Abstract
Purpose of review
Assisted reproductive technology (ART) has continued to emerge as a treatment option for infertility, a condition that affects a significant proportion of couples of reproductive age. The goal of this review is to provide information on the link between adverse pregnancy outcomes (APOs) and ART and long-term cardiovascular consequences associated with these therapies.
Recent findings
Increasing use and advancing maternal age have raised concerns regarding the short- and long-term use of such technologies. ART and associated hormonal therapies are known to cause potential vascular effects and have an association with APOs and cardiovascular disease.
Summary
Our review highlights potential risks associated with ART and the need for pre-treatment awareness and counseling of short-term APO risk and long-term cardiovascular disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infertility, according to the World Health Organization, is defined as failing to achieve a successful pregnancy after at least 12 months of unprotected and regular sexual intercourse [1]. However, this definition varies based on significant factors in a patient’s medical history, clinical presentation, and age. If an individual has a medical, sexual, reproductive history, or physical exam finding, significant for concern regarding reproductive function, or if they are 40 years of age or older, it is recommended to initiate diagnostic testing immediately [1]. Additionally, age itself may warrant expedited workup at 6 months, particularly for women 35 years and older, who have had regular, unprotected intercourse without conceiving [1]. Infertility affects up to 15% of reproductive-aged couples and once identified warrants evaluation. The most common female cause of infertility is ovulatory dysfunction secondary to factors such as obesity, polycystic ovary syndrome (PCOS), hypothalamic and pituitary dysfunction, thyroid disease, and hyperprolactinemia [2, 3]. Women at risk for diminished ovarian reserve include women 35 years or older, those with a family history of early menopause, prior ovarian resection, and a history of pelvic radiation, or chemotherapy [2]. Tubal disease and endometriosis are other common causes of infertility. In 40% of couples, there is a contributing male factor which may also lead to a need for ART. Despite many identifiable causes and risk factors for infertility, as many as 15–20% of couples may ultimately be diagnosed with unexplained infertility [3].
The first live birth using in vitro fertilization (IVF), one type of ART, occurred in 1978 and 3 years later, the first infant in the USA to be conceived with the use of IVF was born [4, 5]. Since then, the use of ART and IVF procedures to treat infertility has increased substantially due to the growing number of fertility clinics, improved success rates, awareness of these procedures, and greater availability of insurance coverage. This is illustrated by the fact that in 2015, out of 4,009,654 infants born in the USA and Puerto Rico, 66,298 (1.7%) of them were conceived using ART procedures [5]. As maternal age continues to advance, the need for ART will only continue to increase [6].
ART encompasses many different treatments and procedures (Table 1) that involve the handling of both human oocyte and sperm, ovarian tissue, testicular tissue, or embryos in vitro with the ultimate goal of establishing a pregnancy [7, 8]. IVF is composed of procedures that encourage the fertilization of gametes outside of the body [7]. Once an embryo is created via IVF, with or without intracytoplasmic sperm injection (ICSI—a procedure involving injecting a single spermatozoon into the cytoplasm of an oocyte), then an embryo is transferred to the uterus, a process known as embryo transfer [7]. Both fresh and frozen embryo transfers are common, and embryos can be created from self or donor eggs or sperm. According to the Society for Assisted Reproductive Technology, about 99% of ART cycles performed are IVF with fresh or frozen embryo transfer (IVF-ET) [9].
ART and impact on pregnancy outcomes
ART offers several treatment options for those experiencing infertility; however, like other diseases and their respective therapies, these treatments are not without risk from both maternal and fetal perspectives (Table 2) [10]. It is known that there are significant adverse pregnancy outcomes (APOs) associated with multiple gestations that result from fertility treatment; however, the focus hereafter will be on the APOs associated with singleton pregnancies resulting from ART. The goal of current IVF treatments is to help infertile individuals have one healthy baby at a time. Even if the pregnancy is limited to a singleton, both the mother and baby have higher risks of complications than singleton babies conceived spontaneously.
