Abstract
The main objective of this study was to understand the effects and relationship amongst four factors, which are reaction temperature, reaction time, enzyme load, and substrate mole ratio with the purpose of producing healthy functional cooking oil for long-term dietary treatment. Lipozyme RM IM lipase-catalyzed esterification of medium- and long-chain triacylglycerols (MLCT) from glycerol and mixtures of capric and oleic acid was optimized using response surface methodology (RSM) with a five-level, four-factorial design. Reaction temperature, reaction time, and substrate mole ratio strongly affected MLCT synthesis (P < 0.01). However, enzyme load did not have a significant (P > 0.01) effect on MLCT yield. Comparison between predicted and experimental value from central composite rotatable design optimization procedures revealed good correlation, implying that the reduced cubic polynomial model with backward elimination statistically expressed the percent MLCT yield obtained. The optimum MLCT yield was 59.76% by using 10 wt% enzyme load, reaction temperature of 70°C, reaction time of 14 h, and substrate mole ratio of 3.5:1. Experiments to confirm the predicted results using the optimal parameters showed an MLCT yield of 56.35% (n = 2). The choice on the types of fatty acids used in MLCT optimization work greatly influenced the physical and chemical properties of MLCT oil produced. The refined MLCT oil characteristics study showed this oil is suitable to be used for cooking/frying purposes as a high-value added product.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medium-chain triglycerides (MCT) were introduced into clinical nutrition for the malabsorption treatment because of their rapid absorption and quick energy release after intake (Seaton et al. 1986). However, high dose of MCT may lead to metabolic acidosis and gastrointestinal symptoms. Recent findings had shown that the use of lipid emulsion containing medium-chain triglycerides/long-chain triglycerides (MCT/LCT) showed a greater recovery of their nutrition status than the traditional MCT or LCT formula in the total parenteral nutrition study (Lai and Chen 2000; Garnacho-Montero et al. 2002; Lin et al. 2002; Socha et al. 2007). MLCT that contained medium-chain fatty acids (MCFA, C6-C10) and long-chain fatty acids (LCFA, C12-C24) in the same triacylglycerol (TAG) are the latest structured lipids being focused nowadays. Most of the MLCT works reported were designed to provide the most effective means of delivery of the desired fatty acid (FA) for nutritive purposes, and for targeting specific disease and metabolic conditions (Fomuso and Akoh 1997; Kawashima et al. 2001, 2004; Mu et al. 2001; Aoyama 2003; Kim and Akoh 2005; Mu and Porsgaard 2005). The FA and their position in the TAG determine the functional and physical properties, metabolic rate, and health benefits of the structured lipid.
The latest approach on structured lipid emulsion which contained both medium- and long-chain fatty acids in the same glycerol backbone has been shown to be a safe and efficient way of providing energy to patients requiring parenteral nutrition on a long-term basis (Rubin et al. 2000; Matulka et al. 2006). This approach was designed to provide simultaneous delivery of MCFA and LCFA, with a more controlled release of the MCFA into bloodstream than that of physical mixture of MCT and LCT. A considerable number of papers on MLCT structured lipids (Swift et al. 1992; Matsuo et al. 2001; Bendixen et al. 2002; Kasai et al. 2003; Matsuo and Takeuchi 2004) had been published and showed that MLCT diets have similar fat accumulation and postprandial thermogenesis as those of MCT. The results obtained showed that long-term substitution of MLCT for LCT will result in reduction in body weight, body fat, and total serum cholesterol. The data obtained from a few Japanese studies on MLCT suggested that enhancement of energy expenditure and medium-chain fatty acids oxidation without activating de novo lipogenesis are responsible for the lower fat accumulation (Shinohara et al. 2002, 2005).
Regiospecificity is one of the major advantages of using lipase technology to produce high-value added products. Enzymatic interesterification with sn-1,3-specific lipases has been used by industry for the production of cocoa butter-like fats, human milk fat substitutes, and so on (Xu 2000a). The most commonly used method for the production of MLCT is acidolysis, using a regiospecific lipase to incorporate the MCFA into the primary positions of TAG (Fomuso et al. 1997; Kawashima et al. 2001, 2004; Mu et al. 2001; Kim and Akoh 2005). The acidolysis reaction proceeds in two steps. The TAG are hydrolyzed into diacylglycerols (DAG) or even to monoacylglycerols (MAG), followed by the esterification of new fatty acids (FA) into the TAG (Xu et al. 1998, 2000b). Acyl migration may occur between these two steps and results in the formation of by-products that may affect product quality (Mu et al. 2001; Kawashima et al. 2004; Kim and Akoh 2005). On the contrary, migration of the acyl groups from positions 1 or 3 to 2 is necessary for the TAG formation in the lipase-catalyzed esterification synthesis as Lipozyme RM IM used to esterify glycerol is a 1,3-specific lipase (Fu and Parkin 2004). Therefore, isomerization reaction is the limiting step for TAG synthesis. So far, to our knowledge, only US Patent no. 0191391 A1 (Takeuchi et al. 2004) focused on the use of MLCT designed for cooking application. The MLCT was produced using lipase or chemical catalysts via transesterification or acidolysis processes. There is no reported work on the use of lipase-catalyzed esterification reaction to produce MLCT oil suitable for both cooking and frying purposes.
