Opinion statement
Several treatment and rehabilitation approaches for sport-related concussion have been mentioned in recent consensus and position statements. These options range from the more conservative behavioral management approaches to aggressive pharmacological and therapeutic interventions. Moreover, clinical decision-making for sport-related concussion changes as symptoms and impairments persist throughout recovery. The current article provides an empirical review of proposed treatment and rehabilitation options for sport-related concussion during the acute, subacute, and chronic phases of injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is estimated that approximately 1.6 to three million sports and recreation mild traumatic brain injuries (i.e., sports-related concussion: SRC), occur every year in the United States [1]. While this likely represents an underestimate in the actual incidence of SRC [2], this injury remains a pressing concern for sports medicine professionals. The documented deleterious cognitive, physical, emotional, and sleep-related effects of this injury have prompted increased research efforts focused on understanding and documenting the time course of recovery following SRC. While approximately 80 % of SRCs resolve within 3 weeks of injury, 20 % of SRCs demonstrate a prolonged recovery longer than this 3-week estimate [3]. The disparity in SRC recovery time has motivated the need for early treatment and rehabilitation interventions in an effort to mitigate the risk of prolonged recovery [4•]. While the clinical practice of managing concussion has begun incorporating several treatment and rehabilitation interventions, the evidence base demonstrating their efficacy is still in its infancy. This article will provide an overview of treatment and rehabilitation interventions that are currently recommended for use in the management of SRC. These interventions will be reviewed in the temporal context of the acute, sub-acute, and chronic phases of recovery, and the evidence supporting the use of these approaches will be critically examined.
Overview of treatment and rehabilitation approaches for acute, sub-acute, and chronic post-concussion recovery
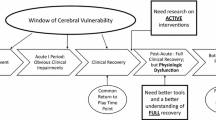
Several consensus statements and review papers present a wide range of treatment and rehabilitation strategies for SRC that include rest (e.g., cognitive and physical), pharmacological interventions (e.g., sleep medication, neurostimulants), therapies (e.g., cognitive, physical, vestibular), and other types of behavioral/environmental modifications (e.g., daily routine, academic accommodations) [4•, 5–7]. Dosage and timing of these treatment and rehabilitation options are often presented in the context of the acute (the first 7 days following injury), sub-acute (8 – 89 days post-injury), and chronic (90 days or more) recovery time periods [8•]. Factor analytic methods applied in a recent study revealed that post-concussion symptoms reported in the first week following SRC represented primarily a “global” symptom presentation that represented a cognitive-fatigue migraine factor along with secondary sleep, physical, and emotional factors [9•]. These results, taken together with other studies suggesting that post-SRC symptomatology begins to delineate into a more distinct cognitive, physical, emotional, and sleep symptom clusters after the first week following SRC [10], support a more conservative approach to concussion management during the acute time period following injury. As symptoms persist into the sub-acute and chronic post-concussion time periods, the clinical approach to determining treatment and rehabilitation options for SRC often becomes more aggressive (e.g., therapies and pharmacological interventions).
Acute treatment of SRC
During the acute stages of recovery following SRC, the “cornerstone” of concussion management is considered to be physical and cognitive rest until acute symptoms of concussion have resolved [6, 7, 11]. However, there are limited evidence-based data to support the utility of rest following concussion [12]. Researchers have observed no benefit of strict activity restriction over 5 days, as compared to 1 – 2 days of activity restriction [13], and provision of rest was associated with longer duration of concussion symptoms [14]. In contrast, other researchers documented decreased concussion symptoms following 1 week of rest, even when implemented weeks to months following concussion [15]. Following the acute period, academic supports may assist in returning the concussed athlete to school [16], including rest periods during the day, excuse from sports or gymnastics, avoidance from physical exertion, and decreased environmental distractions. Unfortunately, while universal policies for such accommodations have been proposed [17], there are as yet no data documenting improved outcomes or efficacy.
