Abstract
In this work, supercritical technology was used to obtain extracts from Ocimum basilicum (sweet basil) with CO2 and the cosolvent H2O at 1, 10, and 20% (w/w). The raw material was obtained from hydroponic cultivation. The extract’s global yield isotherms, chemical compositions, antioxidant activity, and cost of manufacturing were determined. The extraction assays were done for pressures of 10 to 30 MPa at 303 to 323 K. The identification of the compounds present in the extracts was made by GC-MS and ESI-MS. The antioxidant activity of extracts was determined using the coupled reaction of beta-carotene and linolenic acid. At 1% of cosolvent, the largest global yield was obtained at 10 MPa and 303 K (2%, dry basis—d.b.); at 10% of cosolvent the largest global yield was obtained at 10 and 15 MPa (11%, d.b.), and at 20% of cosolvent the largest global yield was detected at 30 MPa and 303 K (24%, d.b.). The main components identified in the extracts were eugenol, germacrene-d, epi–alpha–cadinol, malic acid, tartaric acid, ramnose, caffeic acid, quinic acid, kaempferol, caffeoylquinic acid, and kaempferol 3-O-glucoside. Sweet basil extracts exhibited high antioxidant activity compared to beta-carotene. Three types of SFE extracts from sweet basil were produced, for which the estimated cost of manufacturing (class 5 type) varied from US$ 47.96 to US$ 1,049.58 per kilogram of dry extract.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The diet plays an important role in the four major diseases of society: cardiovascular diseases, cancer, hypertension, and obesity. Diseases of heart and cancer correspond to 70% of all deaths. The scale of importance of the diet on the development of these diseases is still unknown, but estimates accepted by the scientific community show that at least 1/3 of the cases of cancer and half of the cases of arterial diseases and hypertension (coronary heart diseases, CHD) are related to food ingestion (Goldberg 1994; Block et al. 1999; Ness and Powles 1997). The beneficial effects of foods are being partially attributed to the naturally occurring antioxidant substances (Vision et al. 1999).
Ocimum basilicum L. (sweet basil) is a member of the Lamiaceae family that is used as food seasoning. Species of the Laminaceae family are also valuable due to their pharmaceutical properties; for example, the volatile oil produced by their leaves is used as antioxidant (Harsh et al. 2002). Many scientific studies showed that sweet basil extract is a strong radical scavenger and can be considered as a good source of natural antioxidants (Javanmardi et al. 2003; Lee et al. 2005; Abas et al. 2006). Extracts of herbs, vegetables, cereals, fruits, and other vegetal materials rich in phenolic compounds are interesting in the food industry because they delay the oxidation of lipids and thereby improve the quality and the nutritional value of foods (Velioglu et al. 1998), but the Javanmardi et al. (2003) work suggested that 71% of antioxidant capacity of Ocimum is not originated solely by the phenolic compounds—it may also come from the presence of secondary metabolites, such as volatile oils, carotenoids, vitamins, and other substances. Jayasinghe et al. (2003) showed the existence of a synergistic antioxidant effect in sweet basil extract between rosmarinic acid and α-tocopherol. The work of Yamasaki et al. (1998) showed that sweet basil aqueous extracts have an anti-HIV action. Aqueous extracts of sweet basil have an action on the cardiovascular system, and alcoholic extracts have an activity similar to that of atropine (Perez-Guitiérrez et al. 1992).
Supercritical fluid extraction (SFE) is interesting for processing natural products because it produces extracts without organic residues, and the temperature of the process can be low, thus preserving thermo-sensitive compounds. The extraction with supercritical fluids involves a high mass-transfer rate at relatively low temperatures, which is the major reason for the choice of this technique, for extracting essential oils, oleoresins, and other active principles where the final product quality is of great importance. Recently, the use of subcritical water for extraction is being exploited because by varying the temperature and the pressure of the water, the dielectric constant approached very closely the dielectric constants of some organic compounds as methanol, ethanol, butanone, polyaromatic hydrocarbons, phenol, benzene, and others (Hawthorne et al. 1994). Subcritical water can produce extracts with a variety of organic substances with linear or ramified chains, showing the effectiveness of water as a solvent for hydrophobic organic composite extraction, due to the polarity of water measured by its dielectric constant (Miller and Hawthorne 1998). Bavel et al. (1999) concluded that for nonpolar environmental pollutants that can be extracted using water, such method is especially interesting in the development of remediation techniques for contaminated soil. The research for natural sources of compounds with functional activity to be added to foods (functional foods) for specific population groups (diabetics, hypertensives, etc.) is important and necessary.
Leal et al. (2006) reported data on the obtainment of extracts of clove basil (Ocimum gratissimum) by supercritical fluid extraction (SFE). Eugenol was the major compound (41.5–46.6%, area) of SFE extracts obtained from clove basil. Eugenol may also be present in sweet basil extracts, but not as a major compound. Schulz et al. (2003) reported the composition of 11 chemotypes of the basil gender. These authors showed the huge differences in extracts composition of the various chemotypes. Vinã and Murillo (2003) analyzed by GC-MS the volatile oil from 12 basil cultivars and demonstrated the differences in composition. The antioxidant activity of plant extracts is associated with various classes of chemical compounds, and, as mentioned before, the literature reports a synergistic effect among these compounds (Jayasinghe et al. 2003). In spite of some similarities in their usage as food seasoning, the composition and antioxidant activity of sweet basil extracts be quite different from that of the extracts of clove basil reported by Leal et al. (2006). This way, this study investigated the use of supercritical fluid extraction, employing carbon dioxide as solvent and water as cosolvent, with the objective of extracting aqueous soluble as well as volatile oil compounds from sweet basil leaves. To evaluate the effectiveness of the process, i.e., as a quality control tool of the process, the chemical compositions of the extracts were analyzed by gas chromatography and electrospray ionization mass spectrometry, and the antioxidant activity of the extracts was assessed using the coupled reaction of linolenic acid and beta-carotene. Because SFE is associated with high investment costs and because the sweet basil extracts produced by SFE are not commercially available, an estimate of the cost of manufacturing (class 5 type) of extracts by SFE was performed.
