Abstract
There has recently been an increasing interest in seafood products due to the growing awareness of their nutraceutical value. However, marine-based products are highly susceptible to deterioration, mainly because of their high contents of polyunsaturated fatty acids (PUFAs), their high water activity, abundant free amino acids, neutral pH, and the presence of autolytic enzymes. In recent decades, various alternative methods have been developed to address this issue. Among the proposed solutions, chitosan has been highlighted as one of the most promising solutions. Chitosan, a deacetylated derivative of chitin, has attracted high consideration for its nontoxicity, biocompatibility, and biodegradability. Moreover, it is a polymer with versatile functional properties. For this reason, chitosan, which is commercially produced mostly from marine sources (e.g., crustacean shells), has been used to stabilize seafood-based products. In this review, chitosan is highlighted with respect to the various potential applications exploiting its many features, such as antibacterial and antioxidant properties, edible film- and coating-forming ability, the treatment of seafood industry effluent, enhanced gelling properties, micro- and nanocarrier abilities for bioactive compounds, functional foods, and drug compounds from aquaculture and seafood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because consumers are increasingly conscious of the relationship between diet and health, the consumption of marine-based foods has been growing continuously. Consumers identify seafoods as nutritious and complete foods. Hence, they are perceived as an excellent source of high-quality proteins and valuable lipids with high amounts of polyunsaturated fatty acids (PUFAs). These compounds are well known to contribute to the enhancement of human health by different mechanisms, such as reducing the risk of cardiovascular disease, coronary disease, and hypertension. Additionally, marine-based food products are easily digested and constitute an excellent source of essential minerals. Recently, seafoods have been recognized as nutraceuticals, or functional foods. Functional foods, first identified in Japan in 1980, are defined as foods demonstrating a beneficial effect on one or more targeted functions of the human organism (Ross 2000). Marine-based functional foods, or nutraceuticals, include omega-3 fatty acids, chitin and chitosan, fish protein hydrolysates, algal constituents, carotenoids, antioxidants, fish bone, shark cartilage, taurine, and bioactive compounds (Kadam and Prabhasankar 2010).
Despite the aforementioned desirable properties, seafood products are highly susceptible to quality deterioration, mainly due to lipid oxidative reactions, particularly involving PUFAs. These reactions are enhanced (catalyzed) by the presence of high concentrations of heme and nonheme proteins. These proteins are known to contain iron and other metal ions in their structures (Decker and Haultin 1992). Moreover, marine-based food quality is highly influenced by autolysis, bacterial contamination, and loss of protein functionality (Jeon et al. 2002). More recently, the contamination of seafood with various hazardous materials, such as refinery and industrial wastes and heavy metals, has resulted in elevated concern about the consumption of seafood (Kadam and Prabhasankar 2010). Additionally, the aquaculture industry has increasingly attracted much attention for the intensive farming of fish and shellfish, mainly due to the worldwide depletion of wild fish and shellfish stocks. However, this intensive farming entails several difficulties such as stress, which is the most important factor affecting the immune systems of fish (Ledger et al. 2002). To address the aforementioned problems, the use of chitosan as a protective material appears to be a potential alternative.
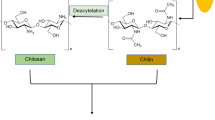
In nature, chitosan is found in the cell walls of fungi of the class Zygomycetes, in the green algae Chlorella, yeast and protozoa as well as insect cuticles and especially in the exoskeletons of crustaceans. Chitosan is a deacetylated derivative of chitin, the second most abundant polysaccharide in nature after cellulose. In 1811, the French scientist Henri Braconnot first discovered chitin in a mushroom. In 1820, chitin was isolated from insect cuticles (Bhatnagar and Sillanpa 2009). In 1859, Rouget reported finding chitosan after boiling chitin in potassium hydroxide. This treatment rendered the material soluble in organic acids. Hoppe-Seyler named it chitosan in 1894 (Khor 2001). Chemically, chitosan is a high-molecular weight, linear, polycationic heteropolysaccharide consisting of two monosaccharides, N-acetyl-glucosamine and d-glucosamine. They are linked by β-(1 → 4) glycosidic bonds. The relative amounts of these two monosaccharides in chitosan vary considerably, yielding chitosans of varying degrees of deacetylation ranging from 75% to 95%, molecular weights in the range of 50–2,000 kDa, different viscosities and pKa values (Tharanathan and Kittur 2003). In addition, chitosan has three functional moieties on its backbone: the amino group on the C2 and the primary and secondary hydroxyl groups on the C3 and C6 positions, respectively. These functional groups play important roles in the various functionalities of chitosan. The amino group is the most important among these moieties, especially in acidic conditions, due to the protonation phenomenon, rendering it able to interact with negatively charged molecules (or sites). Additionally, the chitosan polymer interacts with metal cations through its amino groups, hydroxyl ions, and coordination bonds.
Commercially, chitosan is produced from chitin by exhaustive alkaline deacetylation, involving boiling chitin in concentrated alkali for several hours. Because this N-deacetylation is almost never complete, chitosan is classified as a partially N-deacetylated derivative of chitin (Kumar 2000). From a practical point of view, many commercial interests and applications of chitosan and its derivatives originate from the fact that this polymer combines several features, such as biocompatibility, biodegradability, nontoxicity and bioadhesion, making it a valuable compound for pharmaceutical (Dias et al. 2008), cosmetics (Pittermann et al. 1997), medical (Carlson et al. 2008), food (Shahidi et al. 1999; No et al. 2007; Kumar 2000), textile (El Tahlawy et al. 2005), wastewater treatment (Chi and Cheng 2006), paper finishing, photographic paper (Kumar 2000), and agricultural applications (Hirano 1996).
Although there have been several prior reviews on the use of chitosan in food applications (No et al. 2007; Shahidi et al. 1999), the use of chitosan in seafood applications, especially its novel application in the form of nanocarriers for bioactive compounds for shelf life extension, has not yet been reported. Recently, a study was published on the use of chitosan nanoparticles for stability enhancement of vitamin C in a rainbow trout diet (Alishahi et al. 2011a, b). Hence, this review attempts to survey the applications of chitosan in various fields of marine-based products.
Antibacterial Activity
The modern era of chitosan research was heralded by publications in the 1990s that described the antimicrobial potentials of chitosan and its derivatives, exhibiting a wide spectrum of activities against human pathogens and foodborne microorganisms (Chen et al. 2010; No et al. 2002; Rabea et al. 2003; Raafat et al. 2008; Raafat and Sahl 2009). The first study reporting antibacterial properties was by Allan and Hardwiger (1979). They reported that chitosan showed a broad range of activities and a high inactivation rate against both Gram-positive and Gram-negative bacteria (Allan and Hardwiger 1979). However, although several studies have been published in this area, the exact mechanism of the antimicrobial activity of chitosan remains ambiguous.
Six major mechanisms have been proposed in the literature, as follows (Kong et al. 2010; Raafat and Sahl 2009; Raafat et al. 2008): (1) interactions between the positively charged chitosan amine groups and the negatively charged microbial cell membranes, leading to the leakage of proteinaceous and other intracellular constituents; (2) the activation of several defense processes in the host tissue by the chitosan molecule, acting as a water-binding agent and inhibiting various enzymes by blocking their active centers; (3) the action of chitosan as a chelating agent, selectively binding metals and then inhibiting the production of toxins and microbial growth; (4) the formation, generally by high-molecular weight chitosan, of an impervious polymeric layer on the surface of the cell, thereby altering cell permeability and blocking the entry of nutrients into the cell; (5) the penetration of mainly low-molecular weight chitosan into the cytosol of the microorganism to bind DNA, resulting in interference with the synthesis of mRNA and proteins; and (6) the adsorption and flocculation of electronegative substances in the cell by chitosan, disturbing the physiological activities of the microorganisms, causing their death. However, it is very important to mention that chitosan is soluble only in acidic media and therefore, the effect of pH on microorganisms must be considered together with the effect of chitosan. Thus, the synergetic effect of the chitosan/pH together is probably the most evident explanation of the antimicrobial effect of chitosan.
