Abstract
In the present study, the effects of culture medium and temperature on red pigment production and mycelia growth were evaluated. The maximum red pigment production was found when Monascus purpureus CMU001 was cultivated on potato dextrose broth at 30 °C for 2 weeks. The highest amount of dry weight was achieved when cultivated on tryptone glucose yeast extract medium. Cheap agricultural products and residues were used as substrates for pigment production. Corn meal was the best substrate for pigment production (19.4 U/gds) when compared to peanut meal, coconut residue, and soybean meal. The highest pigment yield (129.63 U/gds) was found when corn meal was supplemented with 8% (w/w) glucose, followed by coconut residue (63.50 U/gds), peanut meal (52.50 U/gds), and soybean meal (22.50 U/gds). Galactose, sorbose, psicose, and mannitol were found to be good supplements next to glucose but not xylitol. Pigment was not stable at high temperature and long exposure to UV. The intensity of red pigment decayed 30.57% and 5.41% after autoclaving and pasteurization, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Monascus purpureus (Van 1884; Went 1895) is a homothallic fungus. It is classified to the Ascomycetes family Monascaceae. The most important characteristic of this fungus is the ability to produce secondary metabolites of polyketide structure which are synthesized by the polymerization of acetyl and propionyl subunits in a similar process to fatty acid synthesis (JÚzlová et al. 1996). Monascus is known to produce at least six molecular structures of pigment which can be classified into three groups depending on their color. They include yellow pigments monascin (C21H26O5) and ankaflavin (C23H30O5), the orange pigments monascorubrin (C23H26O5) and rubropunctatin (C21H22O5), and finally the red pigments monascorubramine (C23H27NO4) and rubropuntamine (C21H23NO4) (Pattanagul et al. 2007). The color specification of the latter depends on the associated amino acid or protein (Lian et al. 2007). Growth of Monascus species would be directly affected by the composition of starch or type of carbon sources (Lee et al. 2007). Aldohexoses such as glucose and dextrose are better carbon sources for growth of M. purpureus than sugar alcohols such as sorbitol and mannitol, while sucrose reduced the growth of the fungus (Babitha et al. 2006).
Some Monascus compounds have applications as pharmaceuticals or food additives (Kraiak et al. 2000). In the former case, monacolin K was found to inhibit cholesterol synthesis thus reducing hypolipidemia (Chairote et al. 2008; Lee et al. 2007), and lovastatin was found to reduce serum cholesterol and triglyceride (Panda et al. 2009). The red pigment has been of increasing interest to the food industry because products are extracellular and water soluble making them easy to use. Applications include the increased red coloring in meat, fish, and ketchup (Hamano and Kilikian 2006). It can also be used in traditional foods to replace nitrate or nitrite for quality improvement. Colorants can be added to fruit-flavored yoghurt for enhancing the natural color of the fruit (Faber et al. 1993).
At present, pigment production at an industrial scale is not economical since the cost of technology used is still high. Therefore, the development of low-cost processes is needed. Up to now, several materials such as jackfruit seed powder, sesame oil cake, coconut oil, wheat bran, palm kernel cake, and grape waste (Babitha et al. 2006; Babitha et al. 2007; Silverira et al. 2008) have been studied as substrates in solid-state fermentation (SSF). This approach gives high pigment productivity at a low cost when compared with liquid fermentation (Cavalcante et al. 2008). These reports indicated that utilization of cheaply available substrates in SSF could be a good strategy for attaining significant pigment production.
The main objectives of this study were to investigate the general conditions for growth and pigment production on six fungal media and exploiting the potential of various agro-industrial materials or residues such as corn meal, peanut meal, soybean meal, and coconut residue, with and without sugar supplements, as substrates for Monascus pigment production by SSF. In addition, stability of red pigment on thermal process was determined.
Materials and Methods
Culture
A culture of M. purpureus CMU001 was isolated from locally available commercial Chinese red yeast rice (Ang-Kak) on Rose Bengal agar plates and deposited in the culture collection at the Biology Department, Faculty of Science, Chiang Mai University. The fungus was maintained on potato dextrose agar (PDA) and incubated at 30–32 °C for 7 days, preserved at 4 °C, and subcultured once every 4 weeks.
