Abstract
Purpose of review The sudden emergence of a change in cognitive abilities or behavior is an important symptom that warrants medical evaluation and may represent the early stages of a rapidly progressive dementia (RPD). To correctly ascertain the cause of RPD in a given patient, the clinician must be methodical and knowledgeable about the range of potential causes and must move forward with supportive treatment, and in some cases empiric treatment, based on clinical features alone.
Recent findings Significant advances in prion disease biomarkers, the molecular features of rapidly progressive Alzheimer’s disease, and new detection of autoimmune limbic encephalitis disease entities have caused a shift in the diagnostic and treatment framework of RPD. Additionally, in the past decade, emerging retrospective data have led to suggested treatments in autoimmune encephalitis that, if instituted early, can protect patients against residual deficits and disease relapse.
Summary Here, we provide an integrative clinical and diagnostic treatment approach that is applicable to the various forms of RPD. We have highlighted the clinical features of selected types of RPD that have experienced advances in the last 10–15 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients experiencing a rapidly progressive neurological decline of central origin, referred to as rapidly progressive dementia (RPD), pose a diagnostic challenge. Generally, RPD describes rapidly progressive neurocognitive decline to dementia or death within 2 years [1]. Several other formal criteria exist to help define rapid progression, particularly in Alzheimer’s disease (AD), that include declines in cognitive scores (e.g., Mini-Mental Status Examination) or functional rating scales (e.g., Clinical Dementia Rating) over a specified time frame [2]. Essentially, the term RPD refers to a rate of progression that is unexpectedly faster than most neurocognitive disorders.
The major guiding diagnostic principle in RPD is to expeditiously detect or exclude treatable conditions such as infections, infarction, metabolic or toxic disturbances, malignancy, or autoimmune etiologies [3•–8••]. Once these etiologies are excluded, etiologies such as prion disease, atypical neurodegenerative causes like rapidly progressive AD, sudden acceleration of disease progression in a patient already diagnosed with a dementing illness, or other neurodegenerative disease process remain. Due to limitation of space, this article will highlight the diagnostic process and currently recommended treatment approaches for selected causes of RPD.
Diagnostic approach
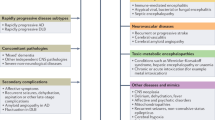
When confronted with a patient experiencing a RPD, the clinician will find himself or herself entertaining a multifarious list of potential causes (Table 1). Several factors aid in arriving at the correct diagnosis, vital among them are the historical and clinical features of the patient’s illness.
The assessment of a patient with RPD parallels the approach to a patient with change in mental status, with the major distinction being that RPD entails a broader work-up. History must include what symptoms were first noticed, which often requires prompting the historian with questions about initial personality changes like bizarre behavior, psychiatric symptoms such as delusions, hallucinations, new anxiety, new depression, or new impulsivity. Historians should be questioned about cognitive symptoms such as increased memory difficulty, apathy, language problems, confusion, problems navigating, or new difficulty using electronic devices or appliances reflecting developing apraxia. Additionally, the clinician should ask about new vision complaints, gait problems or balance difficulties, vertigo, dysarthria, progressive focal weakness, focal numbness or parasthesias, new involuntary movements (tremor, myoclonus, dystonia, chorea), muscle hyperexcitability (fasiculations, cramping), or rigidity.
For patients with RPD, a comprehensive review of systems is important. Clinicians should ask about recent flu-like symptoms, febrile illness, or GI illness, unintentional weight loss, rashes, headaches, and new insomnia. Inquiries must be made regarding the use of new medications, changes in medications, medication compliance, any consumption of supplements or vitamins, exposure to toxins (vocational or environmental), and recent travel. Family history of neurodegenerative diseases, malignancy, autoimmune illnesses, psychiatric, or metabolic disease should be reviewed, as should current or remote substance use.
The recommended testing approach [4••, 6,7,8••] is to begin with basic screening tests, then pursue further testing based on the results of the primary testing battery, all the while administering supportive or therapeutic treatment as dictated by the clinical picture (Fig. 1). The primary testing battery includes urine toxicity screen, urinalysis and culture in addition to basic blood tests: comprehensive metabolic panel, complete blood cell count with differential, thiamine, thyroid stimulating hormone, thyroxine, vitamin B12, relevant medication levels, ethanol level, syphilis IgG, HIV, erythrocyte sedimentation rate, C- reactive protein, coagulation panel, ammonia, and initial rheumatological screening with an ANA that if positive will trigger a full ENA panel. Typically, CNS infection will be high on the differential [9]; therefore, lumbar puncture with CSF studies should be done urgently. In immunocompetent patients, basic CSF studies for detection of viral (HSV 1 and 2, enterovirus, etc.), bacterial, parasitic infections endemic in the patients area, cell count with differential, cytology, protein, glucose, IgG synthesis rate, and the prion panel (RT-QuIC, tau, and 14-3-3) should be sent. Instruct the lab to store one tube of CSF in the event that additional studies (such as CSF paraneoplastic antibodies) become necessary. MRI of the brain with and without contrast is necessary to look for vascular, malignant, or infectious causes. Diffusion-weighted imaging (DWI) will give indication of acute stroke and specific patterns of diffusion restriction are highly suggestive of prion disease. Electroencephalography (EEG) is essential in the evaluation of patients with cognitive impairment, especially if there are fluctuations in neurological symptoms. The clinician should keep in mind that subclinical epileptic events or frontal seizures might only be captured with continuous EEG. Depending on the results of the primary work up, additional testing includes disease-specific markers, and potential further tests are also listed in Fig. 1.