Complicating the issue, a number of studies aimed at determining the antibacterial activities of chitosan on Gram-positive and Gram-negative bacteria have reported antithetical outcomes, making their interpretation difficult. More recently, Kong et al. (2010) showed that chitosan and its derivatives are more powerful antibacterial agents against Gram-negative bacteria than against Gram-positive microorganisms. Conversely, Raafat and Sahl (2009) reported a study in which they demonstrated that Gram-positive bacteria are more sensitive to the antibacterial effect of chitosan than Gram-negative bacteria. Therefore, the interpretation of the sensitivity of bacteria to chitosan is quite difficult.
To address this problem, Kong et al. (2010) proposed that the variation in the bactericidal efficacy of chitosan arises from different parameters that must be considered when evaluating the antibacterial activity of chitosan. These factors can be categorized into four classes, as follows: (1) intrinsic microbial factors, including microbial species and cell age; (2) intrinsic factors of chitosan molecules, namely, the positive charge density, protonation level of the amine group, chitosan molecular weight and concentration, and the hydrophilic/hydrophobic characteristics and chelating capacity of the chitosan molecule; (3) its physical state, i.e., either water-soluble or solid chitosan; and (4) environmental factors including the ionic strength of the testing medium, pH, temperature, and contact time between chitosan and bacterial cells. In addition, it is worth noting that, despite the widely reported antimicrobial properties of chitosan in the literature, the results are mainly based on in vitro experiments. In real-world applications, it is important to consider that most foods are complex matrices composed of different compounds (proteins, carbohydrates, lipids, minerals, vitamins, salts, and others) and many of them may interact with chitosan to varying levels, possibly leading to a loss or enhancement of its antibacterial activity (Devlieghere et al. 2004).
Taking into account the above insights about the antibacterial characteristics of chitosan, the following applications of chitosan in seafood products were considered. Due to the high perishability characteristics of marine-based products, there has been an increased interest in the application of chitosan to extend the shelf life of these products. In this context, chitosan has increasingly gained attention as an antibacterial additive in seafood from both seafood processors and consumers, largely due to a desire to reduce the use of synthetic chemicals in seafood preservation. Cao et al. (2009) reported that chitosan at 5 g/L extended the shelf life of oyster (Crassostrea gigas) from 8 to 9 days to 14–15 days (Table 1). They explained that Pseudomonas and Shewanella are the most prolific microorganisms during cold storage of fish and shellfish, and these bacteria can easily be reduced or eliminated with the addition of chitosan at this concentration.
Fish balls have a high water activity and are prone to the growth of microorganisms, with a relatively short shelf life of 4–5 days at a storage temperature of approximately 5 °C. Kok and Park (2007) reported that fish ball shelf life was increased by adding chitosan, which maintained both aerobic and yeast counts at <1 log CFU/g over 21 days of storage. Kok and Park (2007) also reported that the physical state of chitosan is an important parameter in its antibacterial activity. In the dissolved state, chitosan showed excellent antibacterial activity and contributed to the extension of product shelf life. However, in a study by Lopez-Caballero et al. (2005a and b), it was demonstrated that the addition of powdered chitosan to fish patties had no effect on bacterial growth. Roller and Corvill (2000) reported that spoilage flora were reduced from log 8 CFU/g in the control sample (without chitosan) to log 4 CFU/g throughout a 4-week study at 5 °C with the use of chitosan combined with acetic acid in shrimp salad. However, it is important to consider the effect of acetic acid on the ability of chitosan to extend shelf life. Fernandez-Saiz et al. (2010) studied bacterial growth in two conditions. The first one was a fish soup (ANETO® brand, packaged in TetraBrik® and fabricated by Jamon Aneto, S. L., Barcelona, Spain). The other medium was a model laboratory growth medium, tryptone soy broth. They reported a significant reduction of the growth of Listeria monocytogenes, Staphylococcus aureus, and Salmonella when the products were stored in the presence of chitosan. In fish soup and under laboratory conditions, the effect of chitosan in the tested medium at 4, 12, and 37 °C depended significantly on the type of bacteria, incubation temperature, and food matrix (substrate). The antibacterial effectiveness of chitosan was decreased in the fish soup, suggesting that the constituents of the fish soup had a great influence on the antimicrobial efficacy of chitosan. The authors reported that chitosan was probably irreversibly bound by microbial cells or a negatively charged compound in the soup and therefore rendered it inactive against the remaining unbound microorganisms. In conclusion, the effect of the chitosan-to-cell ratio must be considered. Lopez-Caballero et al. (2005a, b) showed that the addition of chitosan to sausage treated at high pressure yielded a 2-log cycle decrease of total bacterial counts of Pseudomonas and Enterobacteria at 8–11 days storage. Ye et al. (2008) stated that chitosan has an antibacterial activity that is effectively expressed in aqueous systems; however, its antibacterial properties against L. monocytogenes in cold-smoked salmon were negligible when chitosan was in the form of an insoluble film. The growth of L. monocytogenes in salmon samples wrapped in chitosan-coated film and plain films was similar. The authors demonstrated that it is possible that chitosan is ineffective in films because it is unable to diffuse through a rigid food matrix such as salmon. Regarding microbial counts, Lopez-Caballero et al. (2005b) showed that chitosan in the soluble state had no significant effect on high pressure-treated cod sausages. However, when chitosan was added in a dry form, higher counts of microorganisms were recorded. This is an indication that chitosan in soluble form contributed to maintaining product safety. The microbial counts in their study were for lactic acid bacteria, Enterobacteria, Pseudomonas, and Staphylococcus. Duan et al. (2010) reported that the initial total plate count (TPC) of fresh lingcod was 3.67 log CFU/g, which then rapidly increased to 6.16 and 8.36 log CFU/g on days 6 and 14, respectively. When chitosan coatings were used, the results showed a 0.15–0.64 reduction in TPC. Moreover, the TPCs of chitosan-coated samples stored under vacuum or modified-atmosphere packaging were significantly lower than those of the control sample during the subsequent cold storage. The combination of a chitosan coating and vacuum or modified-atmosphere packaging resulted in 2.22–4.25 reductions in TPC for the first 14 days of cold storage. The TPCs of chitosan-coated and MPA samples were lower than 105 CFU/g even after 21 days of cold storage. This result indicated a significant delay of microbial spoilage. Qi et al. (2010) reported that because nonfermenting Gram-negative bacteria are dominant in the initial microbial flora of fish and shellfish sourced from cold seawater, controlling the growth of these Gram-negative bacteria may be important for the preservation of oysters. They demonstrated that combined treatments with chitosan and ozonated water had a better antibacterial effect than either treatment alone. When only aerobic plate count was measured, the authors showed that the product shelf life with the combination of chitosan with ozonated water was at least 20 days, whereas it was only 8 days for the control sample, 10 days for the ozonated samples, and 14 days for the chitosan-treated samples.
The antifungal effect of chitosan was also tested in several experimental works with baker’s yeast (Saccharomyces cerevisiae). The results showed that fermentation was inhibited by a chitosan concentration as low as 3.6 mg/L in a buffer system. Similar activity was shown against the mold Fusarium solani, the growth of which was prevented by a chitosan concentration of 4 mg/L in a model liquid nutrient medium. Variations in sensitivity between closely related microorganisms were illustrated in an experiment in which phytopathogenic fungi were screened for sensitivity to chitosan in liquid media. A Cytosporina sp. isolate was completely inhibited by the addition of 75 mg/L of chitosan to the medium, whereas other Cytosporina spp. isolates were insensitive to chitosan concentrations up to 1,000 mg/L.
Although the mechanism of the antibacterial effect of chitosan is still unclear, the most widely accepted theory on chitosan antibacterial activity is based on the fact that positively charged chitosan can interact with the negatively charged bacterial membrane, which may cause the leaching out of low-molecular weight materials, nucleic acids, and proteins. Other vital molecules can also be affected by the action of chitosan on the bacterial cell membrane. The amounts of nucleic acids and proteins leaching out from the bacterial cells were estimated by measuring absorption (optical density) at 260 and 280 nm. The intensity of the absorption depended on chitosan concentration and incubation time. Most of these studies reported that the antibacterial effect of chitosan is due to the disruption of the cell membrane (Fang et al. 2010). Here, the charge density on the cell surface is a determining factor in establishing the amount of chitosan adsorbed. More adsorbed chitosan would evidently result in greater changes in the structure and permeability of the cell membrane. While the data from the literature are inconclusive as to whether chitosan has higher activity on Gram-positive or Gram-negative bacteria, it has been shown that hydrophilicity is significantly higher in Gram-negative than in Gram-positive bacteria, making them more sensitive to chitosan. Moreover, the cell membrane of Gram-negative bacteria consists essentially of lipopolysaccharides, resulting in high negative charge density in comparison with the Gram-positive bacteria. All these considerations agree with evidence that the leakage of intracellular material observed when chitosan interacts with Gram-negative bacteria is greater than that reported in the case of Gram-positive bacteria. Contradictory information has also been reported; it has been stated that chitosan has stronger effects against Gram-positive than Gram-negative bacteria. This would suggest that the antibacterial mode of action is dependent on the host bacteria, type of chitosan, and experimental conditions (Helander et al. 2001).
