Abstract
Purpose of review
This review highlights issues relevant to women of reproductive age with a history of spontaneous coronary artery dissection (SCAD).
Recent findings
Current topics regarding the care of SCAD patients include multimodality imaging, pregnancy, lactation, SCAD recurrence, reproductive counseling, migraines, cardiac rehabilitation, and mental health. While no single disease-causing gene for SCAD has been discovered, recent genome-wide association studies show promise for future understanding of underlying mechanisms and risk for SCAD.
Summary
Patients with history of SCAD require dedicated, multidisciplinary care, and women of reproductive age necessitate specific discussion regarding pregnancy, contraception, and menses. Imaging to detect fibromuscular dysplasia and other arteriopathies is recommended in all patients. Pregnancy after SCAD is discouraged, although counseling must be tailored. Systemic exogenous hormones have not been shown to definitively increase the risk of SCAD, but nonhormonal treatment approaches are preferred. Triptan therapy should be discouraged in SCAD patients with migraines, and more research is needed to understand the safety of newer agents. Cardiac rehabilitation after SCAD is safe and encouraged. All SCAD survivors should be screened for post-traumatic stress disorder, depression, and anxiety regardless of time from initial event.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
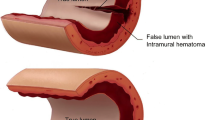
Spontaneous coronary artery dissection (SCAD) is a nonatherosclerotic, non-traumatic separation of the coronary artery wall causing acute coronary syndrome. There are currently two proposed mechanisms of SCAD: a discontinuity of the intima, i.e., a “tear” as the inciting event, versus spontaneous bleeding of microvessels within the vasa vasorum or a combination of both. This results in hematoma formation within the wall of the epicardial vessel compressing the true lumen, a reduction in myocardial perfusion and resulting ischemia or infraction. SCAD disproportionately affects women and causes up to 35% of myocardial infarctions in women ≤ 50 years of age [1••, 2••].
Coronary angiographic appearance of spontaneous coronary artery dissection
SCAD is most commonly diagnosed by invasive coronary angiography. The Yip Saw classification separates angiographic appearance of SCAD into three types and more recently a fourth type has also been proposed. Familiarity with this classification system (Fig. 1) together with a high index of suspicion can help avoid missing the diagnosis of acute SCAD [3••]. In Type 1 SCAD, contrast staining of the arterial wall is seen, and the artery can appear to have multiple lumens. Although this subtype of SCAD is perhaps the most obvious, less than 30% of SCAD in women has angiographic Type I appearance. More recent registry data suggests Type I SCAD is more common in men [4,5,6,7].
Angiographic classification of spontaneous coronary artery dissection. Type 1 spontaneous coronary artery dissection (a), Type 2A spontaneous coronary artery dissection (b), Type 2B spontaneous coronary artery dissection (c), Type 3 spontaneous coronary artery dissection (d), Type 2 spontaneous coronary artery dissection (e), and intermediate Type 1/2 spontaneous coronary artery dissection (f). Republished with permission of European Heart Journal from Adlam et al. [3••]. Permission conveyed through Copyright Clearance Center Inc.
A Type 2 appearance is characterized by a long smooth narrowing. It is the most common angiographic appearance of SCAD and occurs in up to 67% of cases. A Type 2 appearance is likely due to spontaneous bleed into the arterial wall producing an intramural hematoma and compression of the true lumen. Two variants have been described—variant 2A is diffuse narrowing with normal arterial segment distal to the dissected segment. Type 2B is diffuse narrowing that extends to the distal tip of the artery [1••, 4, 5].
Type 3 SCAD is the least common and mimics atherosclerosis. The lesion can be long (11–20 mm) and hazy. The lack of atherosclerosis in other coronary arteries is a clue to the diagnosis of SCAD but cannot be solely relied upon as registry data has shown that women younger than 50 who present with acute myocardial infarction more commonly present with single vessel disease compared to men [8]. In cases with a Type 2 or Type 3 angiographic appearance, intracoronary imaging (OCT or IVUS described below) may be critical for confirmation of the SCAD diagnosis [4, 5]. In 2017, Adlam and colleagues proposed a Type 4 SCAD in which there is a total occlusion of the distal vessel. This is challenging to diagnose in an acute setting but is sometimes considered if SCAD is apparent in other vessels [3••, 9].