Materials and Methods
Plant Cultivation, Analysis, and Preparation
On February 21, 2003, at the Agronomic Institute Experimental Farm in Campinas, Brazil (22°54′S, 47°05′W, 674 m high), a planting experiment was carried with sweet basil (Ocimum basilicum L.) with voucher number 42800 at IAC herbarium. Seedlings of sweet basil 5-cm high were planted in pots in a completely random experimental design with 20 replications. Each plot consisted of two plants in a pot filled with grounded silica as inert material.
Treatments consisted of a modified Hoagland no. 1 solution, developed by Sarruge (1975). Nutrient solutions were delivered to plants by deep irrigation every 5 h during daylight by an automatic solution recycling apparatus, especially developed for plant nutrition research, described by Maia et al. (1999). The automatic hydroponic system (see Fig. 1) promotes the increase in roots aeration, increases the irrigation frequency, and enhances the water availability (Maia et al. 1999). Table 1 shows the nutrient content of the nutrition solution responsible for feeding the hydroponic system. Besides the control, two lots of raw material were cultivated (Table 1): lot 2N, in which the nutrition solution had the double of nitrogen content of the control, and lot 1/2 N, in which the nutrition solution had half of nitrogen content of the control lot. The control solution contained 210 kg/m3 of nitrogen (N); the amounts of the other nutrients (P, K, Ca, and Mg) were kept constant. The studied parameters were dry matter accumulation and nutrient content of leaves and roots. Plants were harvested at 68 days after planting.
Automatic system of nutritive substrate supply used for hydroponic cultivation of sweet basil (Maia et al. 1999)
A sample of sweet basil leaves and roots, 50 g per plot, was delivered to chemical analysis of nutrient content (N, P, K, Ca, Mg, S, B, Cu, Fe, Mn, and Zn). The method described by Sarruge and Haag (1974) was used for the analysis of macronutrients; Bataglia et al. (1983) method was used for the analysis of micronutrients and determination of biomass.
The sample of raw material used in the extraction experiments consisted of a mixture formed of 60% of lot 2N and 40% of lot 1/2N. Sweet basil leaves were dried using a forced air circulation dryer (Fanem, Guarulhos, Brazil) at 318 K up to constant weight. The humidity of the dried leaves was determined by the infrared method (CEM, Model Smart, Matthews, NC). The infrared method was selected because of the restricted amount of raw material; this method was previously used by Leal et al. (2006). Ideally, the humidity should be determined by the xylene distillation method (Jacobs 1981), but the amount of raw material available made it impossible to use. Therefore, the infrared method was used instead because it uses one tenth to one twentieth of the raw material that is used in Jacobs (1981) method. The dried leaves were packed in bags, protected from light to avoid photodegradation, and stored at 291 K. The sweet basil leaves were comminuted in a mill (Tecnal, model TE-631, series 01071, Piracicaba, Brazil) for 7 s at 14,000 rpm and separated according to their sizes (standard testing sieves, Tyler series). Meshes sizes −24 and +48 were selected for the extraction assays.
The particles’ true density was determined by helium pycnometry (Micrometrics, model Multivolume pycnometer 1305, Norcross, GA) in the Analytical Chemistry Facilities of the Institute of Chemistry, IQ/UNICAMP. The apparent bed density was calculated using the mass of feed and the volume of the extractor’s cell. The total porosity (bed + particles) was determined using the particles’ true density and the bed apparent density.
Extraction
The experimental runs were conducted in the SFE unit described by Pasquel et al. (2000), containing a cosolvent pump and an extraction cell of approximately 221 cm3 (length of 37.5 cm and inside diameter of 2.74 cm) and a maximum allowable pressure of 40 MPa using the procedure described by Zancan et al. (2002). The fixed bed was formed inside a nylon basket (mesh 80). A total of 2.5 g of sweet basil particles was added to the extraction basket; glass spheres were used to fill the empty space of the extraction cell. The thermostatic bath controlling the CO2 inlet flow to the pump was set at 263 K. After reaching thermal equilibrium, the system was slowly pressurized by opening the valve at the extractor’s inlet and allowing CO2 + bi-distilled water (1, 10, and 20% w/w) to flow across the extraction basket. Once the system reached the operating pressure and stabilized, the valves from the extractor’s outlet were opened and the extraction process began. CO2 and cosolvent were admitted into the system at flow rates ranging from 5 × 10−5 to 8.0 × 10−5 kg/s; nonetheless, the flow rates were maintained constant during each extraction assay. The solvent + cosolvent flow continued for 1 h; the system depressurization required an average period of 50 min.
The SFE extracts obtained varied, depending on the amount of cosolvent used. For 10 and 20% of cosolvent, the extracts were obtained in a single phase, whereas for 1% of cosolvent, two-phase extracts were obtained. This two-phase extract showed a light phase resembling the volatile oil obtained by hydrodistillation, and a heavy phase similar to aqueous extracts. To separate the light and heavy phases, 3 cm3 of xylene (99.95% purity, Ecibra, lot 15492, São Paulo, Brazil) were added to the extracts of sweet basil obtained using 1% (w/w) of bi-distilled water + CO2; xylene was chosen because of its immiscibility with water. The two phases were denoted as xylene-rich phase containing the xylene phase soluble substances and the aqueous-phase containing the water-soluble substances. After separation of the phases, xylene was eliminated from the xylene phase of the extract using a rotavap (Laborota, model 4001, Vertrieb, Germany) at 313 K with vacuum control (Heidolph Instruments GMBH, Vertrieb, Germany) at 25 × 10−4 MPa. In the tests done using 10 and 20% (w/w) of bi-distilled water, the water contained in the sweet basil extracts was eliminated by freeze-drying (FTS Systems, mod. EZ-DRY, Stone Ridge, NY).