A high fat intake is linked to degenerative diseases such as heart disease, diabetes, high blood pressure, and cancer. With increasing consumer awareness of the risks associated with high fat intake, a market for reduced calorie fats or fat replacers has opened up. Fat replacers based on carbohydrates and proteins were only useful in low temperature applications such as in frozen desserts and cannot be used where elevated temperatures are involved, as in cooking application (Hassel 1993). MLCT is superior for cooking application as opposed to the physical mixtures of MCT and LCT due to its higher smoke point (Matsuo et al. 2001). Therefore, the unique characteristics of MLCT oil which could be used at high temperatures have a bright future in the highly competitive functional oil market.
In this study, the synthesis of MLCT using mixtures of oleic and capric acid, and glycerol, which is an abundant by-product from the oleochemical and biodiesel industry in Malaysia, was carried out using the 1,3-specific Lipozyme RM IM lipase. Capric and oleic acids were used as sources of MCFA and LCFA, respectively. Oleic acid was used because it has less oxidation tendency as compared to other unsaturated fatty acids such as linoleic or linolenic acid, and because of its long chain structure, incorporation of oleic acid also helped to increase the smoke point of the MLCT oil produced. RSM comprising a five-level, four-factorial, central composite rotatable design (CCRD) was used to evaluate the interactive effects and to obtain optimum conditions for the lipase-catalyzed esterification of MLCT. This study aims to understand the effects and relationship amongst all the four factors, which are reaction temperature, reaction time, enzyme load, and substrate mole ratio and to determine the optimum conditions for esterification of the fatty acids to glycerol.
Materials and Methods
Materials
Immobilized lipase from Rhizomucor miehei (Lipozyme RM IM) was purchased from Novozymes A/S (Bagsvaerd, Denmark). Capric acid (99.9%), glycerol (99.8%), and concentrated oleic acid (purity >75%, w/w) were purchased from Cognis Oleochemical (M) Sdn. Bhd. (Banting, Malaysia). Capric acid, oleic acid, monocaprin, monolein, dicaprin, diolein, tricaprin, and triolein were bought from Sigma Chemical Co., USA. All other solvents used were of high performance liquid chromatography (HPLC) grade.
Effect of Capric: Oleic Mole Ratio on the Esterification Synthesis
A preliminary study was conducted prior to the RSM work to understand the effect of different capric/oleic mole ratio on the Lipozyme RM IM lipase-catalyzed esterification process. Capric and oleic acids were used as MCFA and LCFA sources, respectively. The lipase-catalyzed synthesis was carried out by adding 8 wt% of immobilized Lipozyme RM IM lipase into the 250 ml conical flask containing capric and oleic acid mixtures and glycerol mixed in such an amount that yields a total FA to glycerol mole ratio of 3.5:1. The reaction was magnetically stirred for 12 h at 70°C under vacuum conditions (vacuum pressure = −28 psi) to remove the excess water and drive the reaction equilibrium towards esterification. Although the total FA to glycerol substrate mole ratio in the reaction mixtures were fixed at 3.5:1, the mole ratio between the FA, i.e., capric acid (M) and oleic acid (L) mixtures were prepared in such an amount that yields MLL/MLM in the molar ratio of 1:1, 2:1, and 3:1. In order to obtain MLL/MLM in the ratio of 1:1, a mole ratio of capric to oleic acids at 1:1 was prepared, while a mole ratio of capric acid to oleic acid of 4:5 and 5:7 was used to obtain MLL/MLM ratios of 2:1 and 3:1, respectively. After 12 h, the reaction was terminated by cooling the reaction mixture to room temperature, and lipase preparation removed from the reaction product by centrifugation at 4,000 rpm for 5 min. The samples were analyzed using reversed-phase high performance liquid chromatography (RP-HPLC) as described below. All reactions were performed in duplicate. The percentage of TAG obtained was calculated based on the sum percent of MMM, MLCT, and LLL while the percentage of MLCT was calculated based on the sum percent of MLM/MML and MLL/LML obtained, which are the two desired products in this work. The free fatty acid (FFA), MAG, and DAG, which are undesirable by-products, are grouped together and labeled as by-products (BP).
Data obtained were statistically analyzed by one-way analysis of variance (ANOVA) with the SAS 6.12 software package. Duncan’s multiple-range test was applied to determine significant differences, at a level of P < 0.05. Each reported value was the mean of two duplicate tests.