Inoculum Preparation
Inoculum preparation for SSF was performed as described in Babitha et al. (2007) with some modification. Ten milliliters of Tween 20 (0.1% v/v) in sterile distilled water was added to fully sporulated (7–8-day-old) PDA agar slope cultures. The spores were scraped off under aseptic conditions to produce a spore suspension to be used as the inoculum (1 × 106 spores/ml).
Effect of Culture Media on Mycelial Growth
Mycelial growth of M. purpureus CMU001 in six common fungal media was evaluated since little information is available on its biology, including media requirements for growth and pigment production. This information will enable the selection of suitable media for inoculum preparation. Standard and commercially available media commonly used for fungal cultivation were selected such as PDA, yeast malt agar (YM), tryptone glucose yeast extract agar (TGY), Sabourad dextrose agar (SDA), malt peptone agar (MPA), and Czapek yeast extract agar (CYE). M. purpureus CMU001 was grown on PDA at 30 °C for 7 days. Four-millimeter-diameter agar plugs were cut with a sterile cork borer from the leading edges of the colonies, and one plug was placed in the center of the dishes, which were then wrapped with parafilm and incubated at 30 °C for 7 days. Four replicate plates were prepared for each medium. The radius of each developing colony in each plate was measured, from the center of dish, along two perpendicular axes (four measurements per dish) at intervals of approximately 24 h and averaged to give a final value. The morphology of colony was also noted.
Dry Weight Determination
After 7 days, the agar were heated at 900 W for 10 min in a microwave oven and then washed three times with warm distilled water. The plates were then dried, to constant weight, in an oven at 60 °C. They were cooled in a desiccator for 15 min before weighing.
Nutritional and Temperature Effects on Pigment Production
The effect of liquid media and temperature on pigment production was evaluated with the same six agar media used previously. Erlenmeyer flasks (250 ml) were prepared with 100 ml of each medium. A seed culture was prepared by transferring a loop full of spores from the stock PDA slant into a 250-ml Erlenmeyer flask containing 100 ml of potato dextrose broth (PDB). One milliliter of seed culture was pipetted in the flasks and incubated at 25, 30, and 37 °C with orbital shaking at 120 rpm for 7 days. In each temperature (at each incubator), all flasks were arranged in a random complete block design and there were four replicates for each medium at each temperature. After 7 days of incubation, each culture was filtered through two layers of Miracloth membrane (Calbiochem Inc., Germany). Analysis of red pigment production was done by measuring absorbance as described below. Uninoculated media of each type were used as blanks.
Substrate Selection and Solid-State Fermentation
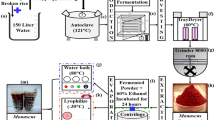
Cheap agricultural products and residues which are abundant in Thailand, e.g., corn meal, coconut residue, peanut meal, and soybean meal, were selected in the hope of adding value to the wastes and reducing their management. Experiments were focused on the evaluation of the best suitable substrates for maximum yield of pigment production. Selected substrates obtained from a local market were dried at 60 °C for 12 h and then ground. Five grams of substrate was added to 6 × 10-in. autoclavable plastic bags and a salt solution (2 ml) containing (g/l): KH2PO4, 2; NH4NO3, 5; NaCl, 1; and MgSO4∙7H2O, 1 (Babitha et al. 2006) was added. Autoclavable plastic tubing was inserted into the bag and plugged with cotton wool. The conditions of culture age, temperature, humidity, and pH were controlled according to Chairote et al. (2007). The contents of the bags were mixed thoroughly and then autoclaved at 121 °C for 15 min. On cooling, they were inoculated with the spore suspension containing 1 × 106 spores/ml of M. purpureus CMU001 and incubated at 30 °C for 14 days. Unless otherwise mentioned, these conditions were maintained throughout the experiments.
The value of adding specific carbon substrates to the wastes to increase pigment production was also investigated. In general, simple molecules consisting of mostly unbranched chains are easily used and decomposed by fungi. However, the use of rare sugars, widely found in diet foods, has not been extensively studied. Thus, 4% and 8% (w/w) of simple sugar, i.e., galactose, glucose, psicose, and sorbose, and rare sugar, i.e., mannitol and xylitol, was added to the natural substrates and assessed for enhancing effects on pigment production.