The amount of the soluble material was calculated as the ratio of the total mass of extract (freeze-dried or xylene phase) to the total initial dry mass of sweet basil. The experiments were run at 303 and 323 K and pressures of 10, 15, 20, 25, and 30 MPa. Carbon dioxide of 99.8% (Gama Gases Industrials, Campinas, Brazil) was used.
Characterization of the Extracts
Gas Chromatography
The sweet basil extract chemical compounds were identified using a GC-MS system (Hewlett Packard, model 5890, series II, Palo Alto, CA) equipped with a fused silica capillary column HP ultra-2. The carrier gas was helium (1.67 × 10−2 cm3/s; 99.99% purity, White Martins Gases Industrials, Brazil); a split ratio of 1:20 was used. The temperatures of the injector and of the detector were 493 and 513 K, respectively. The column was heated from 333 to 573 K at 0.05 K/s. One microliter of the samples was injected. The identification of the substances was based on (1) comparison of substance mass spectrum with GC-MS system data bank (Nist 62 Library), and (2) comparison of mass spectra with data in literature (McLaferty and Stauffer 1989; Adams 1995). To detect the percentage in area of the major compounds, all extracts were also analyzed in a GC–FID system (Shimadzu, QP-5000, Kyoto, Japan), equipped with a fused silica capillary column DB-5 (30 m × 0.25 mm × 0.25 μm; J & W Scientific, Folsom, CA). The carrier gas was helium (2.83 × 10−2 cm3/s; 99.99% purity, White Martins Gases Industriais, Brazil); a split ratio of 1:20 was used. The temperatures of the injector and of the detector were 493 and 513 K, respectively. The column was heated from 333 to 519 K at 0.05 K/s. One microliter of each sample was injected (10–15 μg of extract diluted in 1 cm3 of ethyl acetate).
Electrospray Ionization Mass Spectrometer
For fingerprinting electrospray ionization mass spectrometer (ESI-MS) analysis, a hybrid high-resolution and high-accuracy (5 ppm) micromass Q-TOF mass spectrometer was used. The general conditions were the source temperature of 373 K, capillary voltage of 3.0 kV, and cone voltage of 40 V. For measurements in the negative ion mode, ESI(−)-MS, 0.01 cm3 of concentrated NH4OH was added to the sample mixture, completing a total volume of 1 cm3 and yielding 0.1% as final concentration. For measurements in the positive ion mode ESI(+)-MS, 0.01 cm3 of concentrated formic acid was added, producing a final concentration of 0.1%. ESI-MS was performed by direct infusion with a flow rate of 167 cm3/s using a syringe pump (Harvard Apparatus, South Natick, USA). Mass spectra were acquired and accumulated over 60 s and the spectra were scanned in the range of 50 to 1,000 w/z. The structural analysis of single ions, in the mass spectra from sweet basil extracts obtained with cosolvent, was performed by ESI-MS/MS. The ion with the m/z of interest was selected and submitted to 15–45 eV collisions with argon in the collision quadrupole. The collision gas pressure was optimized to produce extensive fragmentation of the ion under investigation. These unique features of direct infusion ESI-MS have recently been applied for fingerprinting complex mixtures such as bee propolis (Sawaya et al. 2004), beer (Araújo et al. 2005), wine (Cooper and Marshall 2001; Catharino et al. 2006), whisky (Moller et al. 2005), and vegetable oil (Wu et al. 2004; Catharino et al. 2005).
Antioxidant Activity: Coupled Oxidation of Linolenic Acid and Beta-Carotene
The methodology to assess the SFE extracts antioxidant activity was described by Leal et al. (2006). The reaction substrate was prepared using 10 mg of beta-carotene (99%, Acros, B0070834, New Jersey, NJ), 10 cm3 of chloroform (99.0–99.4%, Merck, K31503045 301, 1024451000, Darmstadt, Germany), 60 mg of linolenic acid (99%, Sigma-Aldrich, L2376, 063K3727, St Louis, USA) and 200 mg of Tween 40 (99%, Sigma-Aldrich, P1504, 032K0104, St Louis, USA). This solution was concentrated in a rotary evaporator (Laborota, model 4001, Vertrieb, Germany) at 323 K and afterwards diluted with 50 cm3 of bi-distilled water. The reaction was conducted using the following procedure: 2 cm3 of bi-distilled water was added to 1 cm3 of substrate, and 0.05 cm3 of the sweet basil extract was diluted in bi-distilled water or ethanol (99.8%, Merck, K31865683 312, Darmstadt, Germany; 20 mg of extract/1 cm3 of ethanol). The mixture was set into a water bath (Tecnal, TE 159, Piracicaba, Brazil) at 313 K and the reaction product was monitored using a spectrophotometer (HITACHI, U-3010, Tokyo, Japan) for 0, 1, 2 and 3 h with absorbance reading at 470 nm. The data were reported as the ratio of antioxidant activity of sweet basil extracts to the antioxidant activity of beta-carotene. The antioxidant activity was calculated by Eq. 1 as suggested by Skerget et al. (2005):
where AA is the antioxidant activity, \( {\text{abs}}^{{t - 0}}_{{{\text{extract}}}} \) the absorbance of extract at initial time of reaction, \( {\text{abs}}^{{t - 0}}_{{{\text{control}}}} \) the absorbance of control at initial time of reaction, \( {\text{abs}}^{t}_{{{\text{extract}}}} \) the absorbance of extract at 1, 2, or 3 h, and \( {\text{abs}}^{t}_{{{\text{control}}}} \) the absorbance of control sample at time of reaction (1, 2, or 3 h).