In addition to prescribed cognitive and physical rest, pharmacological interventions (e.g., neurostimulants, sleep aids) have been proposed as options to mitigate severe symptomology experienced during the acute phase of recovery [6]. It has been recommended that individuals avoid medications that might alter mental status during the first 10 h following injury [6]. Moreover, there are no convincing data supporting the efficacy of acute pharmacological intervention over and above the more conservative behavioral management approaches (e.g., appropriate cognitive and physical rest, sleep, diet, hydration) suggested for this initial time period following injury. Future studies examining the efficacy of early pharmacological treatment for SRC are recommended.
Sub-acute treatment and rehabilitation of SRC
Several treatment and rehabilitation strategies for the sub-acute (i.e., persistent impairments longer than 8 days post-concussion) post-injury time period are briefly outlined in current consensus statements [7, 11] and have been expounded upon in recent clinical review papers [4•]. Collins et al. [4•] outlined a new approach for clinical treatment and rehabilitation strategies implemented during the sub-acute presentation of SRC. Using a comprehensive clinical interview with an accompanying symptom, neurocognitive, vestibular, and oculomotor assessment, Collins and colleagues [4•] categorized athletes into a predominant cognitive/fatigue, vestibular, oculomotor, anxiety/mood, post-traumatic migraine, and/or cervical trajectory with recommended treatment and/or rehabilitation approaches specific to the trajectory. While their recommendations are largely based on clinical experience, these authors are among the first to match clinical assessment outcomes with recommended targeted therapies for SRC. These clinical recommendations coupled with the extant evidence outlined in current consensus papers provide a strong foundation for the sub-acute treatment of SRC. More specifically, prescribed and monitored exercise, pharmacological interventions, and therapeutic approaches (e.g., vestibular, oculomotor, and cognitive therapies) are widely proposed as effective approaches for the sub-acute treatment of SRC.
Prescribed exercise
While physical and cognitive rest are currently recommended as a “first line of treatment” during the initial week following SRC, prolonged (i.e., prolonging rest into the sub-acute recovery period) rest may have detrimental effects on the well being of the concussed athlete. If symptoms and impairments persist and do not improve following the acute prescription of physical and cognitive rest, sports medicine professionals should consider the concomitant effects of prolonging physical and cognitive rest on the academic, physical, and social well being of the athlete. In addition, athletes may report feelings of isolation, depression, and anxiety, given that exercise and social interactions with peers and teammates are likely important stress outlets and coping mechanisms (i.e., emotional release) [4•]. In addition, increased cardiovascular activity may help mitigate post-traumatic migraine symptoms (PTM). However, the manner in which exercise is prescribed is critical to avoid further exacerbation of symptoms.
Researchers and clinicians have suggested that “low level” monitored exercise may be beneficial for concussed athletes who are experiencing persistent concussion symptoms and impairment [6, 7, 11]. Prescribed exercise as a means of treatment comes with the caveat that symptoms should not be exacerbated during or following these bouts of activity, and risk of subsequent concussive impacts are eliminated. While the documented neurophysiological benefits of exercise should help mitigate and manage the adverse effects of SRC, few studies have investigated the dose-response relationship between exercise and improved sub-acute recovery outcomes in concussed athletes.
Several related studies support the potential role that exercise may have as a sub-acute treatment strategy for SRC. Exercise interventions in animal models demonstrate improved neurogenesis, neuroplasticity [18], decreased oxidative stress and neuroinflammation, and decreased cognitive dysfunction [19, 20], all of which relate to the neurometabolic and neurophysiological events that underlie SRC [5]. Moreover, exercise has been shown to benefit the treatment and management of depression and anxiety, which often share co-morbidity with SRC [21], in both children and adolescents [22, 23]. While there seems to be a theoretical foundation supporting the role of exercise in the sub-acute treatment of SRC, there are no studies examining its efficacy during this sub-acute stage of recovery. In contrast, this potential SRC treatment has been directly evaluated in patients with chronic PCS, which will be discussed in the following section. Nonetheless, exercise is mentioned by expert consensus and review papers [4•, 7], both as a stand-alone treatment option and as part of the behavioral management treatment approach for athletes experiencing persistent SRC symptoms. Additional research should be targeted at documenting the clinical value of using exercise as a sub-acute treatment option with the hopes of preventing PCS.