Antioxidant Activity
Seafood is considered an excellent source of functional foods for balanced nutrition favorable for promoting good health. The beneficial health effects of marine foods are ascribed to their lipids, mainly the long-chain omega-3 PUFAs, such as eicosapentaenoic acid and docosahexaenoic acid (Newton 2001). However, these valuable compounds in seafood are highly sensitive to oxidative reactions and the development of off-flavors, even during cold storage (Cadwallader and Shahidi 2001). It has been proposed that lipid oxidation in fish and shellfish may be initiated and propagated by a number of mechanisms, namely auto-oxidation, photosensitized oxidation, lipoxygenase, peroxidase, and microsomal enzymes (Hsieh and Kinsella 1989). Additionally, fish and shellfish muscles contain protein-bound iron compounds, for example, myoglobin, hemoglobin, ferritin, transferrin and hemosiderin, as well as other metals. This is a factor that plays an important role in initiating lipid oxidation in marine-based products (Decker and Haultin 1992). Castell et al. (1965) showed that the relative pro-oxidant activity of metal ions in fish and shellfish muscles decreased in the order Cu+2 > Fe+2 > Cd+2 > Li+2 > Mg+2 > Zn+2 > Ca+2 > Ba+2. The iron-bound proteins and other metal ions present in seafood may be released during the storage period and can thus activate and/or initiate lipid oxidative reactions (Decker and Haultin 1992).
Along with the growing consumer demand for seafood devoid of synthetic antioxidants, chitosan has been a booming antioxidant agent in fish and shellfish (Chiang et al. 2000). The antioxidant activity of chitosans of different viscosities (360, 50, and 14 cP) in the cooked, comminuted flesh of herring (Clupea harengus) was investigated (Kamil et al. 2002). The oxidative stability of fish flesh during cold storage at 4 °C with the addition of chitosan at concentrations of 50, 100, and 200 ppm was compared with that of fish treated with conventional antioxidants, such as butylated hydroxyanisole, butylated hydroxytoluene, and tert-butylhydroquinone (all at 200 ppm). Among the three chitosan samples tested, 14 cP chitosan was the most effective in preventing lipid oxidation. The formation of thiobarbituric acid reactive substances (TBARS) in herring samples containing 200 ppm of 14 cP chitosan was reduced by 52% after 8 days of storage compared with the control sample without chitosan. At a chitosan concentration of 200 ppm, the 14 cP chitosan exerted an antioxidant effect similar to that of commercial antioxidants in reducing TBARS values in comminuted herring flesh. A study by Kamil et al. (2002) indicated that the antioxidant capacity of chitosan added to fish muscle depends on its molecular weight (MW) and concentration in the product. Similarly, Kim and Thomas (2007) observed that the antioxidant effects of chitosan in salmon depended on its molecular weight (tested at MW = 30, 90, and 120 kDa) and concentration (evaluated at 0.2%, 0.5%, and 1% w/w). The authors reported that the 30 kDa chitosan showed the highest radical-scavenging activity. The scavenging activities of chitosan were increased by increasing its concentration. However, varying the concentration showed no significant effects when 120 kDa chitosan was used. Ahn and Lee (1992) studied the preservative effect of chitosan film on the quality of highly salted and dried horse mackerel. The product was prepared by soaking the fresh horse mackerel in 15% salt solution for 30 min, coating with or without (control) chitosan and drying for 3 h at 40 °C in a hot air dryer. During cold storage at a temperature of 5 °C for 20 days, the chitosan-coated samples had lower TBARS and peroxide values (PV) than the control samples. Similarly, Lopez-Caballero et al. (2005a, b) also found that coating codfish patties with a chitosan–gelatin blend considerably lowered lipid oxidation. However, being insoluble in powder form at neutral pH, chitosan had no effect on lipid oxidation. Shahidi et al. (2002) reported that chitosans with different molecular weights and viscosities (14, 57, and 360 cP) were effective in controlling the oxidation of lipids in comminuted cod (Gadus morhua) following cooking. Both PV and TBARS values were reduced as a result of treatment of the fish prior to cooking with 50, 100, or 200 ppm of 14, 57, and 360 cP chitosans. Inhibition of the oxidation was concentration-dependent and was the highest for the 14 cP chitosan. The authors stated that the antioxidant activity of chitosans of different viscosities in cooked comminuted cod may be attributed to their metal-chelating capacities. Chitosans with different viscosities were found to protect cooked cod from oxidation at various levels. The observed differences were presumably due to the differing degrees of deacetylation and molecular weights of the chitosan molecules. In the study reported by Qin (1993), it has been indicated that the ion-chelating ability of chitosan is strongly affected by its degree of deacetylation. Highly acetylated chitosan has very little chelating activity. In addition, high-molecular weight chitosan has a compact structure and the effect of intramolecular hydrogen bonding is stronger, which weakens the activities of the hydroxyl and amino groups. As result, the probability of the exposure of these active moieties may be restricted, resulting in less radical scavenging activity. Obviously, low-molecular weight chitosan exhibits higher hydroxyl radical scavenging activity, which is partially attributable to its metal-chelating ability. The Fe+2-chelating ability of chitosan is mainly attributed to the presence of amino groups, which contain free electron pairs that contribute to the formation of a chitosan/Fe+2 complex. The Fe+2-chelating ability of low-molecular weight chitosan is more pronounced than that of high-molecular weight chitosan. Consequently, the amino groups in chitosan can react with free radicals to form additional stable macroradicals. Therefore, active hydroxyl and amino groups in the polymer chains are the origin of the scavenging ability of chitosan (Jeon et al. 2000; Feng et al. 2008; No et al. 2007). Kamil et al. (2002) explained that in the charged state (protonated amino groups), the cationic groups of chitosans impart intramolecular electrical repulsive forces. This phenomenon increases the polymer’s hydrodynamic volume by extending the chitosan chain conformation. This phenomenon may be responsible for the reduced chelating ability of high viscosity (high MW) chitosans. Jeon et al. (2002) demonstrated that the antioxidant activity of chitosan is also effective when it is applied as a protective film. In this kind of application, it retards lipid oxidation by acting as a barrier against oxygen penetration. Sathivel et al. (2007) showed that the TBARS value of coated pink salmon (Oncorhynchus gorbuscha) fillets glazed with chitosan (1.3 mg MDA/kg sample) was significantly lower than that of fillets glazed with lactic acid (3 mg MDA/kg sample) or distilled water (1.8 mg MDA/kg sample). The results indicated that chitosan glazing was the most effective at reducing lipid oxidation among the studied alternatives. Sathivel (2005) also reported that the TBARS values of pink salmon fillets coated with 1% and 2% chitosan were significantly lower than in both the control sample and a protein-coated product after 3 months of frozen storage. The author stated that a higher concentration of chitosan resulted in a lower TBARS value. The latter implies that the antioxidant effect of chitosan in the coating state is highly correlated with coating thickness, thereby hindering the entrance of oxygen into the salmon fillets and slowing the oxidative process. Moreover, the primary amino groups of chitosan would form a stable fluorosphere with volatile aldehydes such as malondialdehyde, which are derived from the breakdown of fats during lipid oxidation (Weist and Karel 1992).
Duan et al. (2009, 2010) showed that the combination of chitosan with modified-atmosphere packaging enhanced the lipid stability of lingcod (Ophiodon elongates) for 21 days of cold storage. When applied on the surface of lingcod fillets, chitosan coatings may act as a barrier between the fillet and the surrounding atmosphere. This is mainly due to the good oxygen barrier properties of chitosan films, which slow the diffusion of oxygen from the surrounding air to the surface of fillet and retard lipid oxidation (Aider 2010). Additionally, Ojagh et al. (2010) reported that chitosan coatings enriched with cinnamon oil could suitably delay lipid oxidation in refrigerated rainbow trout over 16 days of storage and markedly reduced the TBARS and PV values compared with the control product. Mao and Wu (2007) showed that lipid oxidation in kamabako gel prepared from grass carp (Ctenopharyngodon idellus) significantly decreased when a 1% chitosan solution was added.