Comorbidities and risk factors for rapid cognitive decline
When rapid cognitive decline is observed in a patient with dementia, one first needs to rule out alternative disease etiologies and other conditions that may cause a superimposing encephalopathy (e.g., infection) as other comorbid diseases and neuropsychiatric syndromes may influence disease progression. In one study by Aubert and colleagues, greater comorbidity burden with other chronic medical conditions was associated with faster cognitive decline in subjects with mild to moderate AD [10]. Specific medical conditions that have demonstrated accelerated cognitive decline in AD include vascular disease risk factors such as atrial fibrillation, hyperlipidemia, hypertension, microvascular disease, myocardial infarction, and possibly diabetes mellitus [11].
Demographic variables and several neuropsychiatric symptoms, whether from the underlying etiology or a comorbid condition, influence disease progression in dementia. Males, younger patients, and those taking cholinesterase inhibitors were at greater risk of faster cognitive decline in two studies [2, 12]. Several psychiatric symptoms including apathy, psychosis, agitation/aggression, as well as the clinical significance of psychiatric symptoms in general are associated with more rapid disease progression [2, 13•]. Neurologic symptoms such as apraxia, motor signs, seizures, or multiple focal neurological signs are also associated with faster disease progression [2]. It is important to note that it is very difficult to discern if many of the above variables independently contribute to disease progression or are part of an atypical RPD phenotype such as rapidly progressive Alzheimer’s disease (rpAD).
Specific disease etiologies
A summary of key aspects of diagnostic testing and treatment approaches in selected etiologies of RPD is presented in Table 2 and is discussed in the following sections.
Rapidly progressive Alzheimer’s disease
Although most cases of AD demonstrate relatively slow clinical progression, a subset of AD patients experience rapidly progressive dementia (rpAD). While there is variation between studies in the definition of rapid progression (some investigators have permitted a clinical dementia duration of up to about 3 years), an estimated 10–30% of AD cases have been classified as rapidly progressive [2]. Some studies report a younger mean age at onset in rpAD patients, and survival time is generally a couple of years [2, 14, 15].
Diagnosis of rpAD is difficult due to its departure from the typical AD phenotype that presents with short-term memory loss and word-finding difficulties [16]. Conversely, rpAD cases generally present with early executive dysfunction and language impairment [15, 17]. Neurologic findings that are often observed in prion disease such as myoclonus, pyramidal and extrapyramidal symptoms, and gait impairment present much earlier than expected in rpAD [11]. CSF markers of neuronal injury including 14-3-3 and total tau are frequently elevated in rpAD; however, elevated p-tau and reduced amyloid beta1–42 (Aβ1–42) levels are rather specific for rpAD. It must be noted that the majority of studies examining rpAD included subjects referred to prion disease surveillance centers, which could introduce considerable bias in clinical phenotype profiles.
Several recent studies have detected distinct molecular differences between rpAD and typical AD. The structure of Aβ1–42 is different in rpAD, which has fewer particles composed of < 30 monomers and increased levels of particles composed of 30–100 monomers [14]. rpAD also demonstrates greater variability in Aβ1–40 structures compared to typical AD [18]. Finally, amyloid plaques contain a higher number of neuronal proteins and decreased levels of astrocytic proteins in rpAD cases [19]. The above findings suggest that there may be distinct molecular subtypes of AD (e.g., rpAD) that are caused by distinct Aβ strains, similar to what is observed in prion diseases [20•].
Prion diseases
Prion diseases are universally fatal, rapidly progressive, neurodegenerative illnesses and are caused by an abnormal conformer of the normal prion protein (PrPSc). PrPSc can arise sporadically (85%), be inherited through mutations of the prion protein gene (PRNP) (10–15%), or acquired via specific forms of transmission (< 1%).