Pharmacological interventions
Pharmacological treatments for SRC have been identified as an option for the management of specific, prolonged symptoms persisting longer than 10 days post injury [11, 24]. It is important to note that there is currently no FDA-approved pharmacological intervention for the treatment of SRC. Expert and consensus statements recommend that pharmacological interventions can be used to manage specific or prolonged SRC symptoms (e.g., sleep, anxiety) and/or to reduce or shorten severe symptoms of SRC [4•, 6, 7, 11]. Only experienced, licensed medical professionals with prescription privileges and specialized training in SRC should prescribe medications to athletes. To date, there are no randomized clinical trials investigating the effectiveness of pharmacological interventions in athletes following SRC. There are several review papers that include related findings in non-athlete, brain-injured populations with varying degrees of injury severity to support the use of medications for SRC. However, these findings are not necessarily generalizable to athletes with SRC.
Currently, the approach for treating SRC using pharmacological interventions has revolved around matching medications to specific somatic, emotional, sleep, and/or cognitive symptom presentations [10, 25, 26]. For example, an athlete with cognitive-related symptoms might be prescribed a neurostimulant to treatment. Recently, Collins et al. (2014) proposed a new clinical care model that suggested matching an athlete’s SRC clinical trajectories with specific targeted treatments, including pharmacological interventions. This approach extends the current clinical framework for prescribing medication to include impairment in addition to symptoms to inform prescription decisions. A thorough review of pharmacological interventions for SRC can be found in Meehan et al. [25] and Petraglia et al. [26]. A brief overview of the proposed pharmacological interventions outlined in Collins et al. [4•] is provided below.
The cognitive-fatigue, sleep, post-traumatic migraine (PTM), and anxiety/mood clinical trajectories may be effectively treated and managed with medication [4•, 7]. Athletes who present with cognitive-fatigue symptomatology (e.g., decreased energy, headache that increases throughout the day, sleep disruption) may benefit from neurostimulant medications and sleep aids (e.g., melatonin). Amantadine, a dopaminergic neurostimulant has been shown to improve recovery in adolescent athletes with SRC [27]. These researchers reported a significant improvement in post-concussion symptoms, verbal memory, and reaction time for adolescent athletes receiving amantadine compared to historical controls who did not received amantadine. In addition to cognitive-fatigue symptoms, concussed athletes presenting with sleep difficulties may benefit from sleep aids such as melatonin. Melatonin has been linked to improved sleep following traumatic brain injury in several studies and is recommended due to its high safety profile and low risk of toxicity [25, 26]. Combined with proper sleep hygiene [28], melatonin can be an effective first-line of treatment. Other more aggressive medication options for treating sleep dysfunction following SRC include sedative hypnotics, serotonin modulators, and beta-andrenergic antagonists [26].
Longer PTM and anxiety/mood clinical trajectories may also be effectively managed through pharmacological interventions. The PTM symptom cluster is characterized by headache and nausea, with accompanying photo- and/or phono-sensitivity and has been linked to protracted recovery outcomes following SRC [29]. Pharmacological treatment of PTM may include tricyclic antidepressants, anticonvulsants, beta or calcium channel blockers, or triptans. These pharmacological interventions for PTM should be accompanied with appropriate behavioral management approaches commonly prescribed for headache/migraine including proper sleep and nutrition, hydration, and exercise. Not surprisingly, anxiety/mood symptoms following concussion can also be treated pharmacologically with prescribed antidepressants, selective serotonin reuptake inhibitors (SSRIs) and benzodiazepines. Currently, there are no empirical investigations documenting the utility of these medications for SRC. However, several studies demonstrating the efficacy of these treatments in more severe traumatic brain injury (TBI) is documented [25, 30].