Intracoronary imaging in spontaneous coronary artery dissection
Intracoronary imaging can be performed with optical coherence tomography (OCT) or intravascular ultrasound (IVUS). OCT uses time delay and intensity of light that is backscattered from internal structures in tissue to generate high-resolution images of the arterial wall. OCT has excellent spatial resolution (10–20 μm) and can provide clinically helpful information in situations where SCAD is suspected, but more confirmation is necessary (Fig. 2). Intimal tears, hemorrhage within the arterial wall, false lumen, and intraluminal thrombi can be well seen with OCT [4, 10, 11]. OCT techniques used to acquire images include either (1) transient coronary blood flow obstruction from inflation of a balloon and flushing of crystalloid solution (“occlusive technique”) or (2) rapid flushing of contrast through the lumen (“non-occlusive technique”). The technique used may vary according to lab and interventionalist, but there is concern that the flush may propagate SCAD [10, 11]. Intravascular ultrasonography (IVUS) is an older technology with less spatial resolution than OCT but better depth penetration. Similar to OCT, it can help visualize intimal tear, false and true lumens, intraluminal thrombi, and intramural hematoma but does not require the flush with crystalloid or contrast [1••]. Intracoronary imaging with IVUS or OCT should be reserved for cases with diagnostic uncertainty due to the risks associated with coronary instrumentation and when performed should be limited to the proximal segment of the dissection.
Invasive coronary angiogram of a 40-year-old female with history of migraines and anxiety presenting with chest pain and found to have a non-ST elevation myocardial infarction. Coronary angiogram showed mild coronary irregularities predominantly in the left anterior descending (LAD) artery and ramus coronary arteries concerning for Type II spontaneous coronary artery dissection (SCAD) (a, arrows). Coronary computed tomography showed a hazy, sleeve-like appearance adjacent to the mid-LAD coronary suggestive of intracoronary hematoma (b, circle). Repeat coronary angiography at 1 month follow-up due to persistent chest discomfort showed interval improvement of the coronary abnormalities (c, arrows) with evidence of persistent intracoronary hematoma on optional coherence tomography (d, arrows).
Cardiac CT angiography and spontaneous coronary artery dissection
Cardiac computed tomography angiography (CCTA) can be helpful to further elucidate a SCAD diagnosis. While SCAD may be obvious on CCTA (e.g., Type I proximal SCAD), this is not the most common finding. Rather, a small study of acute SCAD imaging on CCTA has described distinct features including abrupt luminal stenosis, intramural hematoma, and tapered luminal stenosis with dissection being least common [12•, 13•]. Another small series of acute SCAD imaging on CCTA described features including diffuse sleeve-like wall thickening, “plaque-like” stenosis, dissection flap, and total occlusion (Fig. 2) [14]. Epicardial fat stranding has also been associated with SCAD on acute imaging, although it can also be seen in patients with other etiologies of ACS [13•]. CCTA can also demonstrate calcified plaque in the coronary arteries which may lower the suspicion for SCAD. CCTA is especially helpful in follow-up, such as when reassessing a known SCAD for resolution or evaluating recurrent chest pain. Inherent limitations to CCTA include a lower spatial resolution than invasive coronary angiography, the possibility of motion artifact, and an unknown sensitivity and specificity in the diagnosis of acute SCAD. Therefore, it is not considered the gold standard for diagnosis of acute SCAD but can be considered on a case-by-case basis [1••, 2••, 5, 12•].
Cardiac MRI and spontaneous coronary artery dissection
Cardiac magnetic resonance imaging (CMR) provides different but helpful and clinically relevant information among SCAD patients as compared to invasive coronary angiography or CCTA. Specifically, CMR can be extremely helpful in cases where SCAD was not initially diagnosed but then suspected based on retrospective review of the coronary angiogram with CMR evidence of delayed gadolinium in that territory [12•]. CMR is a modality that does not expose the patient to radiation which can be used to assess left and right ventricular systolic function and differentiate between infarcted/ischemic tissue versus other diagnoses such as myocarditis. However, a normal CMR does not rule out SCAD specifically if there was transient ischemia nor can it adequately evaluate the coronary anatomy [15•].