Bioactive Coatings
Modern marine-related food industries are encountering new challenges and require specific alternatives to surmount them. Among these, issues related to seafood packaging for products with a short shelf life are of pivotal importance. Although the utilization of conventional packaging materials, such as plastics and their derivatives, are effective for seafood preservation, they create serious and hazardous environmental problems, a situation which presents the seafood industry as a source of pollution and social concern. This problem requires that all stakeholders in this industry and especially scientists specializing in food engineering and packaging seek alternatives to address this serious problem related to packaging materials. As the total cost of the final product is a non-negligible aspect, the cost of the packaging materials must also be considered because it is well known that the contribution of the packaging to the total product cost is highly significant. Thus, the search for more economical packaging materials is a very important subject in the seafood industry (Aider 2010).
Edible biopolymer-based films have been investigated for their abilities to avoid moisture or water absorption by the seafood matrix, reduce oxygen penetration into the food matrix, and prevent aroma loss and solute transport out of the product (Campos et al. 2011; Swapna Joseph et al. 2011; Dutta et al. 2009). Based on these considerations, one of the most promising active biofilms is one based on chitosan. More recently, two review studies have reported the application of chitosan as a bioactive film for use in the food industry (Aider 2010; Dutta et al. 2009). Chitosan films, like many other polysaccharide-based films, tend to exhibit resistance to fat diffusion and selective gas permeability. However, they suffer a serious shortcoming in terms of resistance to the transmission of water and water vapor. This behavior is mainly due to the strongly hydrophilic character of these biopolymers, a property that leads to high interaction with water molecules (Bordenave et al. 2007). For this reason, polymer blending or the use of biocomposites and multilayer systems are potential approaches to prepare chitosan-based bioactive coatings or films with desirable characteristics.
In this context, Ye et al. (2008) suggested that because edible films formed of chitosan are brittle and do not have good mechanical properties, coating chitosan onto a plastic film could overcome these problems. These authors used chitosan-coated plastic films into which they incorporated five antibacterial agents, namely, nisin, sodium lactate (SL), sodium diacetate, potassium sorbate (PS), and sodium benzoate, to create a novel antibacterial edible film active against L. monocytogenes for use on cold-smoked salmon. This approach the solved problems related to food safety because it is well known that L. monocytogenes can grow to high levels on cold-smoked salmon, even at typical refrigeration temperatures. The risk related to L. monocytogenes is particularly high at abusive storage temperatures. Chitosan-coated films containing 4.5 mg/cm2 SL, 4.5 mg/cm2 SL–0.6 mg/cm2 PS, and 2.3 mg/cm2 SL-500 IU/cm2 nisin were the most effective treatments against L. monocytogenes at ambient temperature. These treatments showed long-term antilisterial efficacy during refrigerated storage on vacuum-packed cold-smoked salmon. However, it is important to consider the fact that the antibacterial activity of chitosan may be negligible when it is in the form of insoluble films. In this state, chitosan is ineffective because it is unable to diffuse through a rigid food matrix, such as salmon.
Sathivel et al. (2007) showed that skinless pink salmon (O. gorbuscha) fillets glazed with chitosan at a solution concentration of 1% (w/w) had significantly (p < 0.05) higher yield and thaw yield than lactic acid- and distilled-water-glazed fillets; furthermore, these fillets all had similar moisture contents after thawing. In addition, a rheological characterization showed that chitosan has pseudoplastic and viscoelastic characteristics. The glass transition temperature for the chitosan film was observed at 80.23 °C. The oxygen, carbon dioxide, nitrogen, and water vapor permeabilities of the chitosan film were 5.34 × 10−2 cm3/m day atm, 0.17 cm3/m day atm, 0.03 cm3/m day atm, and 2.92 × 10−10 g water m/m2 Pas, respectively. The authors demonstrated that despite the good barrier properties of chitosan against oxygen, it maintained a low water vapor transmission due to its hydrophilic nature. Likewise, they stated that chitosan films showed shear thinning, viscoelastic characteristics and temperature-dependent viscosity, which allowed for uniform glazing of the salmon fillets and prevented the rupturing of the chitosan glazing during solidification when the glazed fillets were frozen. Therefore, the chitosan glazing applied to the surface of the pink salmon fillets may have acted as a barrier between the fillets and the surrounding air, thus slowing down the diffusion of oxygen into the fillets.
Kester and Fennema (1986) reported that chitosan coatings may act as moisture-sacrificing agents in moisture-maintenance barriers. Thus, moisture loss from the product may be delayed as the moisture contained within the chitosan coating is evaporated. Sathivel (2005) noted that coating pink salmon fillets with chitosan resulted in a significantly higher yield, thaw yield, a similar drip loss and cook yield, higher moisture content after thawing, less moisture loss than the control samples and somewhat less than with protein coatings. Additionally, there were no significant (p < 0.05) effects of the coating on color parameters (the a*, b* and L* values) for cooked fillets after 3 months of frozen storage.
Lopez-Caballero et al. (2005a, b) used chitosan as a base material to form a chitosan–gelatin coating for cod patties. They showed that the use of chitosan either as a coating or a powdered ingredient did not affect the product lightness at the end of the storage period. However, it resulted in an increase of the product yellowness (the b* color parameter). The chitosan coating increased the patty elasticity, whereas the addition of powdered chitosan to the patty mixture increased the other rheological parameters such as gumminess, chewiness, cohesiveness, and adhesiveness. Moreover, the coating prevented the spoilage of cod patties, as reflected by a decrease in total volatile basic nitrogen (TVBN). Conversely, none of these effects on the bacterial spoilage were observed when the chitosan was added to the patty mixture in a powdered form. Ultimately, the authors reported that the coating had good sensory properties and that it melted away on cooking and hence did not impart any taste to the product. The coating provided protection by delaying spoilage. Duan et al. (2010) produced a chitosan–krill oil coating and used it in modified-atmosphere packaging to extend the shelf life of lingcod fillets. They reported that this coating approximately doubled the total lipid and omega-3 fatty acid contents of the lingcod. The reduced chemical changes were reflected by the TVBN values and the coating did not change the color of the fresh fillets nor affect consumer acceptance of either the raw or cooked lingcod fillets. Consumers preferred the overall quality of chitosan-coated, cooked lingcod fillets over the control based on the product’s firm texture and less fishy aroma and flavor. Considering the lower cost of vacuum packaging, it could be applied in combination with chitosan coatings to maintain the omega-3 fatty acid content and extend shelf life of fresh lean fish such as lingcod. Duan et al. (2009) also showed that fish oil incorporated into a chitosan coating decreased the drip loss of frozen samples by 14.1–27.6%. This coating also well protected the omega-3 fatty acids in lean fish. Cao et al. (2009) and Qi et al. (2010) showed that a chitosan coating can remarkably increase the shelf life of highly perishable pacific oyster (C. gigas) during 21 days of storage. This evaluation was based on the TVBN and pH values and a sensory evaluation of the product. The authors stated that the discrepancies between their results and others were derived from the differences in chemical composition of fish and shellfish; oyster contains significant levels of carbohydrate (glycogen) and a lower total quantity of nitrogen. Ojagh et al. (2010) synthesized chitosan coatings enriched with cinnamon oil to extend the shelf life of refrigerated rainbow trout and showed that the sensory characteristics and TVBN of the end product after 16 days of cold storage were drastically improved when the coating was employed on rainbow trout fillets. Similarly, Lopez-Caballero et al. (2005a, b) stated that the addition of dry chitosan led to a noticeable increase in elasticity and product yellowness when cod sausages were enriched with a chitosan solution. The TVBN remained stable for 25 days of storage, and the product elasticity was reinforced. Duan et al. (2009) reported a study indicating that incorporation of fish oil into chitosan coatings significantly lowered the total and psychrotrophic counts in frozen lingcod fillets over 3 months of cold storage. Ojagh et al. (2010) also reported that chitosan coatings enriched with cinnamon oil effectively decreased total viable counts and psychrotrophic bacteria in rainbow trout (Oncorhynchus mykiss) during 16 days of cold storage.