Experimental Design and Optimization by RSM
A five-level, four factorial CCRD was employed in this study, requiring 30 experiments for the estimation of the 15 coefficients of the model. The fractional factorial design consisted of 16 factorial points, 8 axial points, and 6 center points. The variable and their levels selected for the MLCT were as follows: reaction temperature (50–90°C), reaction time (6–14 h), substrate mole ratio [mixtures of capric acid/oleic acid at 5:7 mole ratio to glycerol, (3:1–4:1)], and amount of enzyme (6–14 wt%). The data obtained were fitted to a second-order modified model polynomial equation:
where Y is percentage of yield; b 0, bi, b ii , b ij , b iii were constant coefficients of intercept, linear, quadratic, interaction terms, and cubic, respectively, and x i and x j are independent variables. The data from the experiments performed were analyzed using Design Expert 6.06 version and then interpreted in three main analytical steps: ANOVA, a regression analysis and the plotting of response surface were performed to establish an optimum condition for the esterification reaction. The level of significance for all tests was set at 99% confidence level.
The goodness of fit model was evaluated by the coefficient of determination, R 2, together with absolute average deviation (AAD) values and the ANOVA. The overall predictive capability of the model was explained by the coefficient of determination, R 2, which was calculated from prediction error sum of squares (PRESS) residuals while the AAD analysis was calculated by the following equation:
where y i ,exp and y i ,cal were the experimental and calculated responses, respectively, and p is the number of experimental runs. The 3D surface plots were developed using the fitted reduced cubic (modified model) polynomial equations with backward elimination obtained by holding two of the independent variables at a constant value and changing the levels of the other two variables.
In this RSM study, lipase-catalyzed synthesis of MLCT was carried out in a 50 g batch reaction mixture inside a 250 ml conical flask and magnetically stirred at the desired temperature and linked to a vacuum pump to remove water formed during the esterification process. Lipozyme RM IM lipase was added to the reaction medium composed of capric acid, oleic acid, and glycerol at various ratios. The experiments were done according to the five-level, four factorial CCRD experimental design. At the end of reaction, the lipases were removed by centrifugation. The samples were subsequently analyzed for TAG and MLCT content by RP-HPLC. All the reactions were performed in duplicate.
HPLC Peak Identification
The products were analyzed with a SCL-10A HPLC system (Shimadzu, Japan) equipped with a Sil-10AD Auto Injector (Shimadzu, Japan) and RID-10A Detector (Shimadzu, Japan). Sample preparations were made by dissolving 10% (v/v) reactant in appropriate amount of acetone. An aliquot of 10 μl was used for injection. A precoated silica reversed phase C18 HPLC column, LiChroCART®5 μm (4 mm × 25 cm) from Merck (Darmstadt, Germany) was used as a stationary phase. The column temperature was set at 40°C. The isocratic mobile phase consisted of acetone and acetonitrile (50:50, v/v) with a flow rate of 1 ml/min. The total reaction time was 35 min. The contents of FFA, MAG, DAG, MLCT, and TAG were expressed as weight percent of the total weight of the sample. All analyses were performed in duplicates, and average values were reported.
Peak identification was performed by determining the range of retention times at which the relevant compounds elute, using selected reference standards. ECN defines the order of elution of triglycerides and reflects the contribution of each fatty acid to the chromatographic properties of the total triglyceride molecule (Podlaha and Töregård 1989; Wolff et al. 1991; Lo et al. 2004). According to definition, the elution order can be determined by calculating equivalent carbon numbers as ECN=CN-2n, where CN is the carbon number and n is the number of double bonds. Due to the lack of standards for MLM/MML and MLL/LML, both MLM/MML and MLL/LML were identified based on equivalent carbon number (ECN) and grouped as MLCT.
Model Verification: Scale-up Synthesis
Reaction conditions for the scale-up (500 g scale) esterification process were the same but the scale was increased by a factor of 10. The reaction was carried out in 500 g batch reaction mixtures inside a 2-l conical flask and magnetically stirred at the desired temperature and linked to a vacuum pump to remove water formed during the esterification process. Optimal amounts of Lipozyme RM IM lipase was added to the reaction medium composed of capric acid, oleic acid, and glycerol at optimal ratios and reaction times. At the end of reaction, the lipases were removed by centrifugation. The samples were subsequently analyzed for TAG and MLCT content and compared to the predictive values.
Physical and Chemical Characteristics of Refined MLCT Oil
The crude MLCT oil that produced through 500 g batch process was purified through physical refining like refined, bleached, and deodorization process. The refined MLCT oil was found to contain 76% MLCT-type triglycerides. FFA content (Ca 5a-40), iodine value (Cd 1d-92), cold stability test (Cc 11–53), and smoke point (Cc 9a-48) were determined using AOCS official methods (AOCS 1997). The oil color (PORIM p4.1, 1995) was measured in 5 1/4-inch cell in a Lovibond Tintometer (Salibury, UK). The oxidation stability strength of refined MLCT oil was determined with a 743 Rancimat Apparatus (Metrohm, Switzerland) by measuring the induction period of oils at 120°C, based on conductiometric method. The fatty acid profile analysis was performed by converting free and glyceride fatty acid to their corresponding methyl esters prior to the analysis by gas chromatography (GC; PORIM p3.4 1995). Fatty acid methyl esters (FAME) were prepared by transesterification of oil (50 μl) with n-hexane (950 μl) and sodium methoxide (0.5 N, 50 μl) analyzed on a Perkin Elmer Clarus 500 instrument, equipped with a flame-ionization detector (FID). A capillary column DB-Wax of 0.25 mm internal diameter, 30 m length, and 0.25 μm film thickness was used. FAME peaks were identified by comparison of retention times to a standard mixture. The peak areas were calculated using Totalchrom Navigator software, and percentages of FAME were obtained as area percentages by direct normalization. All analyses were carried out in duplicate.