Many studies, including a meta-analysis of 50 cohort studies showed that singleton pregnancies resulting from ART, compared to singleton pregnancies from spontaneous conception, had a significantly increased risk of developing pregnancy-related complications such as pregnancy-induced hypertension (RR 1.30, 95% CI 1.04–1.62, p = .02), gestational diabetes (GDM) (RR 1.31, 95% CI 1.13–1.53, p = .0004), placenta previa (RR 3.71, 95% CI 2.67–5.16, p < .00001), placental abruption (RR 1.83, 95% CI 1.49–2.24, p < .00001), postpartum hemorrhage (RR 1.29, 95% CI 1.06–1.57, p = .01), polyhydramnios (RR 1.74, 95% CI 1.24–2.45, p = .001), oligohydramnios (RR 2.14, 95% CI 1.53–3.01, p < .0001), and cesarean sections (RR 1.58, 95% CI 1.48–1.70, p = .001) [11•]. Additionally, the same study showed that ART resulting in a singleton pregnancy was associated with APOs including preterm birth, very preterm birth, low birth weight, very low birth weight, small for gestation age, congenital malformations, and perinatal mortality [11•]. Many of these findings were supported in the findings from a retrospective cohort study that showed singleton gestations following ART had a greater incidence of GDM, gestational HTN, preeclampsia, and intra-hepatic cholestasis when compared to spontaneous conceptions [12]. This study also showed higher perinatal complications including placenta previa, placental abruption, premature preterm rupture of membranes (PPROM), and postpartum hemorrhage in ART singleton pregnancies and higher rates of APOs including preterm labor, low birth weight compared to spontaneously conceived singleton pregnancies [12].
The risk of APOs differs depending on the specific type of ART used. For example, several studies have shown there is an increased risk of pregnancy-induced hypertension following ART; however, this risk has been shown to be specific to (IVF) pregnancies that use donor oocytes and frozen embryo transfer or thawed embryos [6, 13]. A population-based Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART) analysis showed that donor oocytes, compared to autologous oocytes, also carried an increased risk of prematurity and occurrence of a primary cesarean section [13]. Interestingly, even the presence or absence of a corpus luteum (CL) has been shown to affect APOs. For example, in IVF protocols that override and do not stimulate the ovaries, such as frozen embryo transfer, there is no corpus luteum. A study demonstrated that pregnancies conceived without a CL showed higher rates of preeclampsia and preeclampsia with severe features when compared to pregnancies conceived in women with one or multiple CLs [14]. This same study also showed that programmed cycles of frozen embryo transfer (FET) were associated with higher rates of preeclampsia when compared to modified natural ovulatory cycles and that modified natural cycle FET showed no significant difference in the frequency of preeclampsia when compared to spontaneous conception. This study adds insights to support the need for further study of maternal physiology and preeclampsia risk in FET and other IVF protocols conducted with and without a CL, particularly given the increasing utilization of FET in clinical practice. If the finding of increased preeclampsia risk after FET in programmed cycles is confirmed, FET performed in a natural cycle might alleviate this increased risk.
Another prospective observational study evaluating the impact of the presence or absence of a corpus luteum compared maternal cardiovascular outcomes in spontaneous pregnancies with a CL versus donor egg IVF or FET (no CL) versus multiple CL after ovarian stimulation [15••]. The expected increase in cardiac output, left atrial dimension, peak left ventricular filling velocity in early diastole (E wave velocity), peripheral/central arterial pulse pressure ratio, as well as a decrease in augmentation index was significantly attenuated or absent during the first trimester in women who conceived without a CL, when compared to the 1 and > 1 CL cohorts, which were comparable. These results provide strong support for a critical role of CL factor(s) in the transformation of the maternal cardiovascular system in early gestation. Regimens that lead to the development of a CL or replacement of missing CL factor(s) may be indicated to improve cardiovascular function and reduce preeclampsia risk in IVF pregnancies [15••].
The risks of APOs can also differ depending on the stage of embryo transfer when comparing day 5 blastocyst transfer versus day 2 or 3 cleavage-stage transfer. A population-based retrospective registry study showed that deliveries after blastocyst transfer had a higher risk of preterm birth and a higher rate of neural tube defects compared to spontaneous conception and that the risk of placenta previa and placental abruption were higher in singleton pregnancies after blastocyst transfer compared to those after cleavage-state or spontaneous conception [16]. Currently, the majority of ART procedures involving fresh or frozen embryo transfer are done at the blastocyst stage. The most current data is related to complications related to this stage of transfer.
