic stress. Moreover, LbL-coated melons demonstrated better gas-exchange properties than both the melons covered with a single chitosan coating and even the non-coated melon plugs.
Water Vapor Transmission Properties
Water loss studies strengthened our observations concerning barrier properties of the coatings. In agreement with literature data (Oms-Oliu et al. 2008), alginate coating significantly increased the resistance of cut melon surface towards water vapor transmission and reduced its weight loss measured after 12 days of storage (Fig. 5). Chitosan application also increased the WVR value determined immediately after melon coating, but caused no significant reduction of weight loss at the end of storage. This discrepancy might be due to the poor adhesion of chitosan layer to the melon surface that might partially peel off and/or degrade during the extended storage. Vargas et al. (2009) showed that efficacy of chitosan coating for weight loss control in fresh-cut carrots depended on its adhesion to produce surface.
While both alginate and chitosan monolayer coatings enhanced the resistance of cut melon surface towards water vapor, paradoxically their LbL combination resulted in WVR decrease and enhancement of the water evaporation (Fig. 5). The obtained results correlated well with the results of atmosphere composition studies, revealing that alginate had the highest barrier properties towards gases and water vapor, while adding a second chitosan layer increased the permeability of the obtained LbL coating, even when compared with the non-coated cut fruit surface.
Possible explanation of these phenomena might be related to the effect of coating materials on the properties of the cut fruit surface. Cross-linking of anionic polysaccharides (alginate, pectin) with calcium results in the formation of a layer with enhanced water holding capacity that behaves as a dry “crust” on cut melon (Luna-Guzmán and Barrett 2000). Development of such a dry layer on a cut melon surface would increase its diffusion resistance similar to the situation described in soil science (Hide 1954; Ham and Heilman 1991) when mass transfer takes place in the depth of a substrate (at the interface between dry and wet layers) rather than on its surface (the substrate–air interface). Furthermore, similar phenomena can take place on a surface of non-coated fresh-cut produce due to the formation of a layer of dehydrated and structurally altered dead cells (Simões et al. 2010).
On the other hand, a layer of chitosan superimposed over the alginate in the LbL system might swell under high humidity conditions and act as a wick facilitating mass transfer between melon tissues and air. Swelling and transport properties of polyelectrolyte multilayer membranes depend, among other factors, on the choice of a capping layer (Miller and Bruening 2005). In examining an alginate-chitosan membrane used in a pervaporation process, Kanti et al. (2004) reported that a swollen and plasticized upstream layer of the membrane allowed unrestricted transport of feed components, while the virtually dry downstream layer performed as a diffusion barrier. Ito et al. (1997) demonstrated that absorption of water vapor caused swelling of chitosan membrane, enhancing its CO2 permeability. Similarly, permeability of chitosan-coated polyethylene film in moist air (RH 96 %) compared to the dry one (RH 0 %) increased 1.85 times for water vapor, 4 to 42 times for O2, and 5 to 238 times for CO2, changing the film selectivity in favor of CO2 permeation (Kurek et al. 2012). As mentioned above, these changes were accompanied by swelling of the chitosan layer. Similar swelling was observed in our trials on the surface of LbL-coated melon pieces giving them an attractive succulent juicy appearance without a tacky touch. In contrast, the non-coated or alginate-coated melon plugs had a visibly dry matte appearance.
Thus, LbL-coated melons demonstrated better gas exchange and water vapor permeability properties than melons coated with a single alginate or chitosan coating and even than the non-coated melons. As a payoff for greater water vapor permeability, the LbL coating was found to offer less protection against water loss. Nevertheless, a higher percentage of water loss in LbL-coated melons did not detract from fruit quality as can be seen from firmness studies and was outweighed by the prevented off-flavor development. In addition, the LbL coating imparted the melon pieces an attractive, succulent, and juicy appearance.
Conclusions
In this report, we present the performance of the layer-by-layer electrostatic deposition approach applied to a food model. Two oppositely charged natural polysaccharides, alginate and chitosan, were utilized to coat fresh-cut melons. To the best of our knowledge, this is the first report of LbL alginate–chitosan edible coating in a food application.