Echocardiography and spontaneous coronary artery dissection
Echocardiography does not expose the patient to radiation and can give immediate information about biventricular function, regional wall motion abnormalities, and valve function, and evaluate for the presence of pericardial fluid. Echocardiography is also valuable for follow-up imaging, particularly for those patients who present with reduced left ventricular function [12•]. More recent data has suggested an overlap between Takotsubo cardiomyopathy (TTC) and SCAD. TTC is a transient cardiomyopathy with characteristic apical ballooning seen on echocardiography or left ventriculography and absent delayed gadolinium enhancement with CMR imaging, more common in women, and occurs in the absence of plaque rupture [16, 17]. Some SCAD patients may have a wrap-around LAD SCAD with associated regional wall motion abnormalities that simply mimic TTC [16]. However, some have suggested that especially in patients with inciting stressful event TTC may occur concurrently with regional wall motion abnormalities out of proportion to the extent of SCAD [17, 18]. While TTC and SCAD may be indistinguishable by echocardiography alone, SCAD should be included in the differential diagnosis of patients with evidence of TTC on echocardiography. CMR can be helpful to distinguish features of TTC (e.g., edema, lack of late gadolinium enhancement despite regional wall motion abnormalities) versus SCAD (e.g., late gadolinium enhancement in the suspected coronary distribution or corresponding with the regional wall motion abnormalities).
Extracardiac imaging in spontaneous coronary artery dissection
SCAD is associated with extracoronary arteriopathies, like fibromuscular dysplasia (FMD). FMD is a nonatherosclerotic, non-inflammatory abnormality of the arteries that can affect multiple vascular beds and can result in stenosis, dissection, or aneurysm. The most common histologic type of FMD involves medial fibroplasia which appears as a string of beads angiographically [8]. The prevalence of FMD in patients with SCAD ranges from 17 to 86% [19,20,21]. Given the frequency of FMD in SCAD and implications of finding extracardiac involvement, current consensus practice is to perform head, neck, abdomen, and pelvis imaging at least once to evaluate for FMD and other arteriopathies such as aneurysm, dissection, and ectasia in patients with SCAD [1••]. CT angiography has excellent spatial resolution to detect FMD and is commonly a preferred modality [20]. If there are concerns about cumulative radiation exposure especially among women of reproductive age, MR angiography is an alternative modality [1••, 2••, 12•, 22]. Duplex ultrasonography may not identify mild abnormalities and is usually limited to the renal and carotid arteries; therefore, while it can be helpful for follow-up imaging, it has limitations as the initial imaging technique [20].
Spontaneous coronary artery dissection and pregnancy
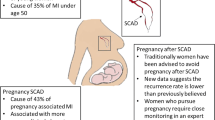
Spontaneous coronary artery dissection can occur antepartum, peripartum, or post-partum with the majority of pregnancy-associated SCAD (P-SCAD) occurring in the third trimester or within 6 weeks of delivery [23]. P-SCAD can also occur after a miscarriage, termination, or still birth. Women with P-SCAD often have a more severe clinical presentation including ST segment myocardial infarction, multi-vessel involvement, and acute heart failure compared with non-pregnancy-associated SCAD (NP-SCAD) [24, 25]. The numerous hormonal and hemodynamic changes that occur during pregnancy including increased estrogen and progesterone levels, increased cardiac output, expansion of total blood volume, arterial wall changes, and reduction in systemic vascular resistance may contribute to vascular fragility in predisposed patients. Whether repeated or prolonged exposure to hormones increases this risk is uncertain, although data from the Mayo Clinic SCAD registry reported women with P-SCAD were more likely to have a history of assisted fertility treatments (28%) and were multiparous (80%) compared to women with NP-SCAD [24].
Spontaneous coronary artery dissection and lactation
In the Mayo Clinic SCAD registry, 6 patients with P-SCAD voluntarily reported symptom onset with lactation. The role of lactation, oxytocin, and prolactin in the pathophysiology of P-SCAD is unknown. For some women, breastfeeding is an opportunity to bond and nourish the infant. Therefore, common practice in the care of women with P-SCAD is to proceed with lactation if the woman desires (with medication modification as indicated) but to stop should there be any concerning symptoms with clinical evaluation as deemed relevant [26].