The use of ART has markedly increased over the past several years. Based on data from the CDC, there were 306,197 ART cycles performed in the USA during 2018 [17]. From these cycles, there were 73,831 live births (defined as deliveries of one or more living infants) and 81,478 live-born infants [17]. Approximately 1.9% of all infants born in the USA every year are conceived using ART [17]. Overall, compared to age-matched singleton pregnancies, there is a higher risk of adverse pregnancy outcomes in women conceiving through ART. It is important that these couples receive pre-treatment counseling so they are aware and prepared for these risks.
Hemodynamics
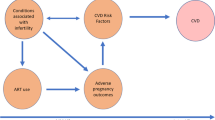
ART treatments such as IVF involve the use of fertility medicines to produce ovarian stimulation associated with multifollicular growth and resulting supraphysiologic levels of estradiol. These changes start to occur within a few days of beginning ovarian stimulation with gonadotropin injections. For frozen or donor embryo transfer, estradiol and progesterone are replaced to create a suitable endometrial environment for embryo transfer. Hormone levels will be closer to those in a natural menstrual cycle, but they will lack a corpus luteum. As a result, the changes in hormone levels that occur during ART have downstream implications on the cardiovascular system through shifts in hemodynamics, endothelial function, and the coagulation cascade (Fig. 1). These effects can have varying clinical significance [18]. Initially, some IVF protocols use a GnRH agonist for initial ovarian suppression and result in estradiol suppression and are associated with increases in blood pressure and peripheral vascular resistance. Subsequently, gonadotropin injections given for ovarian stimulation lead to rising estradiol levels and an increase in cardiac output but also a decrease in mean arterial pressure and peripheral vascular resistance [19]. In the context of IVF, once the embryo is transferred to the uterus, exogenous estrogen, progesterone, or hCG may be given up until the end of the first trimester to allow endometrial development for implantation and ongoing pregnancy support or until the placenta becomes the dominant endocrine organ of the pregnancy. Typically, these changes are well tolerated; however, ovarian hyperstimulation syndrome (OHSS) is a rare but extreme example of potential hemodynamic consequences of ART [18, 20].
OHSS is an exaggerated response to ovarian stimulation that leads to increased systemic capillary permeability and arterial vasodilation resulting in extravascular fluid shift [21]. The signs and symptoms can range from mild to critical in severity and include enlarged ovaries, ascites, pleural and pericardial effusions, and thromboembolic events [22]. Rarely, myocardial infarction or embolic phenomenon such as pulmonary embolus or DVT may occur secondary to OHSS [23]. Although the incidence of moderate to severe OHSS is estimated as occurring in 1–5% of ART cycles, it has been suggested that even lower grade levels of ovarian stimulation may cause long-term cardiovascular effects [22, 24]. The exact mechanism of OHSS is not completely understood; however, the role of estradiol, LH, hCG, inflammatory mediators, and vascular endothelial growth factor (VEGF) has been studied as markers or mediators in OHSS [25]. Additionally, disruption in the renin-angiotensin axis has been documented in several studies finding higher concentrations of angiotensin II in follicular and ascitic fluid of women with OHSS [26]. This is likely a downstream effect of the intravascularly depleted state rather than a direct cause of OHSS [27]. Fortunately, newer stimulation protocols have further reduced this type of complication.
In addition to the hormonal impacts on hemodynamics, ART increases the likelihood of multiple pregnancies, which results in physiologic changes requiring additional circulatory adaptations. Twin pregnancies compared to singleton pregnancies have an increase in cardiac output, ejection fraction, left ventricular end-diastolic and end-systolic dimensions, and a lower total vascular resistance [28]. Women with multiple pregnancies are also more likely to experience hypertensive complications [29]. These challenges with multiple pregnancies may be of particular significance for women with pre-existing cardiovascular disease [18]. New protocols, insurance coverage, and higher success rates have caused a positive trend towards single embryo transfer, with falling rates of multiple gestations.