While alginate and chitosan cannot be applied as a mixed multicomponent coating, the LbL approach allows combining beneficial properties of both polysaccharides. The adhesive, inner alginate substrate resulted in a homogeneous and tight coating and also improved fruit firmness. The outer chitosan layer supplied potent antimicrobial protection against bacteria, yeast, and molds and also inhibited product degradation partially associated with microbial activity, such as the formation of undesirable off-flavor volatiles and fruit softening. All these benefits were achieved without the addition of external antimicrobial agents. The LbL-coated melons were much firmer than their uncoated or single-component-coated analogues. A remarkable and unexpected effect of the LbL strategy was the improvement of the coating gas-exchange and water vapor permeability properties.
To summarize, the layer-by-layer electrostatic deposition of edible coatings had clear benefits with regard to food firmness, gas exchange, and microbiological protection. The method is cheap, simple, and has applied potential. We expect the present findings will contribute to the development of rationally designed edible coatings that improve the quality and safety of food products.
References
Baldwin, E. A., Hagenmaier, R., & Bai, J. (2011). Edible coatings and films to improve food quality (2nd ed.). Boca Raton: CRC.
Baldwin, E. A., Burns, J. K., Kazokas, W., Brecht, J. K., Hagenmaier, R. D., Bender, R. J., et al. (1999). Effect of two edible coatings with different permeability characteristics on mango (Mangifera indica L.) ripening during storage. Postharvest Biology and Technology, 17(3), 215–226.
Ben-Yehoshua, S., Burg, S. P., & Young, R. (1985). Resistance of citrus fruit to mass transport of water vapor and other gases. Plant Physiology, 79(4), 1048–1053.
Brasil, I. M., Gomes, C., Puerta-Gomez, A., Castell-Perez, M. E., & Moreira, R. G. (2012). Polysaccharide-based multilayered antimicrobial edible coating enhances quality of fresh-cut papaya. LWT—Food Science and Technology, 47(1), 39–45.
Campos, C. A., Gerschenson, L. N., & Flores, S. K. (2011). Development of edible films and coatings with antimicrobial activity. Food and Bioprocess Technology, 4(6), 849–875.
Chen, J. (2002). Microbial enzymes associated with fresh-cut produce. In Lamikanra (Ed.), Fresh-cut fruits and vegetables: science, technology and market (pp. 249–266). Boca Raton: CRC.
Cissé, M., Kouakou, A. C., Montet, D., Loiseau, G., & Ducamp-Collin, M. N. (2013). Antimicrobial and physical properties of edible chitosan films enhanced by lactoperoxidase system. Food Hydrocolloids, 30(2), 576–580.
Cook, R. (2011). Trends in the marketing of fresh produce and fresh-cut products, University of California Davis, USA. Available at: ucce.ucdavis.edu/files/datastore/234-2115.pdf.
Corbo, M. R., Speranza, B., Campaniello, D., D’Amato, D., & Sinigaglia, M. (2010). Fresh-cut fruits preservation: current status and emerging technologies. In V. Méndez (Ed.), Current research technology and education topic in applied microbiology and microbial biotechnology (pp. 1143–1154). Badajoz, Spain: Formatex Research Center.
Decher, G. (1997). Fuzzy nanoassemblies toward layered polymeric multicomposites. Science, 277(5330), 1232–1237.
Dhall, R. K. (2013). Advances in edible coatings for fresh fruits and vegetables: a review. Critical Reviews in Food Science and Nutrition, 53(5), 435–450.
Dutta, P. K., Tripathi, S., Mehrotra, G. K., & Dutta, J. (2009). Perspectives for chitosan based antimicrobial films in food applications. Food Chemistry, 114(4), 1173–1182.
Falguera, V., Quintero, J. P., Jimenez, A., Munoz, J. A., & Ibarz, A. (2011). Edible films and coatings structures, active functions and trends in their use. Trends in Food Science and Technology, 22(6), 292–303.
Fallik, E., Shalom, Y., Alkalai-Tuvia, S., Larkov, O., Brandeis, E., & Ravid, U. (2005). External, internal and sensory traits in Galia-type melon treated with different waxes. Postharvest Biology and Technology, 36(1), 69–75.
Fernandez-Salguero, J., Gómez, R., & Carmona, M. A. (1993). Water activity in selected high-moisture foods. Journal of Food Composition and Analysis, 6(4), 364–369.
Ferrari, C. C., Sarantopoulos, C. I. G. L., Carmello-Guerreiro, S. M., & Hubinger, M. D. (2011). Effect of osmotic dehydration and pectin edible coatings on quality and shelf life of fresh-cut melon. Food and Bioprocess Technology, 6(1), 80–91.