Risk of recurrent spontaneous coronary artery dissection
Counseling SCAD patients regarding the risk of recurrent SCAD (R-SCAD) is challenging and more research on how to mitigate this risk is needed. R-SCAD has been defined differently in the literature with some studies including extension of an initial dissection as a recurrent SCAD. According to the 2018 AHA SCAD consensus statement, recurrent SCAD refers to de novo SCAD which is due to a separate SCAD unrelated to the index dissection or event and often occurring later (> 30 days) [1••]. Reported SCAD recurrence among a variety of cohorts has ranged from 4.7 to 29% with a variety of methodologies [27••]. A recent population-based Olmsted county study found the incidence of recurrent SCAD to be 10% at 5 years [28]. Among women with P-SCAD in the Mayo Clinic SCAD registry, 15% (8/54) had a recurrence, 4 of which were within 3 months following delivery. However, 5-year rates of recurrence overall in patients with NP-SCAD vs. P-SCAD were not significantly different [24].
Further investigation into the predisposition for recurrent SCAD and approaches towards prevention are ongoing and much needed. The onset of initial and recurrent SCAD is likely multi-factorial, including a combination of environmental, hormonal, physiological, and genetic factors. Coronary tortuosity and history of hypertension have been associated with increased risk of recurrent SCAD, whereas beta-blockers have been correlated with lower rates of recurrence. A meta-analysis and systematic review of 18 studies including over 2000 patients with SCAD reported the prevalence of baseline hypertension was 45%, and two studies found hypertension is associated with increased risk of R-SCAD [29,30,31]. These findings emphasize the importance of guideline-based blood pressure lowering in women with history of SCAD. Beta-blockers are also used when possible, with a preference for nonselective beta-blockers in hypertensive patients. To date, no conclusive evidence exists that FMD, migraine headaches, or a specific gene mutation increases the risk of R-SCAD and data are limited [27••].
SCAD and reproductive counseling
SCAD commonly affects women of childbearing age which challenges future reproductive decision-making. Sex hormones likely contribute to the mechanism of SCAD, as it is a condition that primarily affects women and is associated with pregnancy [6]. Women with SCAD have reported increasing angina preceding menstruation. The role of fluctuations in levels of female sex hormones during pregnancy, menstruation, and menopause needs to be further understood [32].
In a recently published case series of 23 women with a total of 32 post-SCAD pregnancies, only two women had recurrent SCAD (9%). The first woman had an initial NP-SCAD followed by a P-SCAD, and the second had an initial P-SCAD with a second NP-SCAD > 10 years later. Additional analyses using cohort and nested case-control study designs found no association of pregnancy after SCAD and recurrent SCAD [32, 33]. However, these analyses were limited by the small total number of women who had SCAD after pregnancy, selection bias, and confounders. All of the women in that study had normal or near normal left ventricular ejection fraction at the time of pregnancy, which has been shown to be associated with maternal outcomes in other studies of women with cardiovascular disease [34••]. Our current clinical practice is to discourage pregnancy due to concern that SCAD is associated with pregnancy; P-SCAD is more commonly severe and life-threatening; predictors and prevention for R-SCAD are unknown; and patients may have baseline left ventricular dysfunction, persistent cardiac symptoms, or arrhythmias after their initial event. If a patient is contemplating pregnancy following SCAD, it is pertinent to involve a multidisciplinary pregnancy heart team to review cardiac history and medications and to discuss the care plan regarding the anticipated pregnancy and delivery. Delivery is ideally a planned vaginal delivery with neuraxial anesthesia at a Level IV maternal care facility fully capable of treating emergencies (Fig. 3).
Regarding contraception, nonhormonal approaches are preferred such as intrauterine devices (including those with local progestin delivery) and patient or partner sterilization. Systemic hormonal contraception is less preferred due to the uncertainty of potential harm in patients with SCAD but may be considered when the other options are not feasible, as pregnancy may pose more harm than systemic hormonal birth control (Fig. 3) [6].