Finally, the hemodynamic consequences of abnormal placentation and vascular dysfunction associated with ART result in an elevated risk of hypertensive disorders including preeclampsia [30]. During embryo transfer into the uterine cavity, the changes in the hormonal environment in the endometrium alter the typical maternal-fetal interface and may result in placental insufficiency [29]. The compromised uteroplacental blood flow triggers the release of antiangiogenic factors into the maternal circulation that results in maternal systemic vasoconstriction. Additionally, in IVF pregnancies, the chorion forms in vitro which may predispose to diseased placenta vessels and inadequate uteroplacental circulation. Furthermore, a disruption in the balance of various vasoactive factors during ART may be implicated in hypertensive disorders. For example, in IVF procedures without the formation of corpus luteum, levels of relaxin are lower resulting in impaired vasodilation [29].
Cardiovascular disease and ART
To date, the largest systematic review of data evaluating cardiovascular risk following ART is a meta-analysis by Dayan et al. in 2017 [31•]. Six observational studies including over 40,000 women receiving fertility therapy and over 1,400,000 who did not showed no increased risk of cardiac events in the fertility-treated group (pooled HR 0.91; 95% CI 0.67 to 1.25; I2 = 36.6%). This included various cardiovascular outcomes in different studies such as coronary heart disease, cardiovascular hospitalization, and “CVD not otherwise specified.” Limited data on stroke outcomes showed a potential increased risk for stroke among women receiving fertility therapy (pooled HR1.25; 95% CI 0.96 to 1.63; I2 = 0%). Still, definitive conclusions on cardiovascular outcomes associated with fertility therapy could not be made due to heterogeneity in comparator groups, types of fertility therapy, and varied cardiovascular outcomes reported in these studies. The major utility of this systematic review was to highlight the gaps in knowledge on long-term CV safety of fertility therapy and the need for quality studies in this area [24, 31•].
The association of ART and elevated risks of hypertensive disorders of pregnancy and thromboembolic events have been studied. Thomopoulous et al. reviewed 47 studies and found IVF in particular was associated with increased gestational hypertension and preeclampsia [29]. The 2019 systematic review by Almasi-Hashaiani et al. found the incidence of preeclampsia following ART to be 1.71-fold higher compared to spontaneous conception (RR 1.71, 95% CI 1.11–2.62, p = 0.015) [30]. Additionally, there is a 100-fold increased risk of thromboembolic events specifically in the setting of OHSS following ART [32]. Interestingly, venous thrombosis associated with ART often occurs in upper extremities and head and neck vasculature as opposed to lower extremity venous thrombosis due to poor venous return associated with pregnancy [33].
It is difficult to control for confounding factors associated with cardiovascular risk and fertility therapy. In recent years, an association between adverse pregnancy conditions and long-term cardiovascular outcomes such as myocardial infarction and stroke has been increasingly recognized and incorporated into risk stratification guidelines [34]. APOs as a marker and/or mediator of future cardiovascular risk have been studied in the context of overlapping physiologic mechanisms that include vascular dysfunction, inflammation, and endothelial dysfunction [35]. Although the additive adverse effects fertility therapy may have on cardiovascular risk is unclear, similar pathways resulting in vascular dysfunction and a pro-thrombotic state may be implicated.
There is limited data to speculate on the degree of increased risk of complications following ART in women with pre-existing cardiovascular disease. A retrospective study of 20 women with either congenital heart disease (68%) or acquired cardiovascular disease (32%) who successfully achieved pregnancy with ART was surveyed for pregnancy complications. In this small cohort, OHSS occurred in 18% and adverse cardiac maternal outcomes occurred in 27% of women [36]. Although there are several limitations to this study, it is reasonable that pre-existing cardiovascular disease would further limit the ability to compensate for the hemodynamic changes associated with ART.