As is clear from the preceding overview, additional research on the efficacy of pharmacological interventions for the treatment and management of SRC is warranted. The current knowledge supporting pharmacological interventions is based primarily on findings from more severe forms of brain injury. These findings may not be directly applicable for athletes experiencing the more subtle effects of SRC. As a final comment, it is important for clinicians to note that concussed athletes should be symptom-free without medication in order to determine if they are recovered and ready for RTP. Recently, other therapeutic treatment approaches for SRC including vestibular and oculomotor/vision interventions are becoming more prevalent in the clinical approach to treating athletes with SRC as we develop a better understanding of the clinical trajectories of this injury.
Vestibular therapy
The most common vestibular symptoms include dizziness, imbalance, and vertigo. In fact, approximately 50 % of athletes report vestibular symptoms following SRC [9•]. It is important to note that symptoms such as headache, dizziness, and nausea may only be present following a provoking stimulus. In a recent study, 61 % of patients with SRC reported at least one symptom following a brief vestibular/oculomotor screening (VOMS) exam [4•]. Vestibular impairment may be categorized as vestibulo-ocular (e.g., involving maintenance of visual stability during movement as evident in gaze stability and gait dysfunction); or vestibulo-spinal (e.g., involving postural control and evident in imbalance). Documented approaches to vestibular therapies include eye-head coordination; sitting, standing static, and standing dynamic balance; and ambulation exercises [31]. The vestibular rehabilitation programs reported in the literature are typically 4 – 8 weeks in duration, with each rehabilitation session lasting between 1 – 3 h. Most of the programs involve some sort of at-home rehabilitation component, but adherence is rarely reported. One of the problems in quantifying the effects of specific vestibular exercises on concussion-related outcomes is the number of modifiers (e.g., posture, surface, trunk position, direction of head movement, presence of dual task) that can be manipulated during each exercise. As such, researchers have typically combined multiple vestibular exercises with different parameters into aggregate groups for analysis. Moreover, although the preceding vestibular therapies are suggested as viable treatment interventions for vestibular-related symptoms and impairment following SRC [4•], there are limited empirical studies examining their effectiveness. The evidence that has been published consists primarily of retrospective, cross-sectional, and small cohort studies.
There are few published prospective studies or randomized, controlled trial (RCT) designs of the effectiveness of therapeutic interventions for SRC. However, recently Schneider and colleagues [32] conducted an RCT with a small sample (N = 31) of 12 – 30 year olds with dizziness, neck pain, and/or headache following SRC. The results of their 8-week trial indicated that the group that received cervical spine and vestibular rehabilitation were nearly four times more likely to be medically cleared following the 8-week study period than the control group. Although the results from this study are promising, they are limited by the inability to infer how much of the effect was due to the cervical spine compared to the vestibular component of the intervention. In a retrospective chart review of 114 patients with concussion, Alsalaheen et al. [33] reported that vestibular rehabilitation may reduce dizziness and improve both gait and balance. Researchers indicated that these improvements were similar for both children and adult patients. In one of the first studies to examine the effectiveness of vestibular therapies, Hoffer et al. [34] examined the effectiveness of vestibular therapy in 58 patients with post-traumatic migraine-associated, positional vertigo, and spatial disorientation following mild TBI. Overall, their results indicated that within 1 – 8 weeks of treatment, patients improved, but improvement was dependent on the type of impairment and symptoms. Specifically, they reported that 84 % of patients with post-traumatic migraine-associated dizziness were responsive to treatment compared to only 27 % of the spatial disorientation group. Also, on average the spatial disorientation group required three times longer to return to work. This study was unique in that it further partitioned vestibular trajectory patients into a migraine-variant, disorientation, and positional vertigo groups.