Results and Discussion
Effect of Capric/Oleic Mole Ratio on the Esterification Synthesis
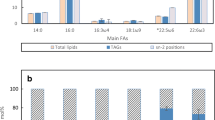
Figure 1a–c showed the time course of the esterification reaction catalyzed by Lipozyme RM IM lipase when different capric/oleic mole ratios of 1:1, 4:5, and 5:7 were used, respectively. As reactions were conducted in vacuum conditions, water was continuously removed to drive the reaction equilibrium towards synthesis of esterification. The amount of undesirable by-products, BP (sum of % FFA + MAG + DAG) reduced as the reaction time increased while the weight percent of triacylglycerols, TAG (sum of percent MMM, MLCT, and LLL) and medium- and long-chain triacylglycerols, MLCT (sum of percent MLM/MML and MLL/LML) increased. Similar trends were observed for all the three reactions.
At the end of 12 h (Table 1), all three mixtures contained almost similar amounts of BP (25–27 wt%), MLCT (58–60 wt%) and TAG (73–75 wt%). No significant differences (P > 0.05) were observed between the different mole ratios. We can conclude from Fig. 1 that as reaction time increased, MLCT and TAG formation also increased. The relationship between reaction time and product yield seem to exhibit an asymptotic approach to equilibrium, where complete conversion to the desired products, MLCT, in this case, will not occur on any time scale. When the mole ratio of capric/oleic increased from 1:1 to 5:7 (more oleic acid as a source of LCFA added), the percent of MMM formed decreased from 8 to 5 wt% while the weight percent of LLL increased from 8 to 11 wt% (Table 1). Both MMM and LLL showed significant differences (P < 0.05) when different capric/oleic molar ratios were used. This observation also revealed that Lipozyme RM IM has higher selectivity towards long-chain fatty acid and showed a preferential incorporation of oleic acid rather than capric acid into the TAG pool during the esterification reaction. A similar observation was also reported in the work of Fu and Parkin (2004).
In designing the experimental protocol for production of MLCT, where the MLCT oil will be used as cooking oil, the percent MMM formed is of importance. In order to design healthy functional cooking oil that has the nutritional benefits of MLCT while having the good properties suitable for frying, the cooking oil should have low percent MMM formed. TAG having three MCFA moieties in the glycerol structure can cause foaming problems during frying (Negishi et al. 2003; Takeuchi et al. 2004). The smoke point of the oil will also be significantly lowered. Based on this criterion, the substrate mole ratios in this experiment were designed so as to yield higher MLCT in order to obtain a significantly lower percent of MMM in the final product. As such, capric/oleic at a mole ratio of 5:7 was selected for the subsequent RSM optimization studies as it had lower percent MMM and higher percent LLL as opposed to other molar ratios.
Model Fitting and ANOVA
Experimental data for the various runs of five-level, four-factorial design and each respective predicted and experimental TAG and MLCT yield were given in Table 2. The predicted values were obtained from the model fitting technique using Design Expert 6.06 and were seen to be closely correlated to the experimental value. Fitting of the data to various models (modified, linear, quadratic and cubic) and their subsequent ANOVA showed that enzymatic esterification of FA to glycerol were most suitably described with reduced cubic polynomial model (modified model), whereby insignificant factors and interactions were deleted from the model. The modified model with backward elimination was also shown in other published studies such as the modeling of lipase-catalyzed acidolysis of sesame oil and caprylic acid (Kim and Akoh 2005) and process optimization of dietary DAG by lipase-catalyzed glycerolysis (Kristensen et al. 2005). These published studies showed a well-fitted modified model after multiple regression analysis with backward elimination.
In order to determine whether this polynomial model was significant, it was necessary to conduct ANOVA. The ANOVA of wt% TAG yield and wt% MLCT yield after esterification, respectively, were presented in Table 3. The model adequacy was evaluated by an F test and the determination coefficient, R 2. The computed model F value of 102.66 in wt% TAG yield and F value of 106.86 in wt% MLCT yield was higher than tabular value of F 0.01(15,14) = 3.656, implying that the model was significant at 99% confidence level. The model also showed no lack of fit, as was evident from the lower computed F value of 7.04 for wt% TAG yield and F value of 5.33 for wt% MLCT yield as compared to tabular F 0.01(9,5) (10.158) at 99% confidence level with no outliers observed.