Effluent Treatment
The use of chitosan as a coagulating agent has been widely investigated for the removal of suspended solids from various processing streams, including cheese whey and dairy wash water and effluents from the processing of poultry and seafood products (Kumar 2000; Savant 2001; Savant and Torres 2000, 2003; Shahidi et al. 1999). Chitosan at a concentration of 10 mg/L reduced the amount of the total suspended solids in shrimp processing wastewater by up to 98% (Bough 1976). Protein recoveries from surimi wash water (SWW) using a 150 mg/L chitosan–alginate complex at a mixing ratio of 0.2 l per liter of SWW resulted in 78–94% protein adsorption after 24 h (Wibowo 2003). The following two chitosan samples were used: a commercial chitosan with a degree of deacetylation of 84% and a viscosity of 2,400 cP, obtained from Vanson Chemicals (Redmond, WA, USA) used to prepare 1% stock solutions in 1 M acetic acid and an experimental chitosan prepared in the laboratory by the author.
Chitosan is a biopolymer that can be used for the preparation of various polyelectrolyte complex products with natural polyanions such as alginate, a polymer with a negative electric charge. The strong electrostatic interaction of the amine groups of chitosan with the carboxyl groups of alginate lead to the formation of a chitosan–alginate complex. Chitosan–alginate gel beads with a chitosan core and a chitosan–alginate skin can be prepared by dropping a solution of chitosan into an alginate solution; conversely, chitosan–alginate gel beads with an alginate core and a chitosan–alginate skin are prepared by dropping a solution of alginate into chitosan solution. Due to the protonation of the amino groups on chitosan and the ionization of carboxylic acid groups on alginate, the stability of chitosan–alginate complexes may be influenced by environmental parameters, such as pH and ionic strength (Mi et al. 2002). This result was higher than the one obtained with 50 mg/L, which yielded 81–90% protein adsorption in the same treatment time (Savant 2001). These reported findings suggest that reaction time and chitosan concentration play an important role in reducing total suspended solids and lowering solution turbidity. Moreover, differences in MW and the degree of deacetylation (DD) among chitosan samples could explain the significant differences observed in protein recovery capacity. At the lowest concentration (20 mg/L SWW) tested in the study by Wibowo (2003), the experimental chitosan yielded higher protein recovery than the commercial sample, which required a fivefold higher concentration for the same effectiveness.
This finding has commercial implications, as this method would reduce processing costs and the chitosan content in the solids recovered by such treatment (Wibowo et al. 2007). If implemented commercially, the use of chitosan–alginate complexes may be an effective alternative not only for the recovery of soluble proteins that would otherwise be discarded into the environment but also as an economically viable downstream effluent treatment process that would be preferable to expensive, commercial membrane treatments, which are of limited use due to fouling problems (Savant 2001). In a subsequent study, surimi wash water protein (SWWP) was precipitated using a chitosan–alginate complex. The precipitate had a crude protein content of 73.1% and a high concentration of essential amino acids (3% histidine, 9.4% lysine, 3.7% methionine, and 5.1% phenylalanine). In a rat-feeding trial, SWWP used as a single protein source yielded higher modified protein efficiency and net protein rations than the casein control. Blood chemistry analysis revealed no deleterious effects from the full protein substitution or the chitosan in SWWP (Wibowo et al. 2005, 2007). Moreover, Guerrero et al. (1998) showed that the utilization of chitosan at a concentration of 10 mg/L and pH 7 in the treatment of fish-meal factory effluent using a coagulation–flocculation process followed by centrifugation decreased the total suspended solids up to 85%. The effectiveness of chitosan in the treatment of seafood plant effluents was mainly attributed to its positive charge and the resulting interactions with negatively charged compounds in the effluents such as protein. Furthermore, the hydroxyl groups on the chitosan molecule act to increase the precipitation of proteins and other suspended solids from these effluents (Savant 2001; Wibowo et al. 2007a, b).
Gelling Enhancer
Surimi is a refined fish protein product prepared by washing mechanically deboned fish to remove blood, lipids, enzymes, and sarcoplasmic protein. The myofibrillar proteins are concentrated in the resulting product and form an elastic gel when solubilized with NaCl and heated (Mao and Wu 2007). The gel-forming properties of the myofibrillar proteins are quickly lost due to degradation by the action of endogenous proteolytic enzymes if the fish is not processed into surimi immediately. The utilization of frozen fish flesh for surimi production is unsuitable due to the rapid loss of protein functionality by freezing denaturation. High-quality surimi is produced from fresh, unfrozen fish. Thus, processing at sea has been required to obtain high-quality surimi. However, the cost of such processing is much higher than land-based processing (Lanier et al. 1992). To prepare a strong and elastic gel from fish species with low commercial value, low-quality surimi is produced onshore with the aid of gel-forming biopolymers such as starch. Chitosan is thus a good option for incorporation into these products to improve their technofunctional quality (Kataoka et al. 1998; Mao and Wu 2007; Li and Xia 2010).
The gel-forming ability of surimi depends on both intrinsic and extrinsic factors, namely, fish species, the physicochemical properties of muscle proteins, the presence of endogenous enzymes, such as proteinase and transglutaminase, and the processing conditions used (Benjakul et al. 2003). The strength of the gels prepared from low-quality walleye Pollock (Theragra chalcogramma) was almost doubled by the addition of 1.5% chitosan when salted surimi pastes were set below 25 °C. The polymerization of the myosin heavy chain was accelerated in the presence of 1.5% chitosan (Kataoka et al. 1998). Along with chitosan, endogenous transglutaminase (TGase) plays an important role in gel formation. The addition of a TGase inhibitor to salted walleye Pollock surimi inhibited gel enhancement by chitosan. The mechanism of chitosan’s effect on enhancing gel formation is unclear. However, the participation of hydrophobic interactions, hydrogen bonding, and electrostatic interactions during the setting process have been proposed as possible mechanisms by which chitosan can enhance the formation of cross-linked myosin heavy chain components during their polymerization by endogenous enzymes (Benjakul et al. 2003: Kataoka et al. 1998; Li and Xia 2010; Mao and Wu 2007). Benjakul et al. (2003) reported that barred garfish (Hemiramphus far) surimi gel showed an increase in breaking force when 1% chitosan was added. However, the gel-forming ability of surimi containing chitosan was inhibited in the presence of EDTA due to the chelating of calcium ions that are necessary for TGase activity. Therefore, the enhancing effect of chitosan was possibly mediated by the action of endogenous TGase during product processing, resulting in the formation of protein–protein and protein–chitosan conjugates. In conjunction with processing and the addition of calcium ions, TGase may play an important role in the cross-linking of protein–protein and protein–chitosan conjugates via the amino groups of chitosan acting as acyl acceptors.
The addition of chitosan did not substantially modify the rheological and microstructural properties of horse mackerel gels (Trachurus spp.). Chitosan addition resulted in a slight reduction in gel elasticity obtained under high-pressure conditions (Gomez-Guillen et al. 2005). Kok and Park (2007) stated that in threadfin bream (Nemipterus spp.) surimi, the balance of protein–chitosan and protein–protein conjugates determined the surimi gel strength. Similarly, Mao and Wu (2007) showed that in the presence of chitosan in a kamaboko gel composed of grass carp (C. idellus), protein–chitosan conjugates were formed between the reactive amino groups of glucosamine and the glutaminyl residue of the myofibrillar proteins. The bonds between chitosan and myofibrillar proteins were associated with the improvement of texture properties in the gels, with the final structure formed by both covalent and noncovalent interactions. The textural effect was also due to modification of the endogenous TGase activity.
More recently, Li and Xia (2010) showed that molecular weight and DD of chitosan have different impacts on the gel properties of salt-soluble meat proteins derived from silver carp. A gel containing chitosan with a DD of 77.3% showed the highest penetration force and storage modulus. The penetration forces of the tested gels increased with increasing molecular weight of the chitosan incorporated in the gel. The interaction between chitosan and salt-soluble meat proteins was mainly stabilized by electrostatic interactions and hydrogen bonds.