Pigment Extraction and Estimation
Five grams of fermented solid substrate was taken for pigment extraction using 25 ml of 95% ethanol (Carvalho et al. 2005), with shaking on a rotary shaker at 200 rpm for 1 h. The extracts were allowed to settle at room temperature and then filtered through Miracloth membrane (Calbiochem). Ethanol extracts of unfermented substrates were kept as blanks. Analysis of pigment production was done by measuring absorbance (spectraMR, Dynex, USA) at 500 nm, near the absorbance peak of red pigments. Pigment yield was expressed as OD per gram dry fermented substrate (John and Stuart 1991; Lin and Demain 1992).
Color Stability
The aqueous phase of extract solutions were stored and investigated for pigment stability. These solutions were incubated under UV light and different temperatures (40, 50, and 60 °C) for 6 h. The pigments were tested for tolerance to sterilization at 15 psi and 121 °C for 15 min and pasteurization at 70 °C for 10 s. The color intensity was read as described above, against water as blank.
Thin Layer Chromatography (TLC) Analysis of the Pigment Extract
Ten microliters of ethanol extracts was applied to Silica Gel 60 F254 plates (Merek, Germany) and developed with solvent mixture containing (ml): chloroform, 90; methanol, 25; and H2O, 4 as described by Babitha et al. (2006). The Rf values of the pigments were determined on developed chromatograms.
Statistical Analyses
All the experimental data were analyzed with SPSS program (SPSS Inc., Chicago, IL, USA). Data mycelial growth and dry weight determinations were subjected to analysis of variance (ANOVA) by the Tukey’s test (p < 0.05). Data gathered to assess nutritional differences of liquid culture media and temperature, on pigment, and production were separated by Random Effect Model (p < 0.01) and ANOVA by using the Tukey’s test (p < 0.05). Data from solid-state fermentation and the effect of supplementation of carbon source were separated by Latin Square Design, with substrates and sugars as fixed factors, and analysis of multiple comparisons by the Tukey’s test (p < 0.05).
Results and Discussion
Effect of Culture Media
The results showed that M. purpureus CMU001 grew rapidly on all media but the color and texture of mycelium produced were dependent on media type. The radial mycelial growth of this strain was affected significantly by culture media. TGY and YM were the best media to promote fungal growth in terms of radial growth with significant difference from other media after 7 days of incubation. The highest dry weight was achieved when M. purpureus was cultivated on TGY medium (Table 1). At 30 °C, compacted colonies with red hyphae were found on these two media. On PDA, the colony was compacted and strongly secreted the red pigment into the medium. The fungus formed abundant and colorless colonies on SDA. The mycelial growth on MPA and CYE was poor with scanty colorless colonies.
The results indicated that a high amount of yeast extract and glucose found in a mixture of TGY and YM promoted faster growth compared to other media. Pisareva and Kujumdzieva (2006) determined the assimilation of carbon sources by measurement dry weight (g/l) of M. pilosus CBS289.34 and Monascus sp. after 7 days of incubation at 30 °C. They reported that glucose gave the highest dry weight of both followed by raffinose, maltose, sucrose, and lactose, respectively.
Nutritional and Temperature Effects on Pigment Production
The interaction of six fungal media and temperature to promote pigment production was evaluated. There was a significant interaction (p < 0.01) between medium and temperature. The various temperatures investigated affected pigment production with significant differences (p < 0.05). At 30 °C, PDB is the best medium suitable for red pigment production by this fungus, followed by TGY, YM, and SDB, while MP and CZY were unfavorable for pigment production (Fig. 1). This result occurred when carried out at 37 and 25 °C. It can be concluded that incubation at 30 °C promotes the highest yield of pigment production in all media with significant differences from other temperatures, followed by 37 and 25 °C, respectively. This result agreed with previous data (Lin et al. 2008) which showed that incubation at 30–35 °C favored of the best propagation and mycelium growth. The maximum red pigment production was obtained at 30 °C and the yellow pigment was maximum at 40 °C (Babitha et al. 2007). Other components in medium such as leucine were reported to interfere with the production of red pigment (Lin and Demain 1994), and the absence of potassium phosphate in culture medium was found to suppress red pigment production in M. pilosus (Lin et al. 2007). Metabolites production by filamentous fungi varies according to the strain, the composition of the growth medium, cultivation conditions, pH, temperature, and the kind of carbon and nitrogen source (Treichel et al. 2009). Cultivation conditions for improved pigment production are reported as initial pH at 6.0 and incubation at 32 °C by using 5% rice powder as carbon substrate and supplemented with 0.5% of sodium nitrate or potassium nitrate as the nitrogen source (Lin 1973; Lin and Suen 1973).