Oculomotor and vision therapies
Oculomotor symptoms and visual impairment following brain injury include accommodative deficiencies, convergence insufficiency, diplopia, fixation defects, nystagmus, phoria, as well as defective gaze stability and saccadic and pursuit eye movements [35]. Almost 30 % of athletes report visual problems following SRC [21]. These symptoms and impairments may manifest as concentration problems, difficulty in busy, high-traffic environments (e.g., shopping center, driving), headaches, reading difficulties, trouble focusing and paying attention, and visual confusion [36]. Functionally, these symptoms and impairments often translate into reduced academic and work performance. Oculomotor and vision therapies might include versional (e.g., fixation, saccades, pursuits), vergence (e.g., fusion, sustained vergence), accommodative (e.g., monocular and binocular) and visual attention (e.g., scanning, gaze) exercises. Many of the current therapies involve Internet-based software programs and specially designed glasses, allowing many patients to perform their vision exercises at home on their own schedule. As with vestibular therapies, the key to the effectiveness in these therapies lies in matching the oculomotor and vision therapy with the specific symptoms and impairments.
Empirical support for oculomotor and vision-related therapies is even more scant than for vestibular therapies, and the level of evidence for the support does not include any RCTs. Nonetheless, initial evidence points to support for targeted oculomotor and vision rehabilitation therapies. In a retrospective study that included 33 patients with mTBI, researchers reported that oculomotor therapies (i.e., combined vergence, versional, and accommodative exercises) resulted in 90 % of patients improving markedly or completely in symptoms and impairment [36]. These improvements were accompanied by subjective reports of enhanced reading following treatment and were sustained out to a 2 – 3-month follow-up time period. More recently, Thiagarajan and colleagues [37, 38] reported improvements in both accommodative responsivity and versional eye movements following oculomotor training in a small sample of patients (N = 12) with mTBI. They employed training targeting the version, vergence, and acommodation components of the oculomotor system. Specifically, saccadic training using visual concentration and perceptual puzzle block tasks. Unfortunately, the assessment of these rehabilitation therapies was conducted in such as a way that the researchers could not infer which therapy resulted in which specific effect. The sample sizes were also very small to infer to the general population. Future approaches would benefit from treatment-withdrawal and true RCT designs with larger samples.
Vestibular and oculomotor symptoms and impairment often accompany SRC and may indicate worse outcomes. There is growing support in the literature for the effectiveness of specific therapeutic interventions and rehabilitation modalities that target these issues. Together these findings suggest that as a result of residual neural plasticity, the vestibular and oculomotor systems are amenable to improvements with appropriately applied and matched exercises and therapies. Although the preliminary evidence for these therapies is promising, researchers need to address several shortcomings moving forward. In the current climate of reduced insurance coverage for therapy following injury, it is important for researchers to determine the minimal and optimal number of therapy sessions, and how soon after injury (e.g., acute, sub-acute phase of SRC) they should be implemented to be effective. In addition, a comparison of the effectiveness of at-home, software-based vision therapy compared to clinic-based therapy sessions is also warranted. Researchers also need to establish whether the effects of therapies have both near and far transfer to other activities such as academic, driving, and sport performance; and whether these effects and the therapies might be influenced by developmental (i.e., age) factors. To date, the research discussed previously assessed the combined effects of multiple therapies used simultaneously. A better approach would be to determine the relative contribution of each therapy to specific outcomes. Finally, all therapies need to be tested using RCT designs, as there is likely to be substantial bias (e.g., placebo effect, halo effect, experimenter bias) in less internally valid research designs of the effectiveness of therapeutic interventions.