Sporadic prion diseases include sporadic Creutzfeldt-Jakob disease (sCJD), sporadic fatal insomnia (sFI), and variably protease sensitive prionopathy (VPSPr). sCJD is by far the most common form of prion disease and is a disease of mid-to-late life with a mean age at onset in the 60s [21]. The vast majority of patients pass within a year of disease onset. The clinical presentation of sCJD is heterogeneous but often includes symptoms of cognitive decline, ataxia, visual disturbances, and psychiatric symptoms [22]. Additionally, sCJD patients develop myoclonus, pyramidal and extrapyramidal symptoms, and akinetic mutism at the end of the disease course. Although the only way to definitely diagnosis prion disease is through neuropathologic examination, preferably through autopsy, several diagnostic tests can aid ante mortem diagnosis [23]. Periodic sharp wave complexes (PSWCs) observed on EEG are suggestive of prion disease. Several cerebrospinal fluid (CSF) tests can also be suggestive of sCJD. Two markers of neuronal injury, 14-3-3 and tau proteins, are often used to help diagnosis sCJD but lack specificity. A more recently developed test, real-time quaking-induced conversion (RT-QuIC), exploits the seeding qualities of PrPSc and is a disease-specific marker. Hence, RT-QuIC is nearly 100% specific and also demonstrates robust sensitivity (95%) [24]. RT-QuIC can also be conducted on olfactory epithelium and may be useful for the few cases that do not have a positive CSF RT-QuIC result [25••]. Brain magnetic resonance imaging (MRI) also frequently demonstrates hyperintensity in the basal ganglia and/or cortex on DWI with attenuation of the signal on attenuated diffusion coefficient (ADC) maps [26].
Unfortunately, the two remaining sporadic prion diseases are much more difficult to diagnose. sFI clinically resembles fatal familial insomnia (FFI) with early sleep and autonomic disturbances and more typical sCJD symptoms later in the disease course [27]. Diagnostic tests used in sCJD are typically negative in sFI, but brain FDG-PET and SPECT imaging typically demonstrate thalamic hypometabolism/hypoperfusion [28]. Polysomnography (PSG) also resembles what is found in FFI with loss of normal sleep structure and total sleep time [29]. VPSPr clinically resembles non-Alzheimer’s dementias such as frontotemporal dementia or dementia with Lewy bodies and has a mean disease duration of 2 years [30]. Diagnostic tests suggestive of prion disease are usually negative and the tests with the highest sensitivity are CSF 14-3-3/tau (21% combined sensitivity) [27]. As such, a diagnosis of VPSPr is often missed clinically and frequently only ascertained at autopsy.
There are several genetic prion diseases (gPDs) including genetic Creutzfeldt-Jakob disease (gCJD), FFI, and Gerstmann-Sträussler-Scheinker syndrome (GSS). Approximately 40 different mutations cause gPD, the majority of which are point mutations. Penetrance varies by mutation but is typically high in most mutations [31]. Age at disease onset is generally mid-life across all mutations [21]. The most common gCJD mutation, E200K, closely resembles the clinical phenotype of sCJD and is diagnosed similarly with EEG, CSF, and brain MRI findings. FFI is due to the disease causing D178N-129M haplotype and presents with sleep disturbances, dysautonomia, and psychiatric symptoms [32]. Later symptoms include ataxia, extrapyramidal symptoms, and myoclonus. PSG demonstrates loss of normal sleep architecture and decreased total sleep time. Brain FDG-PET imaging frequently demonstrates thalamic hypometabolism, sometimes before clinical signs of the disease [33]. CSF RT-QuIC is positive in over 80% of cases [34]. GSS is an atypical prion disease that is caused by several different mutations, the most common of which is P102L. GSS typically presents with either a pure cerebellar syndrome or an early extrapyramidal syndrome (A117V). Illness duration averages 5 years and cognitive impairment typically does not occur until late in the disease course. EEG, CSF 14-3-3/tau, and brain MRI are usually unrevealing but CSF RT-QuIC approaches 80% sensitivity in GSS [34].
Acquired prion diseases are rare and include kuru, iatrogenic CJD (iCJD), and variant CJD (vCJD). iCJD can be transmitted through cadaveric-derived gonadotropins, growth hormone, dura mater grafts, corneal transplants, and through contaminated neurosurgical instrumentation [35]. Incubation periods vary depending on the source of contamination and route of transmission and can range from 1 year to decades. Because of knowledge regarding prion disease transmission risks and recombinant technologies, the incidence of iCJD has declined dramatically in recent years. vCJD is due to consuming meat contaminated with bovine spongiform encephalopathy (BSE). vCJD typically affects young individuals who present with psychiatric or sensory symptoms that later evolve to more typical sCJD symptoms over the course of a year or more [36]. EEG and CSF 14-3-3 protein results are generally unrevealing, but brain MRI often demonstrates hyperintensity in the pulvinar nucleus of the thalamus on fluid attenuated inversion recovery (FLAIR) sequences (i.e., pulvinar or hockey-stick sign) [37]. vCJD causing PrPSc is present in lymphoreticular tissue and is the only prion disease that can be identified via tonsil biopsy [23]. vCJD is also the only prion disease with epidemiologic evidence of transmission through blood transfusion (Transfusion Medicine Epidemiological Review (TMER) http://www.cjd.ed.ac.uk/TMER/TMER.htm).