The overall predictive capability of the model was commonly explained by the coefficient of determination (R 2) which was calculated from PRESS. However, adding a variable to the model will always increase R 2, regardless of whether the additional variable was statistically significant or not. Thus, it is possible for model that has good value of R 2 to yield poor prediction or estimate the mean responses (Myers and Montgomery 1995). Therefore, absolute average deviation (AAD) analysis, which is a direct method for describing the deviation can be used to eliminate these types of error. In this case, the goodness of model fit was expressed by the high values of R 2 and adjusted R 2 with low value of AAD which showed a close agreement between the experimental results and the theoretical values predicted by the reduced cubic polynomial model. The coefficient of determination, R 2 for TAG and MLCT models were 0.9910 and 0.9913, respectively, while the AAD value for TAG and MLCT models were 7.57% and 6.99%, respectively. The R 2 was close to 1.0 with low value of AAD between the predicted and observed data reflecting the true behavior of the system on model equation (Deniz and İsmail 2007). And the pure error was low, indicating good reproducibility of the data obtained. The very small P value (0.0001) from the analysis of ANOVA indicated that reduced cubic polynomial model was highly significant and sufficient to represent the actual relationship between response (wt% TAG and MLCT yield) and the significant variables. The variables of reaction temperature, reaction time and substrate mole ratio were the most significant in the process. The enzyme load had an insignificant effect on the esterification reaction under this experimental design. However, most of the interactions between these variables were found to be insignificant at 99% confidence level. Only interaction of reaction temperature with reaction time and also interaction of reaction time with enzyme load were found to be significant at 99% confidence level.
A multiple regression analysis was performed to fit second order modified model of polynomial equations to the experimental data points. Table 3 showed the similar trends on the main effects of regression coefficients variables on the TAG and MLCT yields. Both TAG and MLCT responses have positive effect on all first-order coefficients except for reaction temperature which showed negative effects. Most of the second-order coefficients for reaction temperature, substrate mole ratio and enzyme have negative effects. Other variables have relatively small effects of coefficients value either on MLCT or TAG yields.
Effect of Parameters
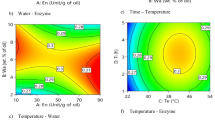
Figure 2 showed the response surface plots as function of reaction time and reaction temperature and their mutual effects on the synthesis of wt% TAG and MLCT at 10 wt% enzyme load and FA to glycerol mole ratio of 3.8:1. Both wt% TAG and MLCT yield increased from 50°C to 80°C and thereafter decreased with a dome shape observed. The wt% TAG and MLCT yield also increased with reaction time. The maximum increase in wt% TAG and MLCT yield indicated that greater enzyme synthesis occurred at 80°C than at 50°C and 90°C with a shorter reaction time (less than 8 h). Figure 3 showed the effect of varying FA to glycerol mole ratio and reaction temperature on the wt% TAG and MLCT yields at constant reaction time of 8 h with the amount of enzyme constant at 10 wt%. It also showed a dome shape as described in Fig. 2. In this case, one axis was in a linear increase in enzymatic synthesis, while in the other axis, the increase was only up to 77.3 wt% for TAG yield and 61.3 wt% for MLCT yield before decreasing. Many lipase-catalyzed esterification systems exhibit this type of plots (Manohar and Divakar 2004). Higher substrate mole ratios are known to drive the reaction equilibrium higher and increase the theoretical yield obtained. However, higher incorporation of FA will have an inhibition effect and also causes the reaction to have a longer time before reaching equilibrium. Both Figs. 2 and 3 exhibited a critical temperature of 80°C, which favored the esterification synthesis under vacuum conditions and became not so after that critical temperature. Lower percent yield was observed at temperatures >80°C while higher yield was shown at high substrate mole ratio (Fig. 3). This indicated the low operational stability of the enzyme at high temperatures because of the inactivation of enzyme at temperatures over 80°C. High temperature has caused changes in the enzymes’ conformation, indicating thermal denaturation as the protein linkages binding the lipase was disrupted.
Figure 4 depicted the response surface plots as function of reaction temperature versus enzyme load at reaction time of 8 h with FA to glycerol mole ratio of 3.8:1. This response surface plot indicated that the amount of enzyme was insignificant (P > 0.01) in conferring any considerable effect on the percent TAG and MLCT yield in the esterification synthesis, as shown in Table 3. Higher esterification rate was observed with an increase in temperature until the critical temperature of 80°C, but then decreased on further temperature rising. There are three reasons that may explain this phenomenon. Higher temperature increase reaction rates based on the rule of Arrhenius equation. It is also related to faster water removal that favored esterification reaction at high temperatures. Higher temperature also reduced the viscosity of the mixtures, and this increased the transfer of substrates and products on the surface or inside the enzyme particles. Above 80°C, the enzyme may also become less active due to protein denaturation at high temperatures. Figure 5 illustrated the effect of varying the amount of enzyme and reaction time at constant temperature of 70°C with FA to glycerol mole ratio of 3.8:1. The 3D surface plot showed maximal wt% TAG and MLCT yield obtained when moderate amounts of enzyme of 8 to 10 wt% was used in the reaction with reaction time less than 12 h. Further increase of enzyme load did not improve much on the percent esterification yield. This indicated that enzyme load was a less significant variable in the optimization of esterification synthesis under this response surface experimental design at the probability level of 99%. Increase in enzyme load will only shorten the reaction time but does not increase the TAG or MLCT yield. Similar observations were observed in the work of Jennings and Akoh (2001) and Zhao et al. (2007) who also reported no significant difference from analysis of variance on the enzyme load. To be cost effective, it is preferable to choose low enzyme load for the subsequent work. In this case, 10 wt% enzyme load was chosen for the 500 g scale up batch reaction which was conducted to verify on the validity of the response model. For future larger-scale MLCT oil production, 10 wt% enzyme load will be packed into the column, and the reaction will be conducted in the continuous packed bed bioreactor to have better enzyme stability.