Substrate Selection and Effect of Carbon Supplementation
Cultivation of Monascus in solid media has a long tradition in Asian countries to produce a red colorant used as a food ingredient. As shown in Fig. 2, the fungus produced the highest pigment (no sugar added) on corn meal substrate (19.40 ± 0.07 OD U/gds), followed by peanut meal (3.03 ± 0.53 OD U/gds) and soybean meal (3.01 ± 0.10 OD U/gds), and the lowest yield was observed from coconut residue (0.59 ± 0.11 OD U/gds) (Fig. 2a–d). The advantage of using corn meal as substrate is that fermentation period was shorter when compared with that of Chairote et al (2007) who used 3 weeks for fermentation of sticky rice (Korkor 6 and Sanpatong 1), but in this study, we still need to improve the yield.
In our experiment with corn meal supplemented with 8% glucose, pigment production was increased six-fold (129.63 ± 0.92 U/gds) after 1-week incubation, followed by coconut residue (63.50 ± 0.98 U/gds), peanut meal (52.50 ± 1.24 U/gds), and soybean meal (22.50 ± 1.09 U/gds). Next to glucose, galactose, sorbose, psicose, and mannitol were found to be good supplements for pigment production. In contrast, xylitol had a negligible effect. As described above, glucose, galactose, sorbose, and psicose are all monosaccharides but mannitol and xylitol are sugar alcohols which may be not suitable as a carbon source in M. purpureus CMU001. Monosaccharides are readily metabolized, sugar alcohols less so, and consequently pigment production is reduced (Babitha et al. 2006). Corn meal with an addition of glucose 4% (56.06 ± 1.24 U/gds) and 8% might be better for pigment production when compared with sticky rice since it induced a three-fold higher pigment production and shorter fermentation period. Although, in fermented RD6, the addition of soybean milk gave a darkened color, fermentation without soybean milk gave the highest yield of momacolin K and compactin (Chairote et al. 2008). Our results agreed with those of Lin et al. (1992) who concluded that the utilization of carbon source for growth and pigment production depended on strain specification. Glucose and its oligopolysaccharide were better than other carbon sources for both growth and pigment production (Lin et al. 1992; Pandey 2003).
Pigment Analysis
Monascus pigments are the group of azaphilones that is the metabolite synthesized from polyketide chromophores and beta-keto acids by esterification; also, red pigment and citrinin are common synthesis pathways in this fungus controlled by the pksCT gene of 7,838 bp with a single intron (Fu et al. 2007). In this study, pigment extracts produced by M. purpureus CMU001 were separated by TLC. Supplementation with 8% and 4% of glucose (Fig. 3a) and sorbose (Fig. 3b) was selected to represent the experiment because glucose (an aldose) and sorbose(a ketose) might affect the pattern of pigment production. The results showed that the Rf value for the yellow pigment was the same for all of extracts, but in contrast the red pigment was present in more than one spot in each of the extracts. The results were different from those of Babitha et al. (2006) who showed that Rf value of yellow pigment is higher than orange pigment and red pigment has the lowest Rf value. This result indicated that, during fermentation, yellow and red pigments were produced and the supplementation of sugar affected pigment production. The various types of red pigment production indicated by Rf value might be dependent on the effect of substrate type. To form pigment, it can easily react with amino group containing compounds in the substrate such as proteins, amino acid, or nucleic acid, according to Dufosse et al. (2005). Lian et al. (2007) reported that the color specification of Monascus red pigment greatly depends on the amino acid or protein which the pigment was associated and they also found that the synthesis of the new red pigment might start with the restraint of the esterification course between beta-keto acid and polyketide chromophore.