Autoimmune encephalitis
Since the 2007 case series description of 12 women with limbic encephalitis and ovarian teratomas [38], later found to be caused by antibodies directed against the N-methyl-D-aspartate (NMDA) receptor, there have been incredible advances in our understanding of autoimmune encephalitis. The molecular identities of neuronal surface antigens have been further elucidated, prime examples of this is the discovery of leucine-rich glioma inactivated-1 (LGI1) and contactin-associated protein-like 2 (CASPR2) as the proteins targeted in most encephalitis cases previously thought to be caused by antibodies against the voltage-gated potassium channel [39,40,41].
Given the emerging knowledge about autoimmune encephalitis, we will give a brief overview of the salient clinical features of a few of the most well-defined types that can present clinically as a RPD: NMDA receptor, LGI1, and CASPR2 antibodies. Within the context of RPD, autoimmune encephalidities are characterized by a high prevalence of seizures, cognitive and psychiatric symptoms, and are potentially treatable. Robust epidemiological data continues to be gathered [42,43,44,45,46,47] as these syndromes are increasingly identified; therefore, it is important for clinicians to consider autoimmune encephalitis even in patients who may seem atypical and certainly should be ruled out in patients under investigation for RPD. Recently, an updated diagnostic guideline was made available for autoimmune encephalitis [48]. Discussion of paraneoplastic etiologies is omitted here due to space constraints; however, several excellent reviews have been published on the topic [49••, 50].
NMDA receptor encephalitis is currently the most common autoimmune cause of limbic encephalitis. The median age of onset is 21; however, age ranges from 1 to 85 years old have been reported, and it is much more common in women (81%) [43]. Patients typically have a febrile flu-like prodrome 1–2 weeks before the development of neurological changes. Behavior/personality changes (bizarre behavior, disinhibition, increased anger), psychiatric symptoms (depression, anxiety, hallucinations, delusions), and cognitive symptoms (memory loss, apraxia, executive dysfunction, language changes) emerge over days to weeks. Generalized tonic-clonic seizures are felt to be the most common seizure-type, and men present with seizures more commonly than women [51••]. The encephalopathy progresses and patients can fluctuate between akinetic mutism and episodes of agitation. Orofacial dyskinesia, trunkal dykinesia, hypoventilation, and autonomic instability then emerge. Patients warrant an intensive care treatment environment not only for seizure control but also for mechanical ventilation and close hemodynamic monitoring in the setting of dysautonomia. Mortality is estimated as 7% [43].
Diagnostic testing can prove frustrating in NMDA encephalitis as CSF shows a lymphocytic pleocytosis in 70–80% of patients [43, 52, 53] and antibody testing takes about 1 week. Brain MRI is usually normal; however, in about a third of the patients, there may be T2/FLAIR hypertintensity in the mesial temporal lobe, basal ganglia, or brain stem [54]. In about 30% of patients, the EEG shows an extreme delta brush pattern (generalized slow delta with overriding beta activity), which is independent of medication effect and felt to be unique to NMDA encephalitis [55].
Similar to NMDA encephalitis, the distinctive clinical features of anti-LGI1-mediated limbic encephalitis are important to recognize. LGI1 autoimmune encephalitis, like GABA-A and GABA-B encephalitis, is also classified as an autoimmune epilepsy, yet it is worthy of our attention within the consideration of RPD as patients develop subacute memory impairment and psychiatric disturbance [40, 41, 56]. Focal seizures with secondary generalization occur in roughly 70% of patients [57], and up to 47% demonstrate the pathognomonic faciobrachial dystonic seizure [47]. Faciobrachial dystonic seizures are coactivation of dystonic hand posturing and ipsilateral hemi-facial movements that last less than 3 s and appear dozens of times throughout a single day [58]. Rarely, patients with LGI1 antibodies can have Morvan’s syndrome (insomnia, peripheral nerve hyperexcitability, weakness, and multiorgan autonomic dysfunction) [40, 47, 59]. CSF is normal more than 50% of the time, and abnormalities are non-specific (e.g., oligoclonal bands, mild protein elevation) [47]. Brain MRI can show hippocampal T2 and FLAIR hyperintensity in about 80% of cases [47, 54]. Patients can also develop hyponatremia from the sydrome of inappropriate anti-diuretic hormone secretion [39].