SCAD and menopausal symptoms
Similarly, nonhormonal treatment approaches to peri- and post-menopausal symptoms are preferred, although the ratio of harm versus benefit is not known. If a woman with history of SCAD is seeking treatment for menopausal symptoms, nonhormonal options should be considered first. These may include lifestyle changes and cognitive behavioral therapy, antidepressants, gabapentin, or oxybutynin. Sometimes symptoms are significant enough to consider hormonal therapy, in which case topical therapies are preferred to systemic therapy. Certainly, further understanding regarding risk versus potential benefit of exogenous hormones for SCAD patients is needed and a current gap in knowledge (Fig. 3).
SCAD and menorrhagia
Particularly among women taking dual antiplatelet therapy or anticoagulation, menorrhagia can contribute to significant anemia and as a result fatigue, dyspnea, or chest pain. Review of the necessity of these medications is an initial approach. However, if the antiplatelet or anticoagulation therapy cannot be de-escalated, other treatment options to consider in collaboration with gynecology may include progestin intrauterine device, endometrial ablation, or surgical treatments in cases where structural abnormalities such as uterine fibroid may be predisposing to excessive bleeding (Fig. 3).
Genetic basis of spontaneous coronary artery dissection
The genetic underpinnings of SCAD are complex and no single disease-causing gene has yet been identified. Studies have reported SCAD in genetically triggered connective tissue disorders such as Marfan’s syndrome, vascular Ehlers-Danlos syndrome, and Loeys-Dietz syndrome [35, 36]. Only 3 of patients in a series of 116 patients evaluated in the Mayo Clinic genetics clinic were diagnosed with an inherited genetic disease, although 12/59 patients with genetic testing had a variant of unknown significance [35]. A study of 73 patients that underwent genetic evaluation in the Massachusetts General Hospital found 6 (8.2%) patients with a molecularly identifiable disorder associated with vascular disease and the most common diagnosis was vascular Ehlers-Danlos syndrome [37•].
Similar to SCAD, no single disease-causing gene for FMD has been identified. Recent targeted genotyping studies identified a non-coding allele in the phosphatase and actin regulator 1 gene (PHACTR1) as the first genetic susceptibility locus for FMD [38]. Given the association of FMD with SCAD, this has been a focus for additional research. PHACTR1 is a genetic locus on chromosome 6q24 and the common allele rs9349379-A is associated with an increased risk of FMD by up to 40%. Interestingly, it has also been associated with migraine headaches, and cervicocerebral artery dissections. PHACTR1 is present on endothelial and smooth muscle cells and is considered to play a key role in vessel structure, although the exact mechanisms are yet to be determined [38]. In a study of 1055 patients with SCAD and 7190 controls, the rs9349379-A allele was more common among SCAD patients (0.72 compared to 0.56 in the controls), and in a subgroup analysis, it was more common among SCAD patients than in FMD patients [39••].
Genome-wide association studies (GWAS) have provided further insight into the genetics of SCAD. In a GWAS of 484 white women with SCAD compared with 1477 controls in the discovery cohort, 3 genetic risk loci reached genome-wide significance for an association with SCAD: 1q21.3, 6p24.1, and 12q13.3 [27••, 40•]. A second GWAS study of 270 SCAD patients compared with 5263 controls identified the following loci associated with SCAD: 1q21.2, 12q13.3, 21q22.11 and the previously reported 6p24.1 (PHACTR1 gene). The authors of this study developed and tested a genetic risk score and found an association with alleles identified through the GWAS associated with SCAD occurrence in an independent FMD cohort [41•]. SCAD genetics is an exciting area of research with the potential to better understand what drives the risk of an initial and recurrent event.
Migraine headache and SCAD
Migraine headaches have been associated with increased risk of cardiovascular adverse events and occur commonly among young women. Patients with vascular abnormalities such as fibromuscular dysplasia, carotid or vertebral dissection, or intracranial aneurysm have been observed as having higher prevalence of migraine. Similarly, cohorts of patients with SCAD have a higher prevalence of migraine history as compared to non-SCAD cohorts. In a study of 585 SCAD patients, those persons with migraine were more often female and younger; they also had a higher prevalence of depression, recurrent chest pain in the 1-month timeframe following SCAD, and arterial abnormalities such as aneurysms, pseudoaneurysms, or dissections. However, risk of recurrent SCAD was not increased among those with history of migraines [42].
Assessing for migraine history when caring for a woman with SCAD is pertinent for medication management decisions. For example, although triptan therapy is a commonly used abortive medication for migraine headaches, there is concern triptans may increase risk for epicardial contractions and as a result vasospasm or onset of dissection [43,44,45].