Encapsulation
Currently, the value of functional foods and bioactive compounds is increasing due to consumer awareness and consciousness. However, many of these compounds are very sensitive to environmental factors, such as oxygen, light, and temperature. In addition, when incorporated into foods and drug delivery systems, these bioactive components are often hydrolyzed by harsh conditions in the gastrointestinal tract (Alishahi et al. 2011a, b). Schep et al. (1999) stated that many oral delivery systems for bioactive aquaculture compounds meet three major barriers in passing through the gastrointestinal tract, namely enzymatic barriers from the host luminal and membrane bound enzymes, immunological cells present within both the enterocytes and underlying connective tissue and the physical barrier of the epithelial cells.
Based on these considerations, the encapsulation of bioactive compounds and functional foods could be a promising way to overcome these problems. Encapsulation is a process in which thin films, generally consisting of polymeric materials, are applied to small solid particles, liquids, or gas droplets (Figs. 1 and 2). This method is used to entrap active components and release them under controlled conditions (Deladino et al. 2008). Numerous materials are encapsulated for use in the food industry such as vitamins, minerals, antioxidants, colorants, enzymes, and sweeteners (Shahidi and Han 1993).
SEM micrographs of the surface of chitosan and chitosan–gelatin blend films at different concentrations; a chitosan, b chitosan and gelatin (1%), c chitosan and gelatin (2%), and d chitosan and gelatin (4%). Adapted from Vargas et al. (2009) with permission
Chitosan can act as an encapsulating agent due to its nontoxicity, biocompatibility, mucus adhesiveness, and biodegradability (Alishahi et al. 2011a, b: Kumar 2000). Recently, Alishahi et al. (2011a, b) showed that a chitosan/vitamin C nanoparticle system successfully increased the shelf life and delivery of vitamin C in rainbow trout during 20 days of storage (Fig. 3). The authors also showed that the shelf life of vitamin C was significantly (p < 0.05) increased in rainbow trout feed for 20 days at ambient temperature, whereas the control, which was fed vitamin C alone, lost significant vitamin C content in a few days at ambient temperature. Moreover, the controlled release behavior of vitamin C, both in vitro and in vivo, showed that vitamin C was released in the gastrointestinal tract of rainbow trout in a controlled manner (up to 48 h) and that chitosan nanoparticles protected vitamin C from the harsh conditions of acidic and enzymatic hydrolysis in the gastrointestinal tract of the rainbow trout. The authors also showed that chitosan nanoparticles containing vitamin C significantly (p < 0.05) induced the nonspecific immunity system of the rainbow trout compared with the control.
Rajeshkumar et al. (2009) demonstrated that chitosan nanoparticles could be used to encapsulate DNA, which was then beneficially incorporated into shrimp feed to protect them from white spot syndrome virus. Their results showed that these nanoparticles increased the survival rates of shrimp with white spot syndrome for 30 days post-treatment. Likewise, Rajeshkumar et al. (2008) incorporated chitosan nanoparticles containing a DNA vaccine into Asian sea bass (Lates calcarifer) feed. Their results indicated that sea bass orally vaccinated with chitosan–DNA (pVAOMP38) complex showed moderate protection against experimental Vibrio anguillarum infection. Similarly, Tian et al. (2008) reported that chitosan microspheres loaded with plasmid vaccine were used to orally immunize Japanese flounder (Paralichthys olivaceus). They explained that the release profile of DNA from chitosan microspheres in phosphate-buffered saline buffer (pH 7.4) was extended up to 42 days after intestinal imbibition. Aydin and Akbuga (1996) showed that salmon calcitonin, prepared for clinical use, was suitably encapsulated in chitosan beads, and the results confirmed that salmon calcitonin-loaded chitosan beads could be prepared by gelling the cationic chitosan with an anionic counterpart, providing a controlled release property.
Shark liver oil was efficiently encapsulated in calcium alginate beads coated with chitosan to mask its unpleasant taste (Peniche et al. 2004). The chitosan coating allowed for the control of the permeability of the capsules and prevented leakage. Chitosan/calcium alginate capsules loaded with shark liver oil were initially resistant to the acidic environment of the stomach, but after 4 h at intestinal pH (7.4), the capsule wall weakened and thereby was easily deteriorated and disintegrated by the mechanical and peristaltic movements of the gastrointestinal tract. Likewise, Klinkesorn and Mcclements (2009) stated that the encapsulation of tuna oil droplets with chitosan affected their physical stability and digestibility when they were evaluated in an in vitro digestion model using pancreatic lipase. The amount of free fatty acids released from the emulsions decreased as the concentration of chitosan was increased. However, the release was independent of chitosan MW. These results showed that chitosan reduced the amount of free fatty acids released from the emulsion; this may be attributable to a number of different physiological mechanisms, including the formation of a protective chitosan coating around the lipid droplets, the direct interaction of chitosan with lipase, or fatty acid binding by the chitosan. Additionally, the authors showed that pancreatic lipase was able to digest chitosan and release glucosamine, with important implications for the utilization of chitosan coatings for the encapsulation, protection and delivery of omega-3 fatty acids. They suggested that encapsulation with chitosan could be used to protect emulsified polyunsaturated lipids from oxidation during storage while allowing the capsules to release functional lipids after they are consumed. Industrially, tuna oil encapsulation with chitosan using an ultrasonic atomizer was shown to be a promising technique for the near future (Klaypradit and Huang 2008).
Conclusions
Chitosan, a deacetylated derivative of chitin, has attracted a great deal of attention in the seafood industry due to its nontoxicity, biodegradability, biocompatibility, and mucus adhesiveness properties. Chitosan has various useful characteristics, such as antibacterial and antioxidant properties, film-forming ability, gel enhancement, and encapsulating capacity and has been used as a tissue engineering scaffold, a wound dressing, and a coagulating agent. Considering these uses, chitosan could successfully be incorporated into seafood products to improve both seafood quality and enhance human nutrition. Due to its outstanding characteristics, chitosan could be used as a functional ingredient in marine-based products and this merits further study in the future.
References
Ahn, C. B., & Lee, E. H. (1992). Utilization of chitin prepared from the shellfish crust.2. Effect of chitosan film packaging on quality of lightly-salted and dried horse mackerel. Bulletin of the Korean Fisheries Society, 25, 51–57.
Aider, M. (2010). Chitosan application for active bio-based films production and potential in the food industry: a review. LWT-Food Science and Technology, 43, 837–842.
Alishahi, A., Mirvaghefi, A., Rafie-Tehrani, M., Farahmand, H., Shojaosadati, S. A., Dorkoosh, F. A., et al. (2011a). Shelf life and delivery enhancement of vitamin C using chitosan nanoparticles. Food Chemistry, 126, 935–940.
Alishahi, A., Mirvaghefi, A., Tehrani, M. R., Farahmand, H., Koshio, S., Dorkoosh, F. A., et al. (2011b). Chitosan nanoparticle to carry vitamin C through the gastrointestinal tract and induce the non-specific immunity system of rainbow trout (Oncorhynchus mykiss). Carbohydrate Polymers, 86, 142–146.
Allan, C. R., & Hardwiger, L. A. (1979). The fungicidal effect of chitosan on fungi of varying cell wall composition. Experimental Mycology, 3, 285–287.
Aydin, Z., & Akbuga, J. (1996). Chitosan beads for delivery of salmon calcitonin: preparation and release characteristics. International Journal of Pharmaceutics, 131, 101–103.
Benjakul, S., Visessanguan, W., Phatchrat, S., & Tanaka, M. (2003). Chitosan affects transglutaminase-induced surimi gelation. Journal of Food Biochemistry, 27, 53–66.
Bhatnagar, A., & Sillanpa, M. (2009). Application of chitin- and chitosan-derivatives for water and wastewater—a short review. Advances in Colloid and Interface Science, 55, 9479–9488.
Bordenave, N., Grelier, S., & Cama, V. (2007). Water and moisture susceptibility of chitosan and paper-based materials: structure–property relationships. Journal of Agriculture and Food Chemistry, 55, 9479–9488.
Bough, W. A. (1976). Chitosan—a polymer from seafood waste, for use in treatment of food processing wastes and activated sludge. Process Biochemistry, 11, 13–16.
Cadwallader, K. R., & Shahidi, F. (2001). Identification of potent odorants in seal blubber oil by direct thermal desorption-gas chromatography-olfactometry. In F. Shahidi & J. W. Finley (Eds.), Omega-3 fatty acids: chemistry, nutrition and health effects (Vol. Symposium series 788, pp. 221–234). Washington, DC: American chemical society.