CASPR2 encephalitis appears to have a greater range in symptoms and is more rare thus making it more diagnostically challenging. A 2016 retrospective study of 38 cases of antibody confirmed CASPR2 limbic encephalitis showed a clear male predominance (89%). The median age of onset was 66 years of age (ranged 25–77) and affected women were younger with a median age of 49 years. A thymoma was identified in 19% of patients. In patients being treated for limbic encephalitis, 53% developed epilepsy, 29% of them had Morvan’s syndrome, and another 15% had peripheral nerve hyper excitability syndrome. The authors proposed that the “core symptoms” of CASPR2 are cerebral symptoms (cognition and epilepsy), cerebellar symptoms, peripheral nerve hyperexcitability, autonomic dysfunction, insomnia, neuropathic pain, and weight loss. Three or more of these symptoms were present in 77% of their cohort [44]. Additionally, some patients with CASPR2 may have anti-acetylcholine receptor antibodies [60].
General principles of RPD treatment
Of the disease categories discussed above, infections, metabolic/toxic disturbances, and autoimmune encephalitis have effective treatments available, and if treated early, some patients may escape residual cognitive deficits. In neurodegenerative etiologies of RPD, palliative symptom management and ultimately hospice care are indicated. As discussed earlier, there is a subset of patients with an underlying typical dementia who then develop an increased rate of deficit accumulation that appears as “pseudo-rapid progression,” who should be given symptomatic treatment targeting the culprit comorbidity.
Autoimmune encephalitis
The first-line treatment for autoimmune encephalitis is high-dose steroids, plasma exchange, or IVIg with some patients receiving all three. When autoimmune encephalitis is suspected, one should have a low threshold for starting steroids given IV as 1 g of methylprednisolone/day for 5 days followed by oral prednisolone 60 mg daily for 2 weeks. If no response, administer IVIg 2 g/kg over 5 days or PLEX for 5–7 days. Patients who do not respond to these therapies should be considered for immunosuppressive therapies, most commonly rituximab (CD 20 anti-B cell monoclonal antibody, IV 1 g per week for 2 weeks) or cytoxan (T cell specific target, 500 mg IV every 2 weeks for 4 months) [57]. Slight variations on this approach have been published [61, 62].
Future treatment of prion diseases
Unfortunately, treatment trials of prion diseases have not been successful. Several compounds have been examined, including flupirtine, quinacrine, and doxycycline, and have not demonstrated any effect on survival time [5, 63]. Pentosan polysulphate (PPS) may affect progression of vCJD but poses challenges by being a heparin mimetic and requiring intraventricular administration [64]. Experience from these studies revealed that patients may be diagnosed so late in the disease that they and/or their families are reluctant to receive experimental treatment unless it is curative [65]. Thus, the prospect of finding a meaningful treatment for symptomatic prion disease will necessitate early diagnosis and/or reversal of neurologic damage.
Although the treatment of symptomatic prion diseases poses many challenges, the prophylactic treatment of mutation carriers for gPD may be more feasible. An example of this strategy is the Italian study examining the use of doxycycline in FFI mutation carriers [66]. Having a biomarker to help assess treatment efficacy will be important for these types of trials, as they would otherwise take generations to complete. In FFI, brain FDG-PET holds the potential to be such a biomarker and RT-QuIC performed on CSF or olfactory epithelium may prove to be a useful biomarker in other prion diseases [33]. Since the presence of the normal prion protein is necessary for prion disease to occur, knocking down its production may be sufficient to delay or prevent illness onset in mutation carriers [67]. There are several ways to accomplish this clinically such as lentivirus-mediated RNA interference (RNAi), PrPc directed antibodies and antisense oligonucleotides [68,69,70].
Palliative care in end stage dementia
Neurodegenerative etiologies of RPD are not reversible, and treatment with cholinesterase inhibitors or NMDA receptor antagonists only slows the rate of cognitive or functional decline in certain disease etiologies (i.e., AD and PDD). Additionally, these agents have not been studied in patients with RPD as a group, so their efficacy is unclear.
Once a diagnosis is determined, proper support of the caregiver and patient is crucial, with the goal being maintaining quality of life. Palliative and ultimately hospice care can provide appropriate symptom management and enduring support for family members during the course of their loved one’s illness. It is also important to recognize that RPD has a tremendous social and financial impact on families that requires guidance beyond the expertise of a medical professional. Disease-specific patient advocacy organizations such as the CJD Foundation (www.cjdfoundation.org), the Alzheimer’s Association (www.alz.org), the Association for Frontotemporal Degeneration (www.theaftd.org), the Autoimmune Encephalitis Alliance (USA, https://aealliance.org), and the Encephalitis Society (UK, https://www.encephalitis.info) have comprehensive resources and guidance for the serious non-medical aspects of these illnesses.