Over the counter medications such as acetaminophen may not provide satisfactory relief. While nonsteroidal anti-inflammatory drugs such as ibuprofen may be considered, there is concern as many patients are also taking aspirin and some may have chronic heart failure following SCAD. Therefore, it is imperative to explore alternative options for migraine headache control, which may be exacerbated by the nitrate therapy commonly used to treat angina in this cohort. Fortunately, beta-blockers and calcium channel blockers commonly used for cardiac indications may concurrently prevent migraine headaches. Antidepressants may also be helpful for migraine prevention. Botox injections and trigger point injections may be effective in certain patients and help to avoid the systemic side effects or medication interactions from an oral medication; however, they often require repeated injections to remain effective.
New anti-calcitonin gene-related peptide (CGRP) therapies such as galcanezumab have become a promising therapy for patients with refractory migraines. CGRP receptor activation is associated with a reflex microvascular vasodilatory response and could present a theoretical concern to patients with cardiac disease including SCAD. Due to such concerns, participants with cardiac disease were not included in the clinical trials. However, the vasodilatory mechanism of CGRP is likely redundant, especially since other receptors may also contribute vasodilation. In a review of the 6-month treatment trial, adverse cardiac events were uncommon and no different between the placebo and treatment groups [46•]. Therefore, patients who are considering this medication should be made aware of these concerns, although, for some with debilitating migraines, the benefit of the therapy may outweigh the perceived risks after individualized discussion and acknowledging that the specific risk in the context of SCAD is largely unknown.
Cardiac rehabilitation and physical activity after SCAD
Recommendations on the appropriate type and intensity of exercise after SCAD can be challenging for both the clinician and patient. There is a lack of data supporting highly restricted physical activity after SCAD but a general consensus that extreme sports, exercise in extreme cold or hot temperatures, or prolonged Valsalva should be avoided. Fear of resuming exercise should be balanced with the enormous benefits including mental and bone health, as well as overall cardiovascular fitness [2••]. Cardiac rehabilitation is an underutilized but highly effective resource in helping patients with SCAD regain confidence and demonstrate safety of resuming exercise. Participation can be lower in younger patients due to lack of referral, logistical challenges in keeping multiple appointments, and not meeting the specific needs of SCAD patients [47]. Saw and colleagues demonstrated a SCAD tailored cardiac rehab protocol consisting of 6 months of monitored aerobic exercise, light resistance training, weekly sessions that incorporate education, stress management, and peer-to-peer support was safe and associated with an improvement in exercise capacity, cardiac symptoms, and depression/anxiety scores [48, 49••]. Although advice regarding specific heart rate or weight limits could be considered, there is no data to suggest which of these thresholds should be safe, which likely varies among patients. Instead, recommendations on resuming exercise should be titrated to the individual patient with a focus on staying within the moderate range of perceived exertion, good form with minimal straining, and discussion of safe and heart healthy habits [2••].
Mental health after SCAD
Uncertainty around predicting, diagnosing, treating, and preventing SCAD can contribute significantly to the degree of emotional and mental stress after an event in addition to concern that stress may precipitate SCAD in some patients. Mental health assessments of a large cohort of women enrolled in the Mayo Clinic SCAD registry demonstrated that SCAD is associated with significant rates of post-traumatic stress disorder, anxiety, and depression. Younger age at the time of SCAD was associated with more severe symptoms. PTSD and depression scores using the PTSD Diagnostic Scale for Diagnostic and Statistical Manual of Mental Health Disorders, Fifth Edition (PDS-5) and Patient Health Questionnaire-9 survey scores (PHQ-9) respectively were independent of time from event. Generalized Anxiety Disorder-7 (GAD-7) scores were lower with increased time from event [50].
A small series found high prevalence of stress, anxiety, insomnia, depression, and PTSD among SCAD patients with patterns of perceived control more similar to cancer patients than other cardiac patients (e.g., SCAD patients ranked chance to more likely impact medical outcomes than self-control) [50]. Another study of participants with self-reported SCAD completed a questionnaire and described moderate-high perceptions of stress, receiving inadequate information at the time of diagnosis, and generally positive experiences in cardiac rehabilitation with further interest in patient support groups and patient education [51].