Economical Analysis
The methodology of Turton et al. (1998) was used to estimate the cost of manufacturing (COM). This methodology defines COM as a weighed sum of five factors: fixed capital investment (FCI), the cost of operational labor (C OL), the cost of the raw material (C RM), the cost of waste treatment (C WT), and the cost of utilities (C UT). The details and considerations of each cost were presented by Rosa and Meireles (2005). COM can be calculated using Eq. 2.
COM estimated using Eq. 2 is associated 0 to 2% of definition of entire project of process design (Turton et al. 1998), thus, it is used to help the decision makers on deciding to advance or to stop the project.
Fixed Cost of Investment. The investment of the supercritical extraction process is composed basically by the cost of the extraction unit (Fig. 2). The industrial scale unit contains (Rosa and Meireles 2005) (1) two extractors (E-1 and E-2) that are heated by using either electrical or water heating jackets (volume: 0.4 m3; maximum operational pressure: 60 MPa), (2) the basket used to accommodate the raw material, (3) high pressure separator (S-1), (4) low pressure separator (S-2), (5) back pressure regulator (high pressure), (6) back pressure regulator (low pressure), (7) heat exchanger (shell and tube heat exchanger), (8) condensing heat exchanger (C-2) (recycle of CO2), (9) receiver of CO2 (R-1), (10) pre-cooling heat exchanger (C-1), (11) high pressure carbon dioxide pump (P-1), (12) co-solvent pump (P-2), (13) mixer, and (14) control system. This unit costs approximately US$ 2 million. The annual depreciation rate was considered to be 10%.
Cost of Raw Material. The commercialization cost of dried comminuted Ocimum basilicum was US$ 6.90 kg−1. The specific cost of solvent was US$ 0.15 kg−1 of CO2 and the cost of water was US$ 5.32 × 10−3 kg−1.
Cost of Operational Labor. The cost of operational labor was calculated using the tables presented by Ulrich (1984). For the supercritical extraction unit two operators per shift would be needed. The cost of operational labor was US$ 3.00 h−1 and the total annual operational cost was US$ 47,520.00, considering 330 days, working 24 h per day.
Cost of Waste Treatment. It was assumed that the particles after extraction can be sold by the price to remove them from the extraction unit and; therefore, there will be no solid waste to be treated. The gas that leaves the extraction section is CO2, and there will be no treatment. Therefore, the C WT was assumed equal to zero.
Cost of Utility. The cost of the utilities used in the extraction section is presented in Table 2. The main utility cost is the condensation of the CO2 after the flash separation vessel. The energy consumption at the flash tank, condenser, pump, and heat exchanger is in Table 3. The costs of electrical power, cooling water, and saturated steam were US$ 16.8/GJ, US$ 19.99/kJ, and US$ 3.18/kJ, respectively.
Scale-up and Extraction Information. To estimate the COM, the benchmark SFE unit selected was a unit containing two extractor vessels of 0.4 m3 each; this unit has a reasonable size for industrial processing and would be capable of handling numerous raw material. To estimate the industrial scale parameters using the laboratory scale data, the following procedure was used (Rosa and Meireles 2005): the scale-up procedure assumes that both yield and extraction time of the industrial process will be similar to that of the laboratorial scale if the ratio between the mass of the particles inside the extractor and the solvent flow rate is kept constant. The use of two columns is necessary to maintain the unit operating continuously; when the extraction is carried out in one column, the other one is depressurized, the exhausted particles are unloaded, the column is cleaned, the fresh particles are loaded, and the column is pressurized. As this value was assumed to remain constant in the industrial scale, the amount of raw material used per extraction cycle ranged from 54 to 56 kg. The mass flow rates of solvent in the laboratorial scale unit were 8 × 10−5 and 5 × 10−5 kg/s for extraction with 1% (w/w) and 10–20% (w/w) of cosolvent, respectively. Therefore, the mass flow rates of solvent in the industrial scale unit were 1.6 and 1.1 kg/s for extraction with 1 (w/w), 10, and 20% (w/w) of cosolvent, respectively. The extraction time was assumed to be equal to 60 min, so the number of extraction batches per year was calculated as 7,920.
Statistical Analysis
The analyses of variance (ANOVA) were done with SAS 8.2 (SAS 2006).
Results and Discussions
Table 4 shows the nutrient composition of the leaves and roots of lots 1/2N and 2N. The biomass yields for lots 1/2N and 2N were 0.359 and 0.723 kg, respectively. Reducing the amount of nitrogen in the nutrient solution had small effect on the amount of dry matter, as compared to the control lot, whereas doubling the amount of nitrogen resulted in doubling the amount of dry matter. Nonetheless, with respect to the nutrient content of the sweet basil leaves, the effects were less important, except for boron, iron, and zinc. The amount of boron for both nutrient solutions was approximately the same and about 62% of that of the control lot. The iron content in the 1/2N nutrient solution was 50% larger than that of the control and 2N nutrient solutions; the zinc contents of the 1/2N and 2N nutrient solutions were equal and approximately 50% of that in the control lot. Unfortunately, because the amount of sweet basil available was small, lots 1/2N and 2N were mixed together for the SFE experiments; therefore, it was not possible to associate the composition and antioxidant activity of the extracts to the nutrient solutions used to cultivate sweet basil.
The real density of the sweet basil leaves was 1,600 kg/m3 and the apparent density varied from 135 to 139 kg/m3, thus the total porosity of the bed varied from 0.92 to 0.91. The raw material humidity was 10.1% (wet basis).