The most important and difficult question is how we as clinicians counsel patients undergoing evaluation for ART. Women should be counseled that the use of IVF, even when performing single embryo transfer, carries a higher risk for maternal pregnancy complications including gestational diabetes, hypertension, preeclampsia, and cesarean section. This is true even for women without pre-existing cardiovascular disease. For women with pre-existing cardiovascular disease, first, it is important to thoughtfully consider their ability to tolerate the hemodynamic changes associated with a healthy pregnancy. A decision to proceed or offer ART should be made in conjunction with maternal-fetal medicine and cardiology. Risk assessment models such as the World Health Organization classification of maternal cardiovascular risk can be used to stratify patients [37]. It is reasonable to consider WHO class III and IV which includes mechanical valve, complex congenital heart diseases, severe left ventricular dysfunction (EF < 30%, NYHA class III–IV heart failure), severe symptomatic aortic stenosis, and other CV diseases, as relative contraindications to ART [18, 37]. Still, it is important to recognize the limitations within the evidence we use to counsel patients during a pivotal time in their lives. Our aim should be the early identification of potential risk factors and supporting patients through this decision-making process. If it is considered an acceptable risk for pregnancy and ART, there should be close monitoring of metabolic derangements (i.e., gestational diabetes) and hypertension, among other complications.
Conclusions
ART has been increasingly utilized to treat infertility and encompasses a broad range of different techniques. Hormonal therapies associated with ART are associated with vascular changes and ART has been associated with a higher risk of adverse pregnancy outcomes, although this varies by type of ART. The data to support an association between ART and CVD is not definitively established. However, counseling patients should involve discussion of the risks and benefits as it relates to the use of ART and cardiovascular consequences. A team approach to pre-ART counseling and care is important if the woman has pre-existing cardiovascular risk factors.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2020;113(3):533–5. https://doi.org/10.1016/j.fertnstert.2019.11.025.
Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2015;103(6):e44–50. https://doi.org/10.1016/j.fertnstert.2015.03.019.
Infertility workup for the women’s health specialist: ACOG Committee Opinion, Number 781. Obstet Gynecol. 2019;133(6):e377–e84. https://doi.org/10.1097/AOG.0000000000003271.
Eskew AM, Jungheim ES. A history of developments to improve in vitro fertilization. Mo Med. 2017;114(3):156–9.
Sunderam S, Kissin DM, Crawford SB, Folger SG, Boulet SL, Warner L, et al. Assisted reproductive technology surveillance - United States, 2015. MMWR Surveill Summ. 2018;67(3):1–28. https://doi.org/10.15585/mmwr.ss6703a1.
Luke B, Brown MB, Eisenberg ML, Callan C, Botting BJ, Pacey A, et al. In vitro fertilization and risk for hypertensive disorders of pregnancy: associations with treatment parameters. Am J Obstet Gynecol. 2020;222(4):350 e1–e13. https://doi.org/10.1016/j.ajog.2019.10.003.
Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Hum Reprod. 2017;32(9):1786–801. https://doi.org/10.1093/humrep/dex234.
Practice Committee of the American Society for Reproductive Medicine, Practice Committee of the Society for Assisted Reproductive Technology, Practice Committee of the Society of Reproductive Biologists and Technologists. Minimum standards for practices offering assisted reproductive technologies: a committee opinion. Fertil Steril. 2020;113(3):536–41. https://doi.org/10.1016/j.fertnstert.2019.11.024.
Society for Assisted Reproductive Technology. Assisted reproductive technologies. Available at: https://www.sart.org/patients/a-patients-guide-to-assisted-reproductive-technology/general-information/assisted-reproductive-technologies/. Accessed Feb 12, 2021.
Perinatal risks associated with assisted reproductive technology: ACOG Committee Opinion, number 671. Obstet Gynecol. 2016;128(3):e61–8. https://doi.org/10.1097/AOG.0000000000001643.
Qin J, Liu X, Sheng X, Wang H, Gao S. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: a meta-analysis of cohort studies. Fertil Steril. 2016;105(1):73–85 e1–6. https://doi.org/10.1016/j.fertnstert.2015.09.007. The paper provides a comprehensive and detailed review of the utilization of ART and the major maternal and fetal risks of the most used forms of ART such as fresh IVF, donor egg IVF, and frozen embryo transfer. Subfertility, with or without IVF or other infertility treatments to achieve pregnancy, is associated with increased risks of adverse maternal and perinatal outcomes. The lingering question is whether the adverse outcomes are related to something specific with infertility or the type of ART process.