Conclusion
There has been increased recognition of the wide-ranging clinical features of numerous disease entities that can present as RPD. Diagnostic guidelines, cancer screening recommendations, therapy algorithms, and research toward discovery of new treatments are appearing ever more frequently in the literature. With this burgeoning medical knowledge, there is hope for improvements in care and quality of life for patients with RPD and their families.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Shrestha R, Wuerz T, Appleby BS. Rapidly progressive young-onset dementias: neuropsychiatric aspects. Psychiatr Clin North Am. 2015;38(2):221–32.
Schmidt C, Wolff M, Wietz M, Bartlau T, Korth C, Zerr I. Rapidly progressive Alzheimer disease. Arch Neurol. 2011;68(9):1124–30.
• Geschwind MD, Shu H, Haman A, Sejvar JJ, Miller BL. Rapidly progressive dementia. Ann Neurol. 2008;64(1):97–108.Includes an expanded discussion of the clinical features of causes of RPD.
•• Day G, Tang-Wai D. When dementia progresses quickly: a practical approach to the diagnosis and management of rapidly progressive dementia. Neurodegener Dis Manag. 2014;4(1):41–56. The review article features detailed clinical assessment necessities including an expanded discussion of physical exam components. Valuable charts and figures highlight ways of sorting through competing disease etiologies based on a patient's demographic information, speed of decline and testing data
Appleby BS, Lyketsos CG. Rapidly progressive dementias and the treatment of human prion diseases. Expert Opin Pharmacother. 2011;12(1):1–12.
Paterson R, Takada L, Geschwind M. Diagnosis and treatment of rapidly progressive dementias. Neurol Clin Pract. 2012;2(3):187–200.
Mead S, Rudge P. CJD mimics and chameleons. Pract Neurol. 2017;17(2):113–21.
•• Geschwind M. Rapidly progressive dementia. Continuum. 2016;22(2 Dementia):510–37. The most updated review of RPD with excellent illustrative cases and demonstrations of relavant brain MRI findings
Armangue T, Leypoldt F, Dalmau J. Autoimmune encephalitis as differential diagnosis of infectious encephalitis. Curr Opin Neurol. 2014;27(3):361–8.
Aubert L, Pichierri S, Hommet C, Camus V, Berrut G, de Decker L. Association between comorbidity burden and rapid cognitive decline in individuals with mild to moderate Alzheimer’s disease. J Am Geriatr Soc. 2015;63(3):543–7.
Schmidt C, Redyk K, Meissner B, Krack L, von Ahsen N, Roeber S, et al. Clinical features of rapidly progressive Alzheimer’s disease. Dement Geriatr Cogn Disord. 2010;29(4):371–8.
Sona A, Zhang P, Ames D, Bush AI, Lautenschlager NT, Martins RN, et al. Predictors of rapid cognitive decline in Alzheimer’s disease: results from the Australian imaging, biomarkers and lifestyle (AIBL) study of ageing. Int Psychogeriatr. 2012;24(2):197–204.
• Peters ME, Schwartz S, Han D, Rabins PV, Steinberg M, Tschanz JT, et al. Neuropsychiatric symptoms as predictors of progression to severe Alzheimer’s dementia and death: the Cache County Dementia Progression Study. Am J Psychiatry. 2015;172(5):460–5. This is a high quality longitudinal study that draws attention to the prognostic implications of psychiatric comorbidities in Alzheimer’s dementia
Cohen ML, Kim C, Haldiman T, ElHag M, Mehndiratta P, Pichet T, et al. Rapidly progressive Alzheimer’s disease features distinct structures of amyloid-beta. Brain. 2015;138(Pt 4):1009–22.
Tosto G, Gasparini M, Brickman AM, Letteri F, Renie R, Piscopo P, et al. Neuropsychological predictors of rapidly progressive Alzheimer’s disease. Acta Neurol Scand. 2015;132(6):417–22.
Grau-Rivera O, Gelpi E, Nos C, Gaig C, Ferrer I, Saiz A, et al. Clinicopathological correlations and concomitant pathologies in rapidly progressive dementia: a brain bank series. Neurodegener Dis. 2015;15(6):350–60.
Mann UM, Mohr E, Chase TN. Rapidly progressive Alzheimer’s disease. Lancet. 1989;2(8666):799.
Qiang W, Yau WM, Lu JX, Collinge J, Tycko R. Structural variation in amyloid-beta fibrils from Alzheimer’s disease clinical subtypes. Nature. 2017;541(7636):217–21.
Drummond E, Nayak S, Faustin A, Pires G, Hickman RA, Askenazi M, et al. Proteomic differences in amyloid plaques in rapidly progressive and sporadic Alzheimer’s disease. Acta Neuropathol. 2017;133(6):933–54.
• Cohen M, Appleby B, Safar JG. Distinct prion-like strains of amyloid beta implicated in phenotypic diversity of Alzheimer’s disease. Prion. 2016;10(1):9–17. Presents evidence toward prion-like behavior of certain amyloid beta molecular subtypes in different Alzheimer disease phenotypes
Appleby BS, Appleby KK, Rabins PV. Does the presentation of Creutzfeldt-Jakob disease vary by age or presumed etiology? A meta-analysis of the past 10 years. J Neuropsychiatry Clin Neurosci. 2007;19(4):428–35.