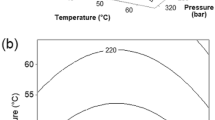
As explained in the “Materials and Methods” section, the extracts obtained using 1% of cosolvent had two phases. Figure 3 shows the xylene phase global yield isotherms (303 and 323 K) obtained with 1% of cosolvent (w/w). The yields ranged from 1.1 to 2.0% (w/w, d.b.), and these results are in agreement with data reported in literature. Lachowicz et al. (1997) reported yields for sweet basil cultivated in Australia. For the five varieties, the yield obtained by hydrodistillation ranged from 0.3 to 1.2% (w/w), while the yields obtained using liquid and supercritical CO2 ranged from 0.42 to 1.54% (w/w) and 0.27 to 1.90% (w/w), respectively. The largest global yields at 303 and 323 K were obtained at 10 MPa (2.0 and 1.9% d.b., respectively). Figure 3 shows that the isotherms 303 and 323 K cross each other at ∼11, ∼18.5, and 27 MPa. These are the inversion pressures that are related to the retrograde behavior of systems containing supercritical fluids (McHugh and Krukonis 1994). In general, this phenomenon is shown in a solubility-pressure diagram, but, as discussed by Moura et al. (2005), the retrograde behavior can also be observed in the global yield against pressure diagram. Because the SFE extracts are multicomponent mixtures, multiple inversion pressures are usually observed in such diagram. Figure 4 shows the global yield 303 K isotherm obtained using 10 and 20% of cosolvent. Increasing the amount of cosolvent from 1% to 10 and 20% changed drastically the behavior of the system: no inversion pressures were observed for 10 or 20% cosolvent. For the 10% cosolvent isotherm, the global yields varied in a narrow interval (∼7 to ∼11%), whereas for the 20% cosolvent isotherm, the global yield varied in a wider interval (∼14 to ∼24%). In addition, for the 20% cosolvent isotherm, a sharp minimum was observed at 20 MPa, whereas for the 10% cosolvent isotherm a discrete minimum was observed at the same pressure.
Figure 5 shows the overall extraction curves (OEC) for extractions at 303 K and pressures of 10 to 30 MPa using 10 and 20% of the cosolvent water. The OEC generally presents three stages (Brunner 1994): (1) the constant extraction rate period (CER), (2) the falling extraction rate period (FER), and (3) the diffusion controlled rate period (DC). In spite of these periods being observed in all the OECs of Fig. 5, they can be seen more clearly in the OEC obtained at 10 MPa with 20% of cosolvent. The system had the same behavior along time, regardless of the cosolvent amount. For extractions with 10% (w/w) of cosolvent at 303 K, the largest global yield was obtained at 10 and 15 MPa (21% d.b.). For extractions with 20% (w/w) of cosolvent at 303 K, the largest global yield was obtained at 30 MPa (24%). The relative position of the 15 and 25 MPa OECs is consistent with the results previously shown (Fig. 4) for the global yields isotherms.
Yields of sweet basil extracts (d.b.) as a function of extraction time obtained at 303 K and 10% H2O (w/w) at 10 MPa (open diamond), 15 MPa (plus symbol), 20 MPa (open circle), 25 MPa (open triangle) and 30 MPa (open square) and 20% H2O (w/w) at 10 MPa (filled diamond), 15 MPa (cross mark), 20 MPa (filled circle), 25 MPa (filled triangle) and 30 MPa (filled square)
Characterization of Extracts
Table 5 shows the major compounds identified and detected in the xylene phase of the sweet basil extract obtained with 1% of cosolvent (w/w). The identified compounds were eugenol, germacrene-d, epi–alpha–cadinol, phytol, and neophytadiene. Eight compounds detected by GC were not identified. The retention times of these compounds varied from as low as 6 to 57.6 min. The major compound of the SFE extract was detected at retention time of 50.7 min and was not identified. For this compound, the area percent ranged from 23.5% at 323 K and 30 MPa up to 34.5% at 303 K and 20 MPa. The largest eugenol content (14.6% in area) was obtained at 10 MPa and 323 K. The analysis of variance (ANOVA) detected that the effects of pressure and temperature on the eugenol content (percent in area) were significant (p = 0.036 and p = 0.025, respectively). The eugenol contents of the sweet basil extracts were lower than the contents in clove basil extracts (Leal et al. 2006).
The water from the SFE extracts obtained with 10 and 20% of cosolvent was removed in a freeze-dryer. To certify that the volatile oil components (terpenoids) were not present in these extracts, some of the freeze-drying assays were done using a column filled with porapak type Q 80–100 mesh (Supelco, Bellefonte, PA) and assembled at the water outlet. Afterwards, it was confirmed (gravimetrically) that no compounds were retained in the packed column. The 10 and 20% cosolvent SFE extracts were analyzed by GC-MS using the Kovats program, but no compound was detected; therefore, we conclude that no compound of the terpenoid class was extracted by the process that used 10 and 20% of cosolvent. This can be explained by the strong affinity to water of some compounds present in sweet basil. To identify the chemical constituents of these extracts, the electrospray ionization mass spectrometer was used. The electrospray ionization mass spectrometer method provides a sensitive and selective way to identify polar organic compounds with acidic sites, such as phenolic, organic acid, and sugar compounds found in sweet basil extract obtained by SFE using water as cosolvent (10 and 20% w/w). Deprotonated forms of the compounds of interest were then selected and their ESI-MS/MS were compared to standards. Figure 6 shows an ESI(−)-MS of sweet basil extract obtained with 10% (w/w) of cosolvent at 30 MPa and 303 K. The same chemical profile was observed in all extracts obtained with 10% (w/w) of cosolvent at 10, 15, 20, and 25 MPa, and 303 K. Sugars, organic acids, and phenolic compound anions are observed as [malic acid–H2O–H]− of m/z 115, [malic acid–H]− of m/z 133, [tartaric acid–H]− of m/z 149, [ramnose–H]− of m/z 163, [caffeic acid–H]− of m/z 179, [quinic acid–H]− of m/z 191, [kaempferol–H]− of m/z 285, [caffeoylquinic acid–H]− of m/z 353 and [kaempferol 3-O-glucoside–H]− of m/z 447. The chemical composition of extracts obtained with 20% of cosolvent was analyzed but could not be identified either by gas chromatography or by electrospray ionization mass spectrometer.