Zhu L, Zhang Y, Liu Y, Zhang R, Wu Y, Huang Y, et al. Maternal and live-birth outcomes of pregnancies following assisted reproductive technology: a retrospective cohort study. Sci Rep. 2016;6:35141. https://doi.org/10.1038/srep35141.
Luke B. Pregnancy and birth outcomes in couples with infertility with and without assisted reproductive technology: with an emphasis on US population-based studies. Am J Obstet Gynecol. 2017;217(3):270–81. https://doi.org/10.1016/j.ajog.2017.03.012.
von Versen-Hoynck F, Schaub AM, Chi YY, Chiu KH, Liu J, Lingis M, et al. Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a corpus luteum. Hypertension. 2019;73(3):640–9. https://doi.org/10.1161/HYPERTENSIONAHA.118.12043.
Conrad KP, Petersen JW, Chi YY, Zhai X, Li M, Chiu KH, et al. Maternal cardiovascular dysregulation during early pregnancy after in vitro fertilization cycles in the absence of a corpus luteum. Hypertension. 2019;74(3):705–15. https://doi.org/10.1161/HYPERTENSIONAHA.119.13015. This prospective observational study compared changes in early pregnancy cardiovascular function and responses in 3 groups—spontaneous conceptions with a single CL, ovum donation IVF pregnancies without a CL and IVF pregnancies with multiple CLs. The findings support the assertion that the higher risk of preeclampsia is related to poorer early pregnancy cardiovascular adaptation. The study supports the concept that CL factors may directly influence early pregnancy maternal cardiovascular adaptation, or to seek to determine if the observed cardiovascular derangements may also have predated the development of the CL.
Ginstrom Ernstad E, Bergh C, Khatibi A, Kallen KB, Westlander G, Nilsson S, et al. Neonatal and maternal outcome after blastocyst transfer: a population-based registry study. Am J Obstet Gynecol. 2016;214(3):378 e1–e10. https://doi.org/10.1016/j.ajog.2015.12.040.
Centers for Disease Control and Prevention. 2018 Assisted reproductive technology fertility clinic success rates report. Atlanta: US Dept of Health and Human Services; 2020. Available at: https://www.cdc.gov/art/reports/2018/fertility-clinic.html. Accessed Feb 12, 2021
Rossberg N, Stangl K, Stangl V. Pregnancy and cardiovascular risk: a review focused on women with heart disease undergoing fertility treatment. Eur J Prev Cardiol. 2016;23(18):1953–61. https://doi.org/10.1177/2047487316673143.
Manau D, Balasch J, Arroyo V, Jiménez W, Fábregues F, Casamitjana R, et al. Circulatory dysfunction in asymptomatic in vitro fertilization patients. Relationship with hyperestrogenemia and activity of endogenous vasodilators. J Clin Endocrinol Metab. 1998;83(5):1489–93. https://doi.org/10.1210/jcem.83.5.4796.
Farquhar C, Marjoribanks J. Assisted reproductive technology: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2018;8(8):Cd010537. https://doi.org/10.1002/14651858.CD010537.pub5.
Goldsman MP, Pedram A, Dominguez CE, Ciuffardi I, Levin E, Asch RH. Increased capillary permeability induced by human follicular fluid: a hypothesis for an ovarian origin of the hyperstimulation syndrome. Fertil Steril. 1995;63(2):268–72. https://doi.org/10.1016/s0015-0282(16)57353-1.
Practice Committee of the American Society for Reproductive Medicine. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril. 2016;106(7):1634–47. https://doi.org/10.1016/j.fertnstert.2016.08.048.
Al-Sadawi M, Chowdhury R, Asun S, Ray J, Soni L, Bahtiyar G, et al. Ovarian hyperstimulation syndrome and myocardial infarction: a systematic review. Int J Clin Endocrinol Metab. 2019;5(1):009–12. https://doi.org/10.17352/ijcem.000035.
Pepine CJ, Park K. Fertility therapy and long-term cardiovascular risk: raising more questions than answers?∗. J Am Coll Cardiol. 2017;70(10):1214–5. https://doi.org/10.1016/j.jacc.2017.07.731.