Cognitive therapies
In addition to physical therapies, cognitive therapies may also have application for athletes in the sub-acute recovery phase of SRC. In the American Academy of Neurology (AAN) consensus statement, Giza and colleagues [11] recognize the value of the education, reassurance, and reattribution of SRC symptom components of cognitive restructuring. These researchers also recognize the potential utility of this approach to mitigate the risk of developing chronic post-concussion syndrome (PCS). Although the literature on using cognitive therapies during the sub-acute recovery phase following SRC is scant and primarily prescribed in patients with PCS, this therapeutic alternative has been linked to improving insomnia [39], depression [40, 41], and anxiety [42] in non-injured youth and may be an effective means of management in earlier phases of recovery. Moreover, providing psychoeducation early in the recovery time course following concussive injury has been linked to reduced symptom reports. Ponsford et al. [43] reported improved recovery outcomes at 3 months post injury in children with mTBI who received an educational coping intervention 1 week following injury than injured children without the 1-week intervention. These results are in concordance with Mittenberg et al. [44] who also reported that early psychoeducational intervention can reduce the risk of PCS.
Chronic PCS treatment and rehabilitation of SRC
Several definitions (see World Health Organization: WHO, 2010, F07.2, and the Diagnostic and Statistical Manual of Mental Disorders: DSM: APA, 2013, pp. 624.ff for PCS definitions) have been proposed in the literature and by different professional organizations in an effort to encapsulate the chronic presentation of SRC symptoms and impairments that unfortunately warrants the “label” of chronic PCS. While most athletes with SRC recover within a 7 – 10 day period, approximately 10 % take longer than 14 days to recover [45]. Other studies have reported that in non-sports-related concussion, 33 % of patients require longer than 3 months to recover [46]. As previously mentioned, for the purpose of the current article, chronic PCS will be defined as SRC symptoms and impairments lasting 90 days or more [8•]. Several clinical and research efforts have been targeted at identifying efficacious treatments for this “miserable minority” [47] as this syndrome can adversely influence all areas of the athletes life (e.g., social, academic, athletic, physical, emotional).
As with most clinically recommended treatments for SRC, there is a paucity of empirically supported literature on treatment interventions for PCS. Several of the aforementioned pharmacological approaches and therapies targeted for the sub-acute phase of recovery, are also viable options for this chronic recovery period. According to a recent literature review by Leddy and colleagues [48], early education [44], cognitive behavioral therapy [43], aerobic exercise therapy [49], and targeted medications are potential treatment interventions for athletes with PCS.
A systematic review of psychological interventions for PCS revealed evidence that cognitive-behavioral therapy (CBT) may be effective in decreasing PCS symptoms [50, 51], as compared to informational or educational programs. Furthermore, the implementation of CBT following post-acute discharge was shown to reduce symptom duration, frequency, and severity [44]. Other studies support the use of controlled aerobic exercise (e.g., using a treadmill to achieve 80 % systolic heart rate) as a safe and effective approach to PCS treatment, with greater reductions in PCS symptoms as compared to controls [49]. These results were supported by a recent study using functional magnetic resonance imaging (fMRI), which revealed that controlled exercise promoted normal cerebral blood flow in PCS patients. These findings, taken together, highlight the underlying physiological dysfunction associated with the persistent chronic symptoms of SRC and the supporting role for aerobic treatment [52]. In contrast to exercise, a period of physical and cognitive rest was also shown to alleviate PCS symptoms, even when applied months following the concussive injury [15]. Hyperbaric oxygen was found to have some immediate effects with respect to cognitive functioning and quality of life, in a sample of mTBI patients 1 – 5 years post injury; however, these effects did not last beyond the treatment period [53].
In conclusion, the framework for treatment options for SRC is evolving. On-going research efforts that identify and document the clinical presentation of SRC in the days, weeks, and months following injury will continue to inform treatment options for injured athletes. Moreover, the treatment paradigm is shifting from a “one size fits all” to a more targeted, specific treatment and rehabilitation plan. These efforts will not only expedite recovery, but also ensure a complete and safe return to activity for the concussed athlete.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–8.