Antioxidant Activity
Table 6 shows AA values of sweet basil extracts that were obtained with 1% of cosolvent (w/w): xylene and aqueous phases, and extracts obtained with 10 and 20% of cosolvent. In Table 6, dashes indicate that either the extract had no AA or the antioxidant activity was smaller than that of beta-carotene. Extracts obtained with 10 and 20% of cosolvent (w/w) showed AA superior to that of the aqueous phase and approximately equal to the xylene phase extracts obtained with 1% of cosolvent at the same temperature of extraction. For 1% of cosolvent, the extracts obtained at 323 K had an AA superior to that of extracts obtained at 303 K. Between the xylene and the aqueous fractions of the extracts obtained with 1% of cosolvent, the xylene fraction had an AA greater than that of the aqueous fraction. The AA of the aqueous fractions was poor as compared to that of beta-carotene. This is expected, since the method employed to measure the AA is not appropriate to detect antioxidant activity of hydrophilic fractions, and for this fraction, the DPPH method would be indicated (Mellors and Tappel 1966). The AAs of the xylene fractions varied greatly with reaction time. At 1% of cosolvent the largest AAs were obtained at 323 K. The AAs were as large as 96% for extracts obtained at 10 MPa and reached 100% for extracts obtained at 30 MPa. At 10 MPa, the AA of the extracts were stable for the first 2 h of reaction, whereas at 30 MPa, the AAs were not stable and oscillated from 100 to 63%. At 20% of cosolvent, the largest AAs were ∼67% (for reaction times of 1, 2, and 3 h), determined for the extract obtained with 20 MPa at 303 K; at 10% of cosolvent, the largest AA was 51% for extracts obtained with at 25–30 MPa and 303 K. The results show that antioxidant activity of sweet basil extracts obtained with 10 and 20% (w/w) of cosolvent had little variation with time of oxidation reaction. It is an indicative that besides having good values of AA, these extracts are stable. Therefore, sweet basil extracts can be used as a natural antioxidant with prolonged action, being able to increase the shelf life of a product. Table 7 shows the antioxidant activity of sweet basil extracts as a function of extraction time (15, 30, 45, 60 min) and for samples collected during the depressurization of the SFE system. The AA of the extracts varied to some extent with the extraction conditions and extraction time interval, except at 10 and 25 MPa, 303 K, and 10% of cosolvent and at 15 MPa, 303 K, and 20% of cosolvent. Towards the end of the extraction, the extracts contain larger and less-soluble molecules, and for these extracts, the largest AA was detected. At 30 MPa and 20% of cosolvent, the fractions F60 and Desp exhibited high AA. At 25 MPa, F45 and Desp showed higher AA while F60 at 30 MPa.
Economical Analysis
The values of COM estimated using Eq. 2 are shown in Table 8. Three different types of products were considered: a product denoted as P1 obtained by SFE with 1% of cosolvent H2O containing nonpolar compounds like volatile oil and oleoresin; a product denoted as P2 obtained by SFE with 10% of cosolvent H2O that contains polar compounds, predominantly sugar and phenols, and a product P3 obtained with 20% of cosolvent H2O containing polar compounds with antioxidant activity larger than that of products P1 and P2. The cost of manufacturing of the products (per kilogram of dry extract) ranged from US$ 572.82 kg−1 to US$ 1,049.58 kg−1 for P1; from US$ 107.37 kg−1 to US$ 152.45 kg−1 for P2, and from US$ 47.96 kg−1 to US$ 85.83 kg−1 for P3. FCI represents ∼12.5% of the estimated COM while the raw material costs contributed to ∼80% of the estimated COM. Utilities and labor represents ∼4.0–6.3% and 2.5%, respectively. These figures, in particular the cost of raw material, demonstrate that SFE is not an expensive technique per se and can be competitive with conventional process, as long as the acceptable economical analysis is performed. The cost of manufacturing analysis indicates that research in the agronomic areas related to the decrease in the production cost of raw material and to increase the content of the soluble substances (CO2 and aqueous soluble substances) is the key issue to a successful decrease in COM regardless of the extraction technique used.
Conclusion
The largest global yield was obtained at 10 MPa for extraction with 1% of the cosolvent water (w/w) in both temperatures studied (303 and 323 K). For extracts obtained with 10% of cosolvent at 303 K, the largest global yields were obtained at 10 and 15 MPa. For extracts obtained with 20% of cosolvent at 303 K, the largest global yield was obtained at 30 MPa.
The compounds identified in sweet basil extracts obtained with 1% of cosolvent were eugenol, germacrene-d, epi–alpha–cadinol, phytol, and neophytadiene. In extracts obtained with 10% of cosolvent, other classes of compounds were identified, like caffeic acid, quinic acid, malic acid, tartaric acid, ramnose, kaempferol, caffeoylquinic acid, and kaempferol 3-O-glucoside. All compounds that were identified possess antioxidant activity.