Optimization and Model Verification
The optimal conditions for the lipase-catalyzed esterification synthesis of TAG and MLCT were predicted using optimization function of the Design Export Software. Table 4 showed some of the solutions predicted for the maximal percent TAG and MLCT yield under optimization conditions at which the temperature was targeted at 70°C and enzyme load was set at 10 wt% due to cost consideration, while the other variables were set in the range such as reaction time in between 11.5 and 14 h, FA to glycerol in the mole ratios of 3:1 and 4:1. All the optimum conditions can be used to produce high wt% of TAG and MLCT. The temperature of 70°C was chosen instead of 80°C as enzyme was sensitive to heat and lower reaction temperature resulted in less harsh conditions to the esterification reaction. In addition, the enzyme cost is the most expensive variable compared to others. Therefore, lower temperature is preferred to allow better operational stability of the enzymes and this allows the enzyme to be used repeatedly for a longer period of time.
To verify the predicted results of this model, the experiment was scaled-up to ten times the total FA (wt% = 500 g) used in the RSM study under optimum conditions. The FA to glycerol mole ratio of 3.5:1 was again chosen for the large-scale production to avoid potential problems caused by the high viscosity of glycerol used. At the targeted temperature of 70°C and enzyme load of 10 wt%, the maximal yield of 75.33 wt% TAG and 59.76 wt% MLCT were predicted to be achieved if the reaction time was 14 h and the FA to glycerol mole ratio was 3.5:1. The experimental results using optimal conditions representing the maximal yield were performed and a TAG and MLCT yield of 70.43 ± 0.64% and 56.35 ± 0.31% (n = 2), respectively, were obtained. The good correlation between the actual and predicted value verified the validity of the response model and the optimal point obtained.
Physical and Chemical Characteristics of Refined MLCT Oil
The refined MLCT oil characteristics were summarized in Table 5. The lighter color and low fatty acid content of MLCT oil with the smoke point value more than 180°C, indicated that this oil was suitable for cooking/frying applications. A relative low rancimat hour in refined MLCT oil was observed, mainly due to the lack of antioxidants in this MLCT oil. Nevertheless, this oxidative weakness can be overcome by using synthetic or natural antioxidants to strengthen its oxidative stability towards thermal degradation. High iodine value of 59.49 g of I2/100 g MLCT oil indicated a larger amount of unsaturated fatty acids (∼65%) than saturated fatty acids (∼35%) in the oil. The medium- and long-chain triacylglycerols structure in MLCT oil with the high iodine value play a key role in preventing crystal growth at low temperature as shown in cold stability test at 5°C, whereby the oil remained crystal clear for more than 24 h.
The distribution of saturated fatty acids (SFA)/monounsaturated fatty acids (MUFA)/polyunsaturated fatty acids (PUFA) gave an interest point whereby type of fatty acid composition ratios can be grouped as 3.5:5.7:0.8. The high amount of unsaturated fatty acids with the lowest amount of PUFA made this MLCT oil suitable to be used as healthy cooking oil as PUFA with the lowest oxidative stability as compared other types of fatty acids has undesirable effects that may develop off-flavor that lower the palatability of fried food (Warner et al. 1997). In general, the refined MLCT oil showed its suitability to be used as healthy cooking oil for long-term obesity management therapy. Further study on the use of synthetic or natural antioxidants in MLCT oil to enhance its thermal-resistant oxidation stability will be conducted in future.
Conclusion
The mathematic modeling of the esterification of capric acid and oleic acid to glycerol, catalyzed by Lipozyme RM IM lipase was attempted by RSM for the production of healthful functional lipids, such as the MLCT cooking oil. Comparison of predicted and experimental R 2 values with low value of AAD revealed good correspondence between them, implying that the reduced cubic polynomial models with backward elimination (modified model) derived from RSM can be used to describe the relationship between the various factors and response (wt% yield) in the lipase-catalyzed esterification synthesis of MLCT cooking oil. The effect of enzyme load was found to be less significant (P > 0.01) while reaction temperature, reaction time, and FA to glycerol mole ratio strongly affected (P < 0.01) the TAG and MLCT yield in this RSM experimental ranges. According to the CCRD optimization, a maximal yield of TAG (75.33%) and MLCT (59.75%) can be obtained using 10 wt% enzyme load, reaction temperature of 70°C, reaction time at 14 h and FA to glycerol mole ratio of 3.5:1. From this work, optimum conditions for the synthesis of TAG and MLCT can be successfully predicted as experimental results showed close correlation to the predicted values obtained. The maximal yield obtained from the experiments was 70.43% for TAG and 56.35% for MLCT yield. The physical and chemical characteristics of refined MLCT oil showed its suitability to be used for cooking/frying applications.