More recent data has identified resilience as a therapeutic target to help manage chronic anxiety and stress. Resilience is a quality that enables an individual to thrive despite adversity and can be measured using the Connor-Davidson Resiliency Scale (CD-RISC) [52]. Studies have shown it is modifiable, and higher levels of resilience correlate to better physical and mental health [53]. Dedicated resiliency training programs are associated with higher CD-RISC scores and have shown benefit in several patient populations [53, 54]. In the SCAD population, higher CD-RISC scores are associated with less severe PTSD, anxiety, and depression [48, 49••]. This is an exciting area of research and suggests tailored programs focused on increasing resiliency may be extremely impactful in the SCAD population.
In summary, a multidisciplinary care model which incorporates routine screening for PTSD, anxiety, and depression in SCAD survivors is strongly recommended. Identifying patients with lower resiliency scores may further help clinicians deliver more intense and targeted behavioral therapy to this subset of patients.
Conclusions
Spontaneous coronary artery dissection is a common cause of acute coronary syndrome among young women and pregnancy-associated myocardial infarction. Clinical care should include a multidisciplinary approach with attention to reproductive concerns among women of childbearing age. These concerns include those regarding SCAD recurrence, pregnancy, lactation, hormones, migraines, physical activity, and mental health. The genetic underpinnings of SCAD are complex, but recent targeted genotype and GWAS studies have discovered new and exciting information. Knowledge gaps remain for predicting, diagnosing, treating, and preventing SCAD. Therefore, increased recognition of SCAD, attention to concerns specific to SCAD, and ongoing research are critical in order ultimately improve the care of this patient population.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, et al. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. 2018;137(19):e523–57. https://doi.org/10.1161/CIR.0000000000000564. Comprehensive document reviewing etiology, pathophysiology, and practice recommendations.
•• Hayes SN, Tweet MS, Adlam D, Kim ESH, Gulati R, Price JE, et al. Spontaneous coronary artery dissection: JACC state-of-the-art review. Am Coll Cardiol. 2020;76(8):961–84. https://doi.org/10.1016/j.jacc.2020.05.084. This review provides clinical update on the diagnosis and management of patients with SCAD, including pregnancy-associated SCAD and pregnancy after SCAD, and highlights high priority knowledge gaps that must be addressed.
•• Adlam D, Alfonso F, Maas A, Vrints C, Writing Committee. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a Position Paper on Spontaneous Coronary Artery Dissection. Eur Heart J. 2018;39(36):3353–68. https://doi.org/10.1093/eurheartj/ehy080. Comprehensive document reviewing etiology, pathophysiology, and practice recommendations.
Saw J, Mancini GB, Humphries K, Fung A, Boone R, Starovoytov A, et al. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter Cardiovasc Interv. 2016;87(2):E54–61. https://doi.org/10.1002/ccd.26022.
Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2014;84(7):1115–22. https://doi.org/10.1002/ccd.25293.
Tweet MS, Miller VM, Hayes SN. The evidence on estrogen, progesterone, and spontaneous coronary artery dissection. JAMA Cardiol. 2019;4:403–4. https://doi.org/10.1001/jamacardio.2019.0774.
Sharma S, Kaadan MI, Duran JM, Ponzini F, Mishra S, Tsiaras SV, et al. Risk factors, imaging findings, and sex differences in spontaneous coronary artery dissection. Am J Cardiol. 2019;123(11):1783–7. https://doi.org/10.1016/j.amjcard.2019.02.040.
DeFilippis EM, Collins BL, Singh A, Biery DW, Fatima A, Qamar A, et al. Women who experience a myocardial infarction at a young age have worse outcomes compared with men: the Mass General Brigham YOUNG-MI registry. Eur Heart J. 2020;41(42):4127–37. https://doi.org/10.1093/eurheartj/ehaa662.
Al-Hussaini A, Adlam D. Spontaneous coronary artery dissection. Heart. 2017;103(13):1043–51. https://doi.org/10.1136/heartjnl-2016-310,320.
Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang IK, et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31(4):401–15. https://doi.org/10.1093/eurheartj/ehp433.