Sweet basil extracts obtained with 10 and 20% of cosolvent showed antioxidant activity larger than that of extracts obtained with 1% of cosolvent at the same temperature of extraction (303 K). Sweet basil extracts can be considered natural antioxidants with prolonged action, being able to increase the shelf life of a product.
The estimated cost of manufacturing of sweet basil extracts by SFE varied from US$ 47.96 to US$ 1,049.58 per kilogram of dry extract. The cost of raw material represented ∼80% of COM, thus indicating that in spite of the association of SFE with high FCI, it is not necessarily true that the fixed cost of investment represents the heaviest fraction of COM.
This study contributed to show that it is possible to obtain extracts from sweet basil with antioxidant activity and three different chemical compositions, according to the amount of cosolvent water. To select the best condition of extraction of sweet basil, the COM of these three extracts must be confronted with their chemical composition and intended application.
References
Abas, F., Lajis, N. H., Israf, D. A., Khozirah, S., & Kalsom, Y. U. (2006). Antioxidant and nitric oxide inhibition activities of selected Malay traditional vegetables. Food Chemistry, 95(4), 566–573.
Adams, R. P. (1995). Identification of essential oil components by gas chromatography/mass spectroscopy. Carol Stream IL, USA: Allured Publishing Corporation.
Araújo, A. S., Rocha, L. L., Tomazela, D. M., Cunha, I. B. S., Bankova, V. S., Marcucci, M. C., et al. (2005). Electrospray ionization mass spectrometry fingerprinting of beer. The Analyst, 130, 884–889.
Bataglia, O. C., Furlani, A. M. C., Teixeira, J. P. F., Furlani, P. R., Gallo, J. R. (1983). Métodos de análise química de plantas. Campinas, S. P., Brazil: Boletim Técnico, 78, Instituto Agronômico de Campinas.
Bavel, B. V., Hartonen, K., Rappe, C., & Riekkola, M. L. (1999). Pressurized hot water/steam extraction of polychlorinated dibenzofurans and naphthalene from industrial soil. The Analyst, 124, 1352–1354.
Block, G., Patterson, B., & Subar, A. (1999). Fruit, vegetable and cancer prevention—A review of the epidemiological evidence. Nutrition and Cancer, 18, 1–29.
Brunner, G. (1994). Gas extraction: An introduction to fundamentals of supercritical fluids and the application to separation process. Darmstadt, Germany: Springer.
Catharino, R. R., Haddad, R., Cabrini, G. C., & Cunha, I. B. S. (2005). Characterization of vegetable oils by electrospray ionization mass spectrometry fingerprinting: Classification, quality, adulteration, and aging. Analytical Chemistry, 77(22), 7429–7433.
Catharino, R. R., Sawaya, A. C. H. F., Cunha, I. B. S., Fogaça, A. O., Facco, E. M. P., Godoy, H. T., et al. (2006). Characterization of must and wine of six varieties of grapes by direct infusion electrospray ionization mass spectrometry. Journal of Mass Spectrometry, 41(2), 185–190.
Cooper, H. J., & Marshall, A. G. (2001). Electrospray ionization Fourier transform mass spectrometric analysis of wine. Journal of Agricultural and Food Chemistry, 49(12), 5710–5718.
Goldberg, I. (Ed.) (1994). Functional foods: Designer foods, pharmafoods, nutraceuticals. New York, USA: Chapman & Hall Inc.
Harsh, P. B., Walker, T. S., Schweizer, H. P., & Vivanco, J. M. (2002). Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root culture of Ocimum basilicum. Plant Physiology and Biochemistry, 40(11), 983–995.
Hawthorne, S. B., Yang, Y., & Miller, D. J. (1994). Extraction of organic pollutants from environmental solids with sub and supercritical water. Analytical Chemistry, 66(18), 2912–2920.
Jacobs, M. B. (1981). The chemical analysis of foods and foods products (pp. 21–22). New York, NY, USA: Robert Krieger Publishing Co.
Javanmardi, J., Stushnoff, C., Locke, E., & Vivanco, J. M. (2003). Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chemistry, 83(4), 547–550.
Jayasinghe, C., Gotoh, N., Aoki, T., & Wada, S. (2003). Phenolics composition and antioxidant activity of sweet basil (Ocimum basilicum L.). Journal of Agriculture and Food Chemistry, 51(15), 4442–4449.
Lachowicz, K. J., Jones, G. P., Briggs, D. R., Bienvenu, F. E., Palmer, M. V., Mishra, V., et al. (1997). Characteristics of plants and plant extracts from five varieties of basil (Ocimum basilicum L.) grown in Australia. Journal of Agriculture and Food Chemistry, 45(7), 2660–2665.
Leal, P. F., Chaves, F. C. M., Ming, L. C., Petenate, A. J., & Meireles, M. A. A. (2006). Global yields, chemical compositions and antioxidant activities of clove basil (Ocimum gratissimum L.) extracts obtained by supercritical fluid extraction. Journal of Food Process Engineering, 29(5), 547–559.
Lee, S. J., Umano, K., Shibamoto, T., & Lee, K. G. (2005). Identification of volatile components in basil (O. basilicum L.) and thyme leaves (Thymus vulgares L.) and their antioxidant properties. Food Chemistry, 91, 131–137.
Maia, N. B., Carmello, Q. A. C., & Marques, M. O. M. (1999). Sistema automático de fornecimento de soluções nutritivas para plantas cultivadas em vaso. Scientia Agricola, 56(1), 103–109.
McHugh, M. A., & Krukonis, V. J. (1994). Supercritical fluid extraction: Principles and practice. London: Butterworth—Heinemann.
McLaferty, F. W., & Stauffer, D. B. (1989). The Wiley/NBS registry of mass spectral data (Vol. 7). New York, USA: Wiley.