References
AOCS (1997). Official methods and recommended practices of the American oil chemist’s society (5th ed.). Champaign, USA: American Oil Chemist’s Society Press.
Aoyama, T. (2003). Fats and oils composition for reducing lipids in blood. U. S. Patent No 0170368 A1.
Bendixen, H., Flint, H. A., Raben, A., Høy, C. E., Mu, H., Xu, X., et al. (2002). Effect of 3 modified fats and a conventional fat on appetite, energy intake, energy expenditure and substrate oxidation in healthy men. American Journal of Clinical Nutrition, 75, 47–56.
Deniz, B., & İsmail, H. B. (2007). Modelling and optimization I: usability of response surface methodology. Journal of Food Engineering, 78, 836–845.
Fomuso, L. B., & Akoh, C. C. (1997). Enzymatic modification of triolein: incorporation of caproic and butyric acids to produce reduced-calorie structured lipids. Journal of the American Oil Chemists’ Society, 74, 269–272.
Fu, X., & Parkin, K. L. (2004). Selectivity of fatty acid incorporation into acylglycerols in esterification reactions using Rhizomucor miehei and Burkholderia cepacia lipases. Food Research International, 37, 651–657.
Garnacho-Montero, J., Ortiz-Leyba, C., Jiménez-Jiménez, F. J., Garcia-Garmendia, J. L., Jiménez-Jiménez, L. M., Garnacho-Montero, M. C., et al. (2002). Clinical and metabolic effects of two lipid emulsions on the parenteral nutrition of septic patients. Nutrition, 18, 134–138.
Hassel, C. (1993). Nutritional implication on fat substitutes. Cereal Foods World, 38, 142–144.
Jennings, B. H., & Akoh, C. C. (2001). Lipase catalyzed modification of fish oil to incorporate capric acid. Food Chemistry, 72, 273–278.
Kasai, M., Nosaka, N., Maki, H., Negishi, S., Aoyama, T., Nakamura, M., et al. (2003). Effect of dietary medium- and long-chain triacylglycerols (MLCT) on accumulation of body fat in healthy humans. Asia Pacific Journal of Clinical Nutrition, 12, 151–160.
Kawashima, A., Nagao, T., Watanabe, Y., Kobayashi, T., Ikeda, I., Tominaga, Y., et al. (2004). Preparation of regioisomers of structured TAG consisting of one mole of CLA and two moles of caprylic acid. Journal of the American Oil Chemists’ Society, 81, 1013–1020.
Kawashima, A., Shimada, Y., Yamamoto, M., Sugihara, A., Nagao, T., Komemushi, S., et al. (2001). Enzymatic synthesis of high-purity structured lipids with caprylic acid at 1,3-positions and polyunsaturated fatty acid at 2-position. Journal of the American Oil Chemists’ Society, 78, 611–616.
Kim, B. H., & Akoh, C. C. (2005). Modelling of lipase-catalyzed acidolysis of sesame oil and caprylic acid by response surface methodology: optimization of reaction conditions by considering both acyl incorporation and migration. Journal of Agriculture and Food Chemistry, 53, 8033–8037.
Kristensen, J. B., Xu, X., & Mu, H. (2005). Process optimization using response surface design and pilot plant production of dietary diacylglycerols by lipase-catalyzed glycerolysis. Journal of Agriculture and Food Chemistry, 53, 7059–7066.
Lai, H. S., & Chen, W. J. (2000). Effects of medium-chain and long-chain triacylglycerols in pediatric surgical patients. Nutrition, 16, 401–406.
Lin, M. T., Yeh, S. L., Kuo, M. L., Liaw, K. Y., Lee, P. H., Chang, K. J., et al. (2002). Effects of medium-chain triglyceride in parenteral nutrition on rats undergoing gastrectomy. Clinical Nutrition, 21, 39–43.
Lo, S. K., Baharin, B. S., Tan, C. P., & Lai, O. M. (2004). Analysis of 1,2(2,3)- and 1,3-positional isomers of diacylglycerols from vegetable oils by reversed-phase high-performance liquid chromatography. Journal of Chromatographic Science, 42, 145–154.
Manohar, B., & Divakar, S. (2004). Applications of surface plots and statistical design to selected lipase-catalyzed esterification reactions. Process Biochemistry, 39, 847–853.
Matsuo, T., Matsuo, M., Taguchi, N., & Takeuchi, H. (2001). The thermic effect is greater for structured medium- and long-chain triacylglycerols versus long-chain triacylglycerols in healthy young women. Metabolisme, 50, 125–130.
Matsuo, T., & Takeuchi, H. (2004). Effects of structured medium- and long-chain triacylglycerols in diets with various levels of fat on body fat accumulation in rats. British Journal of Nutrition, 91, 219–225.
Matulka, R. A., Noguchi, O., & Nosaka, N. (2006). Safety evaluation of a medium- and long-chain triacylglycerol oil produced from medium-chain triacylglycerols and edible vegetable oil. Food and Chemical Toxicology, 44, 1530–1538.