Appleby BS, Appleby KK, Crain BJ, Onyike CU, Wallin MT, Rabins PV. Characteristics of established and proposed sporadic Creutzfeldt-Jakob disease variants. Arch Neurol. 2009;66(2):208–15.
Organization WH. Global surveillance, diagnosis and therapy of human transmissible spongiform encephalopathies: report of a WHO consultation. 1998:1–30.
Foutz A, Appleby BS, Hamlin C, Liu X, Yang S, Cohen Y, et al. Diagnostic and prognostic value of human prion detection in cerebrospinal fluid. Ann Neurol. 2017;81(1):79–92.
•• Bongianni M, Orru C, Groveman BR, Sacchetto L, Fiorini M, Tonoli G, et al. Diagnosis of human prion disease using real-time quaking-induced conversion testing of olfactory mucosa and cerebrospinal fluid samples. JAMA Neurol. 2017;74(2):155–62. Demonstrates the sensitivity and specificity of real-time quaking-induced conversion assay for detection of prion disease in comparison with currently recommended disease defining diagnostic criteria
Vitali P, Maccagnano E, Caverzasi E, Henry RG, Haman A, Torres-Chae C, et al. Diffusion-weighted MRI hyperintensity patterns differentiate CJD from other rapid dementias. Neurology. 2011;76(20):1711–9.
Puoti G, Bizzi A, Forloni G, Safar JG, Tagliavini F, Gambetti P. Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol. 2012;11(7):618–28.
Hamaguchi T, Kitamoto T, Sato T, Mizusawa H, Nakamura Y, Noguchi M, et al. Clinical diagnosis of MM2-type sporadic Creutzfeldt-Jakob disease. Neurology. 2005;64(4):643–8.
Gambetti P, Kong Q, Zou W, Parchi P, Chen SG. Sporadic and familial CJD: classification and characterisation. Br Med Bull. 2003;66:213–39.
Zou WQ, Puoti G, Xiao X, Yuan J, Qing L, Cali I, et al. Variably protease-sensitive prionopathy: a new sporadic disease of the prion protein. Ann Neurol. 2010;68(2):162–72.
Minikel EV, Vallabh SM, Lek M, Estrada K, Samocha KE, Sathirapongsasuti JF, et al. Quantifying prion disease penetrance using large population control cohorts. Sci Transl Med. 2016;8(322):322ra9.
Krasnianski A, Sanchez Juan P, Ponto C, Bartl M, Heinemann U, Varges D, et al. A proposal of new diagnostic pathway for fatal familial insomnia. J Neurol Neurosurg Psychiatry. 2014;85(6):654–9.
Cortelli P, Perani D, Montagna P, Gallassi R, Tinuper P, Provini F, et al. Pre-symptomatic diagnosis in fatal familial insomnia: serial neurophysiological and 18FDG-PET studies. Brain. 2006;129(Pt 3):668–75.
Sano K, Satoh K, Atarashi R, Takashima H, Iwasaki Y, Yoshida M, et al. Early detection of abnormal prion protein in genetic human prion diseases now possible using real-time QUIC assay. PLoS One. 2013;8(1):e54915.
Brown P, Brandel JP, Sato T, Nakamura Y, MacKenzie J, Will RG, et al. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis. 2012;18(6):901–7.
Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, et al. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;347(9006):921–5.
Zeidler M, Sellar RJ, Collie DA, Knight R, Stewart G, Macleod MA, et al. The pulvinar sign on magnetic resonance imaging in variant Creutzfeldt-Jakob disease. Lancet. 2000;355(9213):1412–8.
Dalmau J, Tuzun E, Wu HY, Masjuan J, Rossi JE, Voloschin A, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25–36.
Vincent A, Buckley C, Schott JM, Baker I, Dewar BK, Detert N, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127(Pt 3):701–12.
Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. 2010;133(9):2734–48.
Lai M, Huijbers MG, Lancaster E, Graus F, Bataller L, Balice-Gordon R, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9(8):776–85.
Lee S, Lee S. Laboratory diagnosis of autoimmune encephalitis. J Epilepsy Res. 2016;6(2):45–52.
Titulaer MJ, McCracken L, Gabilondo I, Armangue T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157–65.
van Sonderen A, Arino H, Petit-Pedrol M, Leypoldt F, Kortvelyessy P, Wandinger K, et al. The clinical spectrum of Caspr2 antibody-associated disease. Neurology. 2016;87(5):521–8.