Campos, C. A., Gerschenson, L. N., & Flores, S. K. (2011). Development of edible films and coatings with antimicrobial activity. Food and Bioprocess Technology, 4, 849–875.
Cao, R., Xue, C. H., & Liu, Q. (2009). Change in microbial flora of pacific oysters (Crassostera gigas) during refrigerated storage and its shelf life extension by chitosan. International Journal of Food Microbiology, 131, 272–276.
Carlson, R. P., Taffs, R., Davison, W. M., & Steward, P. S. (2008). Anti-biofilm properties of chitosan coated surfaces. Journal of Biomaterial Science Polymer, 19, 1035–1046.
Castell, C. H., Maclean, J., & Moore, B. (1965). Rancidity in lean fish muscle. IV. Effect of sodium chloride and other salts. Journal of the Fisheries Research Board of Canada, 22, 929–944.
Chen, L. C., Kung, S. K, Chen, H. H., & Lin, S. B. (2010). Evaluation of zeta potential difference as an indicator for antibacterial strength of low molecular weight chitosan. Carbohydrate Polymers, 82, 913–919.
Chi, F. H., & Cheng, W. P. (2006). Use of chitosan as coagulant to treat wastewater from milk processing plant. Journal of Polymer and the Environment, 14, 411–417.
Chiang, M. T., Yao, H. T., & Chen, H. C. (2000). Effect of dietary chitosans with different viscosity on plasma lipids and lipid peroxidation in rats fed on a diet enriched with cholesterol. Bioscience, Biotechnology, and Biochemistry, 5, 965–971.
Decker, E. A., & Haultin, H. O. (1992). Lipid oxidation in muscle foods via redox ion. In A. J. Angelo (Ed.), Lipid oxidation in food (pp. 33–54). Washington, D.C.: American Chemical Society.
Deladino, L., Anbinder, P. S., Navarro, A. S., & Martino, M. N. (2008). Encapsulation of natural antioxidants extracted from Ilex paraguariensis. Carbohydrate Polymer, 71, 126–134.
Devlieghere, F., Vermeulen, A., & Debevere, J. (2004). Chitosan: antimicrobial activity, interactions with food components and applicability as coating on fruit and vegetables. Food Microbiology, 21, 703–714.
Dias, F. S., Querroz, D. C., Nascimento, R. F., & Lima, M. B. (2008). Simple system for preparation of chitosan microspheres. Quimica Nova, 31, 160–163.
Duan, J., Cherian, G., & Zhao, Y. (2009). Quality enhancement in fresh and frozen lngcod (Ophiodon elongates) fillets by employment of fish oil incorporated chitosan coatings. Food Chemistry, 119, 524–532.
Duan, J., Jiang, Y., Cherian, G., & Zhao, Y. (2010). Effect of combined chitosan–krill oil coating and modified atmosphere packaging on the storability of cold-stored lingcod (Ophiodon elongates) fillets. Food Chemistry, 122, 1035–1042.
Dutta, P. K., Tripathi, S., Mehratra, G. M., & Dutta, J. (2009). Perspectives for chitosan based antimicrobial films in food applications. Food Chemistry, 114, 1173–1182.
El Tahlawy, K. F., El Benday, M. A., Elhendawy, A. G., & Hudson, S. M. (2005). The antimicrobial activity of cotton fabrics treated with different crosslinking agents and chitosan. Carbohydrate Polymer, 60, 421–430.
Fang, Y., Lou, M. M., Li, B., Xie, G. L., Wang, F., Zhang, L. X., et al. (2010). Characterization of Burkholderia cepacia complex from cystic fibrosis patients in China and their chitosan susceptibility. World Journal of Microbiology and Biotechnology, 26, 443–450.
Feng, T., Du, Y., Li, J., Hu, Y., & Kennedy, J. F. (2008). Enhancement of antioxidant activity of chitosan by irradiation. Carbohydrate Polymer, 73, 126–132.
Fernandez-Saiz, P., Soler, C., Lagaron, J. M., & Ocio, M. J. (2010). Effects of chitosan films on the growth of Listeria monocytogenes. Staphylococcus aureus and Salmonella spp. in laboratory media and in fish soup. International Journal of Food Microbiology, 137, 287–294.
Gomez-Guillen, M., Montero, P., Solas, M. T., & Perez-Mateos, A. G. (2005). Effect of chitosan and microbial transglutaminase on the gel forming ability of horse mackerel (Trachurus spp.) muscle under high pressure. Food Research International, 38, 103–110.
Guerrero, L., Omil, F., Mendez, R., & Lema, J. M. (1998). Protein recovery during the overall treatment of wastewaters from fish-meal factories. Bioresources Technology, 63, 221–229.
Helander, I. M., Nurmiaho-Lassila, E. L., Ahvenainen, R., Rhoades, J., & Roller, S. (2001). Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. International Journal of Food Microbiology, 71, 235–244.
Hirano, S. (1996). Chitin biotechnology applications. Biotechnology Annual Review, 2, 237–258.
Hsieh, R. J., & Kinsella, J. E. (1989). Oxidation of polyunsaturated fatty acids: mechanism, products and inhibition with emphasis on fish. Advance in Food and Nutrition Research, 33, 233–241.
Jeon, Y. J., Kamil, J. Y. V. A., & Shahidi, F. (2002). Chitosan as an edible invisible film for quality preservation of herring and Atlantic cod. Journal of Agriculture and Food Chemistry, 50, 67–78.
Jeon, J. J., Shahidi, F., & Kim, S. K. (2000). Preparation of chitin and chitosan oligomers and their applications in physiological functional foods. Food Review International, 16, 159–176.
Kadam, S. U., & Prabhasankar, P. (2010). Marine foods as functional ingredients in bakery and pasta products. Food Research International, 43, 1975–1980.
Kamil, J. Y. V. A., Jeon, J. J., & Shahidi, F. (2002). Antioxidative activity of chitosans of different viscosity in cooked comminuted flesh of herring (Clupea harengus). Food Chemistry, 79, 69–77.
Kataoka, J., Ishizaki, S., & Tanaka, M. (1998). Effects of chitosan on gelling properties of low quality surimi. Journal of Muscle Foods, 9, 209–220.
Kester, J. J., & Fennema, O. (1986). Edible films and coatings: a review. Food Technology, 00, 47–59.
Khor E. (2001). The relevance of chitin. In: Chitin: fulfilling a biomaterial promise. New York: Elsevier. pp. 1–8
Kim, K. W., & Thomas, R. L. (2007). Antioxidative activity of chitosans with varying molecular weights. Food Chemistry, 101, 308–313.
Klaypradit, W., & Huang, Y. W. (2008). Fish oil encapsulation with chitosan using ultrasonic atomizer. LWT-Food Science and Technology, 41, 1133–1139.
Klinkesorn, U., & McClement, D. J. (2009). Influence of chitosan on stability and lipase digestibility of lecithin-stabilized tuna oil-in-water emulsions. Food Chemistry, 114, 1308–1315.
Kok, T. N., & Park, J. W. (2007). Extending the shelf life of set fish ball. Journal of Food Quality, 30, 1–27.
Kong, M., Chen, X. G., Xing, K., & Park, H. J. (2010). Antimicrobial activity of chitosan and mode of action: A state of the art review. International Journal of Food Microbiology, 144, 51–63.
Kumar, M. N. V. (2000). A review of chitin and chitosan applications. Reactive Functional Polymer, 46, 1–27.
Lanier, T. C., Manning, P. K., Zetterling, T., & Macdonald, G. A. (1992). Process innovations in surimi manufacture. In T. C. Lanier & C. M. Lee (Eds.), Surimi tyechnology (pp. 167–179). New York: Marcel Decker.
Ledger, R., Tucker, I. G., & Walker, G. F. (2002). The metabolic barrier of the lower intestinal tract of salmon to the oral delivery of protein and peptide drugs. Journal of Controlled Release, 85, 91–103.
Li, X., & Xia, W. (2010). Effect of chitosan on the gel properties of salt-soluble meat proteins from silver carp. Carbohydrate Polymer, 82, 958–964.
Lopez-Caballero, M. E., Gomez-Guillen, M. C., Perez-Mateos, M., & Montero, P. (2005a). A chitosan-gelating blend as a coating for fish patties. Food Hydrocolloids, 19, 303–311.
Lopez-Caballero, M. E., Gomez-Guillen, M. C., Perez-Mateos, M., & Montero, P. (2005b). A functional chitosan-enriched fish sausage treated by high pressure. Journal of Food Science, 70, 166–171.