Mu, H., Kurvinen, J. P., Kallio, H., Xu, X., & Høy, C. E. (2001). Quantitation of acyl migration during lipase-catalyzed acidolysis, and of the regioisomers of structured triacylglycerols formed. Journal of the American Oil Chemists’ Society, 78, 959–964.
Mu, H., & Porsgaard, T. (2005). The metabolism of structured triacylglycerols. Progress in Lipid Research, 44, 430–448.
Myers, R. H., & Montgomery, D. C. (1995). Response surface methodology: Process and product optimization using design experiments. New York, USA: John Wiley & Sons.
Negishi, S., Itakura, M., Arimoto, S., Nagasawa, T., & Tsuchiya, K. (2003). Measurement of foaming of frying oil and effect of the composition of TG on foaming. Journal of the American Oil Chemists’ Society, 80, 471–474.
Podlaha, O., & Töregård, B. (1989). Some new observations on the equivalent carbon numbers of triglycerides and relationship between changes in equivalent carbon number and molecular structure. Journal of Chromatography, 482, 215–226.
PORIM Test Methods (1995). Palm Oil Research Institute of Malaysia, Ministry of Primary Industries, Malaysia.
Rubin, M., Moser, A., Vaserberg, N., Greig, F., Levy, Y., Spivak, H., et al. (2000). Structured triacylglycerol emulsion, containing both medium- and long-chain fatty acids, in long-term home parenteral nutrition: a double-blind randomized cross-over study. Nutrition, 16, 95–100.
Seaton, T. B., Welle, S. T., Warenko, M., & Campbell, R. G. (1986). Thermic effect of medium-chain and long-chain triglycerides in man. American Journal of Clinical Nutrition, 44, 630–634.
Shinohara, H., Ogawa, A., Kasai, M., & Aoyama, T. (2005). Effect of randomly interesterified triacylglycerols containing medium- and long-chain fatty acids on energy expenditure and hepatic fatty acid metabolism in rats. Bioscience, Biotechnology and Biochemistry, 69, 1811–1818.
Shinohara, H., Shimada, H., Noguchi, O., Kubota, F., & Aoyama, T. (2002). Effect of medium-chain fatty acids-containing dietary oil on hepatic fatty acid oxidation enzyme activity in rats. Journal of Oleo Science, 51, 621–626.
Socha, P., Koletzko, B., Demmelmair, H., Jankowska, I., Stajniak, A., Bednarska-Makaruk, M., et al. (2007). Short-term effects of parenteral nutrition of cholestatic infants with lipid emulsions based on medium-chain and long-chain triacylglycerols. Nutrition, 23, 121–126.
Swift, L. L., Hill, J. O., Peters, J. C., & Grene, H. L. (1992). Plasma lipids and lipoproteins during 6 d of maintenance feeding with long-chain, medium-chain and mixed-chain triglycerides. American Journal of Clinical Nutrition, 56, 881–886.
Takeuchi, H., Itakura, M., Kubota, F., & Taguchi, N. (2004). Oil or fat composition, U. S. Patent No 0191391 A1.
Warner, K., Orr, P., & Glynn, M. (1997). Effect of the fatty acid composition of oils and flavour. Journal of the American Oil Chemists’ Society, 74, 347–356.
Wolff, J. P., Mordret, F. X., & Dieffenbacher, A. (1991). Determination of triglycerides in vegetable oils in terms of their partition numbers by high performance liquid chromatography. Pure and Applied Chemistry, 63, 1173–1182.
Xu, X. (2000a). Production od specific-structured triacylglycerols by lipase-catalyzed reactions: a review. European Journal of Lipid Science and Technology, 102, 287–303.
Xu, X., Balchen, S., Høy, C. E., & Adler-Nissen, J. (1998). Pilot batch production of specific-structured lipids by lipase-catalyzed interesterification: Preliminary study on incorporation and acyl migration. Journal of the American Oil Chemists’ Society, 75, 301–308.
Xu, X., Fomuso, L. B., & Akoh, C. C. (2000b). Synthesis of structured triacylglycerols by lipase-catalyzed acidolysis in a packed bed bioreactor. Journal of Agriculture and Food Chemistry, 48, 3–10.
Zhao, H., Lu, Z., Bie, X., Lu, F., & Liu, Z. (2007). Lipase catalyzed acidolysis of lard with capric acid in organic solvent. Journal of Food Engineering, 78, 41–46.
Acknowledgement
The authors gratefully acknowledge Mr Azmi and Ms Mary Goh from MARDI, Serdang and Mr Govalan, Mr Radha Krishnan and Mr Vijay Krishnan from Golden Jomalina, Banting for their kind technical assistance. We thank Golden Hope Sdn. Bhd. for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koh, S.P., Tan, C.P., Lai, O.M. et al. Enzymatic Synthesis of Medium- and Long-Chain Triacylglycerols (MLCT): Optimization of Process Parameters Using Response Surface Methodology. Food Bioprocess Technol 3, 288–299 (2010). https://doi.org/10.1007/s11947-008-0073-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-008-0073-y