Chitravas N, Jung RS, Kofskey DM, Blevins JE, Gambetti P, Leigh RJ, et al. Treatable neurological disorders misdiagnosed as Creutzfeldt-Jakob disease. Ann Neurol. 2011;70(3):437–44.
Celicanin M, Blaabjerg M, Maersk-Moller C, Beniczky S, Marner L, Thomsen C, et al. Autoimmune encephalitis associated with voltage-gated potassium channels-complex and leucine-rich glioma-inactivated 1 antibodies - a national cohort study. Eur J Neurol. 2017;
van Sonderen A, Thijs RD, Coenders EC, Jiskoot LC, Sanchez E, de Bruijn MA, et al. Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology. 2016;87(14):1449–56.
Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404.
•• Titulaer MJ, Soffietti R, Dalmau J, Gilhus NE, Giometto B, Graus F, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol. 2011;18(1):19-e3. Clearly delineates clinical diagnostic criteria for autoimmune encephalitis
Rosenfeld M, Dalmau J. Paraneoplastic disorders of the CNS and autoimmune synaptic encephalitis. Continuum. 2012;18(2):366–83.
•• Titulaer MJ, Dalmau J. Seizures as first symptom of anti-NMDA receptor encephalitis are more common in men. Neurology. 2014;82(7):550–1. This review gives a thorough and pragmatic discussion of paraneoplastic disorders of the central nervous system
Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091–8.
Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133(Pt 6):1655–67.
Gaspard N. Autoimmune epilepsy. Continuum. 2016;22(1):227–45.
Schmitt SE, Pargeon K, Frechette ES, Hirsch LJ, Dalmau J, Friedman D. Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology. 2012;79(11):1094–100.
Finke C, Pruss H, Heine J, Reuter S, Kopp UA, Wegner F, et al. Evaluation of cognitive deficits and structural hippocampal damage in encephalitis with leucine-rich, glioma-inactivated 1 antibodies. JAMA Neurol. 2017;74(1):50–9.
Bakpa OD, Reuber M, Irani SR. Antibody-associated epilepsies: clinical features, evidence for immunotherapies and future research questions. Seizure. 2016;41:26–41.
Irani SR, Michell AW, Lang B, Pettingill P, Waters P, Johnson MR, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69(5):892–900.
Arino H, Armangue T, Petit-Pedrol M, Sabater L, Martinez-Hernandez E, Hara M, et al. Anti-LGI1-associated cognitive impairment: presentation and long-term outcome. Neurology. 2016;87(8):759–65.
Iwasaki Y, Kinoshita M, Ikeda K, Takamiya K, Shiojima T. Concurrence of myasthenia gravis and choree fibrillaire de Morvan. Eur Arch Psychiatry Neurol Sci. 1990;239(5):335–6.
Lancaster E. The diagnosis and treatment of autoimmune encephalitis. J Clin Neurol. 2016;12(1):1–13.
McKeon A. Autoimmune encephalopathies and dementias. Continuum (Minneap Minn). 2016;22(2 Dementia):538–58.
Haik S, Marcon G, Mallet A, Tettamanti M, Welaratne A, Giaccone G, et al. Doxycycline in Creutzfeldt-Jakob disease: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13(2):150–8.
Bone I, Belton L, Walker AS, Darbyshire J. Intraventricular pentosan polysulphate in human prion diseases: an observational study in the UK. Eur J Neurol. 2008;15(5):458–64.
Collinge J, Gorham M, Hudson F, Kennedy A, Keogh G, Pal S, et al. Safety and efficacy of quinacrine in human prion disease (PRION-1 study): a patient-preference trial. Lancet Neurol. 2009;8(4):334–44.
Forloni G, Tettamanti M, Lucca U, Albanese Y, Quaglio E, Chiesa R, et al. Preventive study in subjects at risk of fatal familial insomnia: innovative approach to rare diseases. Prion. 2015;9(2):75–9.
Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, et al. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73(7):1339–47.
Nazor Friberg K, Hung G, Wancewicz E, Giles K, Black C, Freier S, et al. Intracerebral infusion of antisense oligonucleotides into prion-infected mice. Mol Ther Nucleic Acids. 2012;1:e9.
Nicoll AJ, Collinge J. Preventing prion pathogenicity by targeting the cellular prion protein. Infect Disord Drug Targets. 2009;9(1):48–57.
White MD, Mallucci GR. RNAi for the treatment of prion disease: a window for intervention in neurodegeneration? CNS Neurol Disord Drug Targets. 2009;8(5):342–52.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Dementia
Rights and permissions
About this article
Cite this article
Mahajan, S., Appleby, B.S. Comprehensive and Methodical: Diagnostic and Management Approaches to Rapidly Progressive Dementia. Curr Treat Options Neurol 19, 40 (2017). https://doi.org/10.1007/s11940-017-0474-1
Published:
DOI: https://doi.org/10.1007/s11940-017-0474-1