Gomes, A. P., Mano, J. F., Queiroz, J. A., & Gouveia, I. C. (2012). Layer-by-layer deposition of antibacterial polyelectrolytes on cotton fibres. Journal of Polymers and the Environment, 20(4), 1084–1094.
Guilbert, S., Gontard, N., & Cuq, B. (1995). Technology and applications of edible protective films. Packaging Technology and Science, 8(6), 339–346.
Ham, J. M., & Heilman, J. L. (1991). Aerodynamic and surface resistances affecting energy transport in a sparse crop. Agricultural and Forest Meteorology, 53(4), 267–284.
Han, J. H., & Gennadios, A. (2005). Edible films and coatings. A review. In J. H. Han (Ed.), Innovations in food packaging, chapter 15 (pp. 239–262). Amsterdam: Elsevier Academic.
Hide, J. C. (1954). Observations on factors influencing the evaporation of soil moisture. Soil Science Society of America Journal, 18(3), 234–239.
Huber, K. C., & Embuscado, M. E. (2009). Edible films and coatings for food applications. New York: Springer.
Ito, A., Sato, M., & Anma, T. (1997). Permeability of CO2 through chitosan membrane swollen by water vapor in feed gas. Die Angewandte Makromolekulare Chemie, 248(1), 85–94.
Jacxsens, L., Devlieghere, F., Ragaert, P., Vanneste, E., & Debevere, J. (2003). Relation between microbiological quality, metabolite production and sensory quality of equilibrium modified atmosphere packaged fresh-cut produce. International Journal of Food Microbiology, 83(3), 263–280.
Kanti, P., Srigowri, K., Madhuri, J., Smitha, B., & Sridhar, S. (2004). Dehydration of ethanol through blend membranes of chitosan and sodium alginate by pervaporation. Separation and Purification Technology, 40(3), 259–266.
Krasaekoopt, W., & Mabumrung, J. (2008). Microbiological evaluation of edible coated fresh-cut cantaloupe. Kasetsart Journal (Natural Sciences), 42(3), 552–557.
Kurek, M., Ščetar, M., Voilley, A., Galić, K., & Debeaufort, F. (2012). Barrier properties of chitosan coated polyethylene. Journal of Membrane Science, 403–404, 162–168.
Lester, G. (1997). Melon (Cucumis melo L.) fruit nutritional quality and health functionality. HortTechnology, 7(3), 222–227.
Luna-Guzmán, I., & Barrett, D. M. (2000). Comparison of calcium chloride and calcium lactate effectiveness in maintaining shelf stability and quality of fresh-cut cantaloupes. Postharvest Biology and Technology, 19(1), 61–72.
Medeiros, B. G., de Pinheiro, S. A. C., Carneiro-da-Cunha, M. G., & Vicente, A. A. (2012). Development and characterization of a nanomultilayer coating of pectin and chitosan. Evaluation of its gas barrier properties and application on ‘Tommy Atkins’ mangoes. Journal of Food Engineering, 110(3), 457–464.
Miller, M. D., & Bruening, M. L. (2005). Correlation of the swelling and permeability of polyelectrolyte multilayer films. Chemistry of Materials, 17(21), 5375–5381.
Olivas, G. I., Mattinson, D. S., & Barbosa-Cánovas, G. V. (2007). Alginate coatings for preservation of minimally processed ‘Gala’ apples. Postharvest Biology and Technology, 45(1), 89–96.
Oms-Oliu, G., Soliva-Fortuny, R., & Martín-Belloso, O. (2008). Using polysaccharide-based edible coating to enhance quality and antioxidant properties of fresh-cut melon. LWT—Food Science and Technology, 41(10), 1862–1870.
Pizato, S., Cortez-Vega, W. R., de Souza, J. T. A., Prentice-Hernandez, C., & Borges, C. D. (2013). Effects of different edible coatings on physical, chemical and microbiological characteristics of minimally processed peaches (Prunus persica L. Batsch). Journal of Food Safety, 33(1), 30–39.
Raybaudi-Massilia, R. M., Mosquelda-Melgar, J., & Martín-Belloso, O. (2008). Edible alginate-based coating as carrier of antimicrobials to improve shelf-life and safety of fresh-cut melons. International Journal of Food Microbiology, 121(3), 313–327.