Mao, L., & Wu, T. (2007). Gelling properties and lipid oxidation of kamabako gels from grass carp (Ctenopharyngodon idellus) influenced by chitosan. Journal of Food Engineering, 82, 128–134.
Mi, F. L., Sung, H. W., & Shyu, S. S. (2002). Drug release from chitosan-alginate complex beads reinforced by a naturally occurring cross-linking agent. Carbohydrate Polymers, 48, 61–72.
Newton, I. S. (2001). Long chain fatty acids in health and nutrition. In omega-3 fatty acids: chemistry, nutrition and health effects. In F. Shahidi & J. W. Finley (Eds.), ACS symposium series 788 (pp. 14–27). Washington, DC: American chemical society.
No, H. K., Meyers, S. P., Prinyawiwatkul, W., & Xu, Z. (2007). Applications of chitosan for improvement of quality and shelf life of foods: a review. Journal of Food Science, 72, 87–100.
No, H. K., Park, N. Y., Lee, S. H., & Meyers, S. P. (2002). Antibacterial activity of chitosan and chitosan oligomers with different molecular weights. International Journal of Food Microbiology, 74, 65–72.
Ojagh, S. M., Rezaei, M., Razavi, S. H., & Hossieni, S. M. H. (2010). Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chemistry, 120, 193–198.
Peniche, C., Howland, I., Carrillo, O., Zaldivar, C., & Arguelles-Monal, W. (2004). Formation and stability of shark liver oil loaded chitosan/calcium alginate capsules. Food Hydrocolloids, 18, 865–871.
Pittermann, W., Horner, V., & Wachter, R. (1997). Food applications of high molecular weight chitosan in skin care applications. In R. A. A. Muzzarelli & M. G. Peter (Eds.), Chitin handbook (p. 361). Grottammare, Italy: European Chitin Society.
Qi, C. R., Bang-Zhang, Y., & Lan-Ian, Z. (2010). Combined effect of ozonated water and chitosan on the shelf life of pacific oyster (Crassostrea gigas). Innovativ Food Science and Emerging Technologies, 11, 108–112.
Qin, Y. (1993). Chelating property of chitosan fibers. Journal of Applied Polymer, 49, 727–731.
Raafat, D., Bargen, K., Haas, A., & Sahl, H. G. (2008). Insights into the mode of action of chitosan as an antimicrobial compound. Applied and Environmental Microbiology, 74, 3764–3773.
Raafat, D., & Sahl, H. G. (2009). Chitosan and its antimicrobial potential—a critical literature survey. Microbial Biotechnology, 2, 186–201.
Rabea, E. I., Badawy, M. E. T., Stevens, C. V., Smagghe, G., & Steurbaut, W. (2003). Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules, 4, 1457–1465.
Rajeshkumar, S., Ishaq Ahmed, V. D., Parameswaran, V., Sudhakaran, R., Sarath Babu, V., & Sahl Hameed, A. S. (2008). Potential use of chitosan nanoparticles for oral delivery of DNA vaccine in Asian sea bass (Lates calcarifer) to protect from Vibrio anguillarum. Fish & Shellfish Immunology, 25, 47–56.
Rajeshkumar, S., Venkatesan, C., Sarathi, M., Sarathbabu, V., Thomas, J., & Anver Basha, K. (2009). Oral delivery of DNA construct using chitosan nanoparticles to protect the shrimp from white spot syndrome virus (WSSV). Fish & Shellfish Immunology, 26, 429–437.
Roller, S., & Corvill, N. (2000). The antimicrobial properties of chitosan in mayonnaise and mayonnaise-based shrimp salads. Journal of Food Protection, 63, 202–209.
Ross, S. (2000). Functional foods: the food and drug administration perspective. American Journal of Clinical Nutrition, 71, 1735s–1738s.
Sathivel, S. (2005). Chitosan and protein coatings affect yield, moisture loss, lipid oxidation of pink salmon (Oncorhynchus gorbushcha) fillets during frozen storage. Journal of Food Science, 70, 755–459.
Sathivel, S., Liu, Q., Huang, J., & Prinyawiwatkul, W. (2007). The influence of chitosan glazing on the quality of skinless pink salmon (Oncorhynchus gorbuscha) fillets during frozen storage. Journal of Food Engineering, 83, 366–375.
Savant, V.D. (2001). Protein absorption on chitosan-polyanion complexes: application to aqueous food processing wastes. PhD. Thesis, Food Science and Technology, Oregon State University.
Savant, V. D., & Torres, J. A. (2000). Chitosan based coagulating agents for treatment of cheddar cheese whey. Biotechnology Progress, 16, 1091–1097.
Savant, V. D., & Torres, J. A. (2003). Fourier transform infrared analysis of chitosan based coagulating agents for treatment of surimi waste water. Journal of Food Technology, 1, 23–28.
Schep, L. J., Tucker, I. G., Young, G., Ledger, R., & Butt, A. G. (1999). Controlled release opportunities for oral peptide delivery in aquaculture. Journal of Controlled Release, 59, 1–14.
Shahidi, F., & Han, X. (1993). Encapsulation of food ingredients. Critical Reviews in Food Science and Nutrition, 33, 501–547.
Shahidi, F., Kamil, J. Y. V. A., & Jeon, Y. J. (1999). Food applications of chitin and chitosan. Trends in Food Science and Technology, 10, 37–51.
Shahidi, F., Kamil, J. Y. V. A., & Jeon, J. J. (2002). Antioxidant role of chitosan in a cooked cod (Gadus morhua) model system. Journal of Food Lipids, 9, 57–64.
Swapna Joseph, C., Harish Prashanth, K. V., Rastogi, N. K., Indiramma, A. R., Yella Reddy, S., & Raghavarao, K. S. M. S. (2011). Optimum blend of chitosan and poly-(ε-caprolactone) for fabrication of films for food packaging applications. Food and Bioprocess Technology. doi:10.1007/s11947-009-0203-1.
Tharanathan, R. N., & Kittur, F. S. (2003). Chitin—the undisputed biomolecule of great potential. Critical Review in Food Science and Nutrition, 43, 61–87.
Tian, J., Yu, J., & Sun, X. (2008). Chitosan microspheres as candidate plasmid vaccine carrier for oral immunization of Japanese flounder (Paralichthys olivaceus). Veterinary Immunology and Immunopathology, 126, 220–229.
Vargas, M., Albors, A., Chiralt, A., & González-Martínez, C. (2009). Characterization of chitosan–oleic acid composite films. Food Hydrocolloids, 23, 536–547.
Weist, J. L., & Karel, M. (1992). Development of a fluorescence sensor to monitor lipid oxidation. 1. Florescence spectra of chitosan powder and polyamid powder affect exposure to volatile lipid oxidation products. Journal of Agriculture and Food Chemistry, 40, 1158–1162.
Wibowo, S. (2003). Effect of the molecular weight and degree of deacetylation of chitosan and nutritional evaluation of solid recovered from surimi processing plant. PhD. Thesis. Food Science and Technology, Oregon State University
Wibowo, S., Velazquez, G., Savant, V., & Torres, J. A. (2005). Surimi wash water treatment for protein recovery: effect of chitosan-alginate complex concentration and treatment time on protein adsorption. Bioresoures Technology, 96, 665–671.
Wibowo, S., Savant, V., Cherian, G., Savange, T. F., Velaquez, G., & Torres, J. A. (2007). A feeding study to assess nutritional quality and safety of surimi wash water proteins recovered by a chitosan–alginate complex. Journal of Food Science, 72, 179–184.
Wibowo, S., Velazquez, G., Savant, V., & Torres, A. (2007). Effect of chitosan type on protein and water recovery efficiency from surimi wash water treated with chitosan–alginate complexes. Bioresources Technology, 98, 539–545.
Ye, M., Neetao, H., & Chen, H. (2008). Effectiveness of chitosan-coated plastic films incorporating antimicrobials in inhibition of Listeria monocytogens on cold-smoked salmon. International Journal of Food Microbiology, 127, 235–240.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alishahi, A., Aïder, M. Applications of Chitosan in the Seafood Industry and Aquaculture: A Review. Food Bioprocess Technol 5, 817–830 (2012). https://doi.org/10.1007/s11947-011-0664-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-011-0664-x