Mellors, A., & Tappel, A. L. (1966). The inhibition of mitochondrial peroxidation by ubiquinone and ubiquinole. The Journal of Biological Chemistry, 241(19), 4353–4356.
Miller, D. J., & Hawthorne, S. B. (1998). Solubility of polycyclic aromatic hydrocarbons in subcritical water from 298 K to 498 K. Journal of Chemistry Engineering Data, 43(6), 1043–1047.
Moller, J. K. S., Catharino, R. R., & Eberlin, M. N. (2005). Electrospray ionization mass spectrometry fingerprinting of whisky: Immediate proof of origin and authenticity. Analyst, 130(6), 890–897.
Moura, L. S., Carvalho Jr., R. N., Stefanini, M. B., Ming, L. C., & Meireles, M. A. A. M. (2005). Supercritical fluid extraction from fennel (Foeniculum vulgare): Global yield, composition and kinetic data. The Journal of Supercritical Fluids, 35, 212–219.
Ness, A. R., & Powles, J. W. (1997). Fruit and vegetables and cardiovascular diseases: A review. International Journal of Epidemiology, 26, 1–3.
Pasquel, A., Meireles, M. A. A., Marques, M. O. M., & Petenate, A. J. (2000). Extraction of stevia glycosides with CO2 + water, CO2 + ethanol, and CO2 + water + ethanol. Brazilian Journal of Chemical Engineering, 17(3), 271–282.
Perez-Guitiérrez, R. M., Perez-Gutiérrez, S., Perez-Gonzalez, C., Zavala, M. A., & Perez, C. (1992). Action of extracts from some Mexican plants on the cardiovascular-system. Phyton: Revista Internacional de Botânica Experimental, 53(2), 111–116.
Rosa, P. T. V., & Meireles, M. A. A. (2005). Rapid estimation of the manufacturing cost of extracts obtained by supercritical fluid extraction. Journal of Food Engineering, 67(1–2), 235–240.
Sarruge, J. R. (1975). Soluções nutritivas. Summa Phytopathologica, 1(3), 231–233.
Sarruge, J. R., & Haag, H. P. (1974). Análises químicas em plantas. Piracicaba SP, Brazil: USP/ESALQ.
SAS (2006). SAS user’s guide: Statistic (Version 8.2—SAS Learning). Cary, NC, USA: SAS Institute.
Sawaya, A. C. H. F., Tomazela, D. M., Cunha, I. B. S., Bankova, V. S., Marcucci, M. C., Custodio, A. R., et al. (2004). Electrospray ionization mass spectrometry fingerprinting of propolis. The Analyst, 8, 739–744.
Schulz, H., Schrader, B., Quilitzsch, R., Pfeffer, S., & Kruger, H. (2003). Rapid Classification of basil chemotypes by vibrational spectroscopy methods. Journal of Agricultural and Food Chemistry, 51, 2475–2481.
Skerget, M., Kotnik, P., Hadolin, M., Hrãs, A. R., Simonic, M., & Knez, Z. (2005). Phenols proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chemistry, 89, 191–198.
Turton, R., Bailie, R. C., Whiting, W. B., & Shaeiwtz, J. A. (1998). Analysis synthesis and design of chemical processes. Upper Saddle River, NJ, USA: Prentice Hall, PTR.
Ulrich, G. D. (1984). A guide to chemical engineering process design and economics. New York, USA: Wiley.
Velioglu, Y. S., Mazza, G., Gao, L., & Oomah, B. D. (1998). Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. Journal of Agricultural and Food Chemistry, 46, 4113–4117.
Vinã, A., & Murillo, E. (2003). Essential oil composition from twelve varieties of basil (Ocimum ssp) grown in Colombia. Journal of Brazilian Chemistry, 14(5), 744–749.
Vision, J. A., Jang, J., Yang, J., Dabbagh, Y., Liang, X., Sery, M., et al. (1999). Vitamins and especially flavonoids in common beverages are powerful in vitro antioxidants which enrich lower density lipoproteins and increase their oxidative resistance after ex vivo spiking in human plasma. Journal of Agricultural and Food Chemistry, 47, 2502–2504.
Wu, Z., Rodger, R. O., & Marshall, A. G. (2004). Characterization of vegetable oils; detailed compositional fingerprints derived from Electrospray Ionization Fourier Transform Ion Cyclotron resonance Mass Spectrometry. Journal of Agriculture and Food Chemistry, 52, 5322–5328.
Yamasaki, K., Nakano, M., Kawahata, T., Mori, H., Otake, T., Ueba, N., et al. (1998). Anti-HIV-1 activity of herbs in Labiatae. Biological & Pharmaceutical Bulletin, 21(8), 829–833.
Zancan, K. C., Marques, M. O. M., Petenate, A. J., & Meireles, M. A. A. (2002). Extraction of ginger (Zingiber officinale Roscoe) oleoresin with CO2 and cosolvents: A study of the antioxidant action of the extracts. Journal of Supercritical Fluids, 24, 57–76.
Acknowledgement
P. F. Leal thanks FAPESP for the MS and PhD assistantships (02/12001-7 and 04/09310-3). The authors thank Ms. P. Hirata from the Laboratory of Microwave of FEA–UNICAMP for the determination of the humidity by the infrared method. The authors are grateful to FAPESP (1999/01962-1) and CNPq 472512/2004-8 for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leal, P.F., Maia, N.B., Carmello, Q.A.C. et al. Sweet Basil (Ocimum basilicum) Extracts Obtained by Supercritical Fluid Extraction (SFE): Global Yields, Chemical Composition, Antioxidant Activity, and Estimation of the Cost of Manufacturing. Food Bioprocess Technol 1, 326–338 (2008). https://doi.org/10.1007/s11947-007-0030-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-007-0030-1