Rhoades, J., & Roller, S. (2000). Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods. Applied and Environmental Microbiology, 66(1), 80–86.
Ribeiro, A. J., Silva, C., Ferreira, D., & Veiga, A. (2005). Chitosan-reinforced alginate microspheres obtained through the emulsification/internal gelation technique. European Journal of Pharmaceutical Sciences, 25(1), 31–40.
Rojas-Graü, M. A., Tapia, M. S., Rodríguez, F. J., Carmona, A. J., & Martín-Belloso, O. (2007). Alginate and gellan-based edible coatings as carriers of antibrowning agents applied on fresh-cut Fuji apples. Food Hydrocolloids, 21(1), 118–127.
Sangsuwan, J., Rattanapanone, N., & Rachtanapun, P. (2008). Effect of chitosan/methyl cellulose films on microbial and quality characteristics of fresh-cut cantaloupe and pineapple. Postharvest Biology and Technology, 49(3), 403–410.
SAS Institute (2003). JMP® 5.0.1 User’s Guide, SAS Institute, Inc., Cary NC, USA.
Serdyuk, I. N., Zaccai, N. R., & Zaccai, J. (2007). Methods in molecular biophysics: structure, dynamics, function. Cambridge: Cambridge University Press.
Simões, A. D. N., Ventrella, M. C., Moretti, C. L., Carnelossi, M. A., & Puschmann, R. (2010). Anatomical and physiological evidence of white blush on baby carrot surfaces. Postharvest Biology and Technology, 55(1), 45–52.
Sipahi, R. E., Castell-Perez, M. E., Moreira, R. G., Gomes, C., & Castillo, A. (2012). Improved multilayered antimicrobial alginate-based edible coating extends the shelf life of fresh-cut watermelon (Citrullus lanatus). LWT—Food Science and Technology, 47(1), 1–7.
Tapia, M. S., Rojas-Graü, M. A., Carmona, A., Rodriguez, F. J., Soliva-Fortuny, R., & Martin-Belloso, O. (2008). Use of alginate- and gellan-based coatings for improving barrier, texture and nutritional properties of fresh-cut papaya. Food Hydrocolloids, 22(8), 1493–1503.
Vázquez, E., Dewitt, D. M., Hammond, P. T., & Lynn, D. M. (2002). Construction of hydrolytically-degradable thin films via layer-by-layer deposition of degradable polyelectrolytes. Journal of the American Chemical Society, 124(47), 13992–13993.
Valencia-Chamorro, S. A., Palou, L., Delrío, M. A., & Pérez-Gago, M. B. (2011). Antimicrobial edible films and coatings for fresh and minimally processed fruits and vegetables. A review. Critical Reviews in Food Science and Nutrition, 51(9), 872–900.
Vargas, M., Chiralt, A., Albors, A., & González-Martínez, C. (2009). Effect of chitosan-based edible coatings applied by vacuum impregnation on quality preservation of fresh-cut carrot. Postharvest Biology and Technology, 51(2), 263–271.
Vargas, M., Pastor, C., Chiralt, A., McClements, D. J., & González-Martínez, C. (2008). Recent advances in edible coatings for fresh and minimally processed fruits. Critical Reviews in Food Science and Nutrition, 48(6), 496–511.
Acknowledgments
The research leading to these results has received funding from the Chief Scientist of the Israeli Ministry of Agriculture and Rural Development, Grant No. 421-0227-12 and European Union Seventh Framework Programme (FP7/2007/2013) under grant agreement n. 289719 (Project QUAFETY). We would like also thank Tatiana Yefremov for help in texture analysis. Contribution from the Agricultural Research Organization, The Volcani Center, Bet Dagan, Israel, No 646/13.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sources of Support
1. Chief Scientist of the Israeli Ministry of Agriculture and Rural Development, Grant No. 421-0227-12.
2. European Union Seventh Framework Programme (FP7/2007/2013) under grant agreement n. 289719 (Project QUAFETY).
Rights and permissions
About this article
Cite this article
Poverenov, E., Danino, S., Horev, B. et al. Layer-by-Layer Electrostatic Deposition of Edible Coating on Fresh Cut Melon Model: Anticipated and Unexpected Effects of Alginate–Chitosan Combination. Food Bioprocess Technol 7, 1424–1432 (2014). https://doi.org/10.1007/s11947-013-1134-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-013-1134-4