Stability of Pigment
Stability was measured using a relative level of residual absorbance after incubation for 1 to 6 h. The results indicated that the pigment decayed over time, as shown by an intolerance to high temperature (>40 °C) and long exposure to UV (>3 h) as depicted in Fig. 4. The color intensity of the red pigment after autoclaving and pasteurization decayed 30.57% and 5.41%, respectively (Table 2). The results were similar to those of Faber et al. (1993) who studied the pigment of Monascus ruber and found that it was seriously affected by light exposure, temperature, and pH. The pigment was more stable at basic or neutral pH. These properties suggest that the pigment from M. purpureus CMU001 is useful for some applications in the food industry with products used at low temperatures.
Moreover, the stability of pigment from Monascus has been widely studied by others such as Carvalho et al. (2005) who reported that the pigments are unstable at low pH and high temperature and possible due to the fact that the extract is a mixture of pigments, whose degradation may present decaying behavior. Lin and Demain (1992) reported that the pigments are stable over a wide range of pH and autoclaving.
Conclusions
From the results, it can be concluded that TGY and YM were the most favorable for growth of M. purpureus CMU001. Cultivation on PDB at 30 °C gave the highest pigment yield. Pigment production in this fungus can be achieved by solid-state fermentation technique using agricultural product other than rice. Corn meal supplemented with 8% (w/w) glucose was a suitable substrate for pigment production. However, the application of the pigment in food processing is restricted. Our research has also showed that cheap agricultural products and residues that are readily available in Thailand are a suitable substrate for pigment production and this usage reduces the cost of waste disposal.
References
Babitha, S., Soccol, C. R., & Pandey, A. (2006). Jackfruit seed—a novel substrate for the production of Monascus pigment solid-state fermentation. Food Technology and Biotechnology, 44, 465–471.
Babitha, S., Soccol, C. R., & Pandey, A. (2007). Solid-state fermentation for the production of Monascus pigments from jackfruit seed. Bioresource Technology, 98, 1554–1560.
Carvalho, J. C., Oishi, B. O., Pandey, A., & Soccol, C. R. (2005). Biopigments from Monascus: strain selection, citrinin production and color stability. Brazilian Archives of Biology and Technology, 48, 885–894.
Cavalcante, R. S., Lima, H. L. S., Pinto, G. A. S., Gava, C. A. T., & Rodrigues, S. (2008). Effect of moisture on Trichoderma conidia production on corn and wheat bran by solid state fermentation. Food and Bioprocess Technology, 1, 100–104.
Chairote, E., Chairote, G., Wongpornchai, S., & Lumyong, S. (2007). Preparation of red yeast rice using various Thai glutinous rice and Monascus purpureus CMU001 isolated from commercial Chinese red yeast rice sample. KMITL Science Journal, 7, 28–37.
Chairote, E., Chairote, G., Niamsup, H., & Lumyong, S. (2008). The presence and the content of monacolins in red yeast rice prepared from Thai glutinous rice. World Journal of Microbiology & Biotechnology, 24, 3039–3047.
Dufosse, L., Galaup, P., Yaron, A., Arad, S. M., Murthy, K. N. C., & Ravishankar, G. A. (2005). Microorganism and microalgae as source of pigments for use: a scientific oddity or an industrial reality? Trends in Food Science & Technology, 16, 389–406.
Faber, C. E., Santerre, A. L., Loret, M. O., Baberian, R., Paresllerin, A., Goma, G., et al. (1993). Production and food applications of the red pigments of Monascus ruber. Journal of Food Science, 58, 1099–1110.
Fu, G., Xu, Y., Li, Y., & Tan, W. (2007). Construction of a replacement vector to disrupt pksCT gene for the mycotoxin citrinin biosynthesis in Monascus aurantiacus and maintain food red pigment production. Asia Pacific Journal of Clinical and Nutrition, 16, 137–142.
Hamano, P. S., & Kilikian, B. V. (2006). Production of red pigments by Monascus ruber in culture media containing corn steep liquor. Brazilian Journal of Chemical Engineering, 23, 443–449.
John, M. R., & Stuart, D. M. (1991). Production of pigments by Monascus purpureus in solid culture. Journal of Industrial Microbiology, 8, 23–28.
JÚzlová, P., Martínková, L., & Křen, V. (1996). Secondary metabolites of the fungus Monascus: a review. Journal of Industrial Microbiology, 16, 163–170.