Schatz P, Moser RS. Current issues in pediatric sports concussion. The Clinical Neuropsychologist. 2011;1–16.
Iverson GL et al. Tracking neuropsychological recovery following concussion in sport. Brain Inj. 2006;20(3):245–52.
Collins MW et al. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):235–46. This clinical review paper proposes a new paradigm for SRC treatment that includes specific clinical trajectories with appropriate assessment and rehabilitation recommendations.
Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36(3):228–35.
Harmon KG et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47(1):15–26.
McCrory P et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. J Am Coll Surg. 2013;216(5):e55–71.
Krainin BM et al. Mild traumatic brain injury literature review and proposed changes to classification. J Spec Oper Med. 2011;11(3):38–47. This review paper proposes a new classification to the acute, sub-acute, and chronic phases of SRC recovery.
Kontos AP et al. A revised factor structure for the post-concussion symptom scale: baseline and postconcussion factors. Am J Sports Med. 2012;40(10):2375–84. This study conceptualizes the acute symptom presentation of concussion and forms the basis of current understanding of how symptoms change following SRC.
Pardini J et al. The post concussion symptom scale (PCSS): a factory analysis [abstract]. Br J Sports Med. 2004;38:661–2.
Giza CC et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80(24):2250–7.
Schneider KJ et al. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. Br J Sports Med. 2013;47(5):304–7.
Thomas DG et al. Randomized controlled trial of strict rest following acute concussion. Presented at the American Academy of Pediatrics National Conference, Orlando, FL. 2013.
Gibson S et al. The effect of recommending cognitive rest on recovery from sport-related concussion. Brain Inj. 2013;27(7–8):839–42.
Moser RS, Glatts C, Schatz P. Efficacy of immediate and delayed cognitive and physical rest for treatment of sports-related concussion. J Pediatr. 2012;161(5):922–6.
McGrath N. Supporting the student-athlete’s return to the classroom after a sport-related concussion. J Athl Train. 2010;45(5):492–8.
Popoli DM et al. CHOA concussion consensus: establishing a uniform policy for academic accommodations. Clin Pediatr (Phila). 2014;53(3):217–24.
Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and congnition. Eur J Neurosci. 2004;20(10):2580–90.
Austin MW et al. Aerobic exercise effects on neuroprotection and brain repair following stroke: a systematic review and perspective. Neurosci Res. 2014.
Cotman CW, Engesser-Cesar C. Exercise enhances and protects brain function. Exerc Sport Sci Rev. 2002;30(2):75–9.
Kontos AP et al. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751–6.
Larun L et al. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst Rev. 2006;3.
Rimer J et al. Exercise for depression. Cochrane Database Syst Rev. 2012;7.
McCrory P et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250–8.
Meehan III WP. Medical therapies for concussion. Clin Sports Med. 2011;30(1):115–24.
Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70(6):1520–33. discussion 1533.
Reddy CC et al. Efficacy of amantadine treatment on symptoms and neurocognitive performance among adolescents following sports-related concussion. J Head Trauma Rehabil. 2013;28(4):260–5.
Rao V, Rollings P. Sleep disturbances following traumatic brain injury. Curr Treat Options Neurol. 2002;4(1):77–87.
Kontos AP et al. Posttraumatic migraine as a predictor of recovery and cognitive impairment after sport-related concussion. Am J Sports Med. 2013;41(7):1497–504.
Warden DL et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468–501.
Alsalaheen BA et al. Exercise prescription patterns in patients treated with vestibular rehabilitation after concussion. Physiother Res Int. 2013;18(2):100–8.
Schneider KJ et al. Cervicovestibular rehabilitation in sport-related concussion: a randomised controlled trial. Br J Sports Med. 2014;48(17):1294–8.
Alsalaheen BA et al. Vestibular rehabilitation for dizziness and balance disorders after concussion. J Neurol Phys Ther. 2010;34(2):87–93.