Nastri CO, Ferriani RA, Rocha IA, Martins WP. Ovarian hyperstimulation syndrome: pathophysiology and prevention. J Assist Reprod Genet. 2010;27(2–3):121–8. https://doi.org/10.1007/s10815-010-9387-6.
Fernandez LA, Tarlatzis BC, Rzasa PJ, Caride VJ, Laufer N, Negro-Vilar AF, et al. Renin-like activity in ovarian follicular fluid. Fertil Steril. 1985;44(2):219–23. https://doi.org/10.1016/s0015-0282(16)48740-6.
Manno M, Tomei F. Renin-angiotensin system activation during severe OHSS: cause or effect? Fertil Steril. 2008;2. United States:488.
Kametas NA, McAuliffe F, Krampl E, Chambers J, Nicolaides KH. Maternal cardiac function in twin pregnancy. Obstet Gynecol. 2003;102(4):806–15. https://doi.org/10.1016/s0029-7844(03)00807-x.
Thomopoulos C, Tsioufis C, Michalopoulou H, Makris T, Papademetriou V, Stefanadis C. Assisted reproductive technology and pregnancy-related hypertensive complications: a systematic review. J Hum Hypertens. 2013;27(3):148–57. https://doi.org/10.1038/jhh.2012.13.
Almasi-Hashiani A, Omani-Samani R, Mohammadi M, Amini P, Navid B, Alizadeh A, et al. Assisted reproductive technology and the risk of preeclampsia: an updated systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19(1):149. https://doi.org/10.1186/s12884-019-2291-x.
Dayan N, Filion KB, Okano M, Kilmartin C, Reinblatt S, Landry T, et al. Cardiovascular risk following fertility therapy: systematic review and meta-analysis. J Am Coll Cardiol. 2017;70(10):1203–13. https://doi.org/10.1016/j.jacc.2017.07.753. Dayan et al. present a systematic review of the very limited data on the risk of CV events after exposure to fertility therapy assessing only 6 observational studies consisting of just over 40,000 women who received fertility therapy. The authors found an increased incidence of adverse pregnancy outcomes without an increase in CV events among women exposed to fertility therapy.
Rova K, Passmark H, Lindqvist PG. Venous thromboembolism in relation to in vitro fertilization: an approach to determining the incidence and increase in risk in successful cycles. Fertil Steril. 2012;97(1):95–100. https://doi.org/10.1016/j.fertnstert.2011.10.038.
Stewart JA, Hamilton PJ, Murdoch AP. Thromboembolic disease associated with ovarian stimulation and assisted conception techniques. Hum Reprod. 1997;12(10):2167–73. https://doi.org/10.1093/humrep/12.10.2167.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e143. https://doi.org/10.1161/cir.0000000000000625.
Park K, Wei J, Minissian M, Bairey Merz CN, Pepine CJ. Adverse pregnancy conditions, infertility, and future cardiovascular risk: implications for mother and child. Cardiovasc Drugs Ther. 2015;29(4):391–401. https://doi.org/10.1007/s10557-015-6597-2.
Dayan N, Laskin CA, Spitzer K, Mason J, Udell JA, Wald RM, et al. Pregnancy complications in women with heart disease conceiving with fertility therapy. J Am Coll Cardiol. 2014;64(17):1862–4. https://doi.org/10.1016/j.jacc.2014.07.977.
Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, Cifkova R, Ferreira R, Foidart JM, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011;32(24):3147–97. https://doi.org/10.1093/eurheartj/ehr218.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Ki Park, Emily Allard-Phillips, Gregory Christman, Michelle Dimza, and Alice Rhoton-Vlasak declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Reproductive Health and Cardiovascular Disease
Rights and permissions
About this article
Cite this article
Park, K., Allard-Phillips, E., Christman, G. et al. Assisted Reproductive Technologies and Their Association With Adverse Pregnancy Outcomes and Long-Term Cardiovascular Disease: Implications for Counseling Patients. Curr Treat Options Cardio Med 23, 54 (2021). https://doi.org/10.1007/s11936-021-00932-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s11936-021-00932-3