Kraiak, S., Yamamura, K., Irie, R., Nakajima, M., Shimizu, H., & Chim-Anage, P. (2000). Maximizing yellow pigment production in fed-batch culture of Monascus sp. Journal of Bioscience and Bioengineering, 90, 363–367.
Lee, C. L., Hung, H. K., Wang, J. J., & Pan, T. M. (2007). Improving the ratio of manacolin K to citrinin production of Monascus purpureus NTU568 under Dioscorea medium through the mediation of pH value and ethanol addition. Journal of Agricultural and Food Chemistry, 55, 6493–6502.
Lian, X., Wang, C., & Guo, K. (2007). Identification of new red pigments produced by Monascus ruber. Dyes and Pigments, 73, 121–125.
Lin, C. F. (1973). Isolation and cultural conditions of Monascus sp. for production of pigment in a submerged culture. Journal of Fermentation Technology, 51, 407–414.
Lin, C. F., & Suen, S. J. T. (1973). Isolation of hyperpigment-productive mutants of Monascus sp. F-2. Journal of Fermentation Technology, 51, 757–759.
Lin, T. F., & Demain, A. L. (1992). Fermentation of water-soluble Monascus red pigments by biological and semi synthetic processes. Journal of Industrial Microbiology, 9, 173–179.
Lin, T. F., & Demain, A. L. (1994). Leucine interference in the production of water-soluble red Monascus pigments. Archives of Microbiology, 162, 114–119.
Lin, T. F., Yakushijin, K., Büchi, G. H., & Demain, A. L. (1992). Formation of water-soluble Monascus red pigments by biological and semi synthetic processes. Journal of Industrial Microbiology & Biotechnology, 9, 173–179.
Lin, W. Y., Ting, Y. C., & Pan, T. M. (2007). Proteomic response to intracellular protein of Monascus pilosus growth under phosphate-limited complex medium with difference growth rates and pigment production. Journal of Agricultural and Food Chemistry, 55, 467–474.
Lin, Y. L., Wang, T. H., Lee, M. H., & Su, N. W. (2008). Biologically active components and nutraceuticals in the Monascus-fermented rice: a review. Applied Microbiology and Biotechnology, 77, 965–973.
Panda, B. P., Javed, S., & Ali, M. (2009). Optimization of fermentation parameters for higher lovastatin production in red mold rice through co-culture of Monascus purpureus and Monascus ruber. Food and Bioprocess Technology, doi:10.1007/s11947-008-0072-z.
Pandey, A. (2003). Solid-state fermentation. Biochemical Engineering Journal, 14, 81–84.
Pattanagul, P., Pinthong, R., Phianmongkol, A., & Leksawasdi, N. (2007). Review of angkak production (Monascus purpureus). Chiang Mai Journal of Science, 34, 319–328.
Pisareva, E., & Kujumdzieva, A. (2006). Taxonomic investigation and growth characteristic of citrinin free Monascus pilosus c1 strain. Biotechnology & Biotechnological Equipment, 20, 88–96.
Silverira, S. T., Daroit, D. J., & Brandelli, A. (2008). Pigment production by Monascus purpureus in grape waste using factorial design. Food Science and Technology, 41, 170–174.
Treichel, H., de Oliveira, D., Mazutti, M. A., Luccio, M. D., & Oliveira, J. V. (2009). A review on microbial lipases production. Food and Bioprocess Technology, doi:10.1007/s11947-009-0202-2.
Van, T. P. (1884). Monascus, genre nouveau de l’ordre des Ascomycetes. Bulletin de la Société de France, 31, 26–31.
Went, F. A. F. C. (1895). Monascus purpureus le champignon de l’angquac unenouvelle thelebolee. Annales des Sciences Naturelles Botanique, 8, 1–17.
Acknowledgements
This work was granted by the Commission on Higher Education, Thailand. We thank Ms. Em-on Chairote for providing culture of the M. purpureus CMU001 strain. We are grateful to Prof. John F. Peberdy, Nottingham University for improving the English text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nimnoi, P., Lumyong, S. Improving Solid-State Fermentation of Monascus purpureus on Agricultural Products for Pigment Production. Food Bioprocess Technol 4, 1384–1390 (2011). https://doi.org/10.1007/s11947-009-0233-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-009-0233-8