Hoffer ME et al. Characterizing and treating dizziness after mild head trauma. Otol Neurotol. 2004;25(2):135–8.
Capó-Aponte JE et al. Effectiveness of computerized oculomotor vision screening in a military population: pilot study. J Rehabil Res Dev. 2012;49(9):1377–98.
Ciuffreda KJ et al. Vision therapy for oculomotor dysfunctions in acquired brain injury: a retrospective analysis. Optometry. 2008;79(1):18–22.
Thiagarajan P et al. Versional eye tracking in mild traumatic brain injury (mTBI): effects of oculomotor training (OMT). Brain Inj. 2014;28(7):930–43.
Thiagarajan P et al. Oculomotor neurorehabilitation for reading in mild traumatic brain injury (mTBI): an integrative approach. NeuroRehabilitation. 2014;34(1):129–46.
Cortesi F et al. Controlled-release melatonin, singly and combined with cognitive behavioural therapy, for persistent insomnia in children with autism spectrum disorders: a randomized placebo-controlled trial. J Sleep Res. 2012;21(6):700–9.
Fann JR, Uomoto JM, Katon WJ. Cognitive improvement with treatment of depression following mild traumatic brain injury. Psychosomatics. 2001;42(1):48–54.
Silver JM, McAllister TW, Arciniegas DB. Depression and cognitive complaints following mild traumatic brain injury. Am J Psychiatry. 2009;166(6):653–61.
Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adoloescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527–39.
Ponsford J et al. Impact of early intervention on outcome after mild traumatic brain injury in children. Pediatrics. 2001;108(6):1297–303.
Mittenberg W et al. Cognitive-behavioral prevention of postconcussion syndrome. Arch Clin Neuropsychol. 1996;11(2):139–45.
Willer B, Leddy JJ. Management of concussion and post-concussion syndrome. Curr Treat Options Neurol. 2006;8(5):415–26.
Rimer RW et al. Disability caused by minor head injury. Neurosurgery. 1981;9(3):221–8.
Ruff RM, Camenzuli L, Mueller J. Miserable minority: emotional risk factors that influence the outcome of a mild traumatic brain injury. Brain Inj. 1996;10(8):551–65.
Leddy JJ et al. Rehabilitation of concussion and post-concussion syndrome. Sports Health. 2012;4(2):147–54.
Leddy JJ et al. A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clin J Sport Med. 2010;20(1):21–7.
Al Sayegh A, Sandford D, Carson AJ. Psychological approaches to treatment of postconcussion syndrome: a systematic review. J Neurol Neurosurg Psychiatry. 2010;81(10):1128–34.
Tiersky LA et al. A trial of neuropsychological rehabilitation in mild-spectrum traumatic brain injury. Arch Phys Med Rehabil. 2005;86(8):1565–74.
Leddy JJ et al. Exercise treatment for postconcussion syndrome: a pilot study of changes in functional magnetic resonance imaging activation, physiology, and symptoms. J Head Trauma Rehabil. 2013;28(4):241–9.
Boussi-Gross R et al. Hyperbaric oxygen therapy can improve post concussion syndrome years after mild traumatic brain injury-randomized prospective trial. PLoS One. 2013;8(11):e79995.
Compliance with Ethics Guidelines
Conflict of Interest
R.J. Elbin, Harrison B. Lowder, and Anthony P. Kontos declare no conflict of interest.
Phil Schatz declares that he has served as a consultant for ImPACT Applications, Inc., outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Traumatic Brain Injury
Rights and permissions
About this article
Cite this article
Elbin, R.J., Schatz, P., Lowder, H.B. et al. An Empirical Review of Treatment and Rehabilitation Approaches Used in the Acute, Sub-Acute, and Chronic Phases of Recovery Following Sports-Related Concussion. Curr Treat Options Neurol 16, 320 (2014). https://doi.org/10.1007/s11940-014-0320-7
Published:
DOI: https://doi.org/10.1007/s11940-014-0320-7