Alfonso F, Paulo M, Gonzalo N, Dutary J, Jimenez-Quevedo P, Lennie V, et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol. 2012;59(12):1073–9. https://doi.org/10.1016/j.jacc.2011.08.082.
• Tweet MS, Gulati R, Williamson EE, Vrtiska TJ, Hayes SN. Multimodality imaging for spontaneous coronary artery dissection in women. JACC Cardiovasc Imaging. 2016;9(4):436–50. https://doi.org/10.1016/j.jcmg.2016.01.009. A detailed review of optimal use of echo, CCTA, and MRI in SCAD.
• Gupta S, Meyersohn N, Wood MJ, et al. Role of coronary CT angiography in spontaneous coronary artery dissection. Radiology: Cardiothoracic Imaging. 2020;2(6). https://doi.org/10.1148/ryct.2020200364. An excellent detailed manuscript summarizing CCTA findings in SCAD.
Pozo-Osinalde E, García-Guimaraes M, Bastante T, Aguilera MC, Rodríguez-Alcudia D, Rivero F, et al. Characteristic findings of acute spontaneous coronary artery dissection by cardiac computed tomography. Coron Artery Dis. 2020;31(3):293–9. https://doi.org/10.1097/MCA.0000000000000819.
• Tan NY, Hayes SN, Young PM, Gulati R, Tweet MS. Usefulness of cardiac magnetic resonance imaging in patients with acute spontaneous coronary artery dissection. Am J Cardiol. 2018;122(10):1624–9. https://doi.org/10.1016/j.amjcard.2018.07.043. Detailed information on use of cardiac MRI and nuances in patients with SCAD vs. Takotsubo cardiomyopathy.
Chou AY, Sedlak T, Aymong E, Sheth T, Starovoytov A, Humphries KH, et al. Spontaneous coronary artery dissection misdiagnosed as Takotsubo cardiomyopathy: a case series. Can J Cardiol. 2015;31(8):1073.e5–8. https://doi.org/10.1016/j.cjca.2015.03.018.
Duran JM, Naderi S, Vidula M, Michalak N, Chi G, Lindsay M, et al. Spontaneous coronary artery dissection and its association with Takotsubo syndrome: novel insights from a tertiary center registry. Catheter Cardiovasc Interv. 2020;95(3):485–91. https://doi.org/10.1002/ccd.28314.
Y-Hassan S. Spontaneous coronary artery dissection and Takotsubo syndrome: an often overlooked association; review. Cardiovasc Revasc Med. 2018;19(6):717–23. https://doi.org/10.1016/j.carrev.2018.02.002.
Bolen MA, Brinza E, Renapurkar RD, Kim ESH, Gornik HL. Screening CT angiography of the aorta, visceral branch vessels, and pelvic arteries in fibromuscular dysplasia. JACC Cardiovasc Imaging. 2017;10(5):554–61. https://doi.org/10.1016/j.jcmg.2016.04.010.
Gornik HL, Persu A, Adlam D, Aparicio LS, Azizi M, et al. First international consensus on the diagnosis and management of fibromuscular dysplasia. Vasc Med. 2019;24(2):164–89. https://doi.org/10.1177/1358863X18821816.
Verstraeten A, et al. Collaborators of the European/International Fibromuscular Dysplasia Registry and Initiative (FEIRI). Enrichment of rare variants in Loeys-Dietz syndrome genes in spontaneous coronary artery dissection but not in severe fibromuscular dysplasia. Circulation. 2020;142(10):1021–4. https://doi.org/10.1161/CIRCULATIONAHA.120.045946.
Michelis JC, Olin JW, Kadian-Dodov D, d’Escamard V, Kovacic JC. Coronary artery manifestations of FMD. J Am Coll Cardiol. 2014;64(10):1033–46. https://doi.org/10.1016/j.jacc.2014.07.014.
Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation. 2014;130(21):1915–20. https://doi.org/10.1161/CIRCULATIONAHA.114.011422.
Tweet MS, Hayes SN, Codsi E, Gulati R, Rose CH, Best PJM. Spontaneous coronary artery dissection associated with pregnancy. J Am Coll Cardiol. 2017;70(4):426–35. https://doi.org/10.1016/j.jacc.2017.05.055.
Adlam et al. for the SCAD Collaborative Group. Abstract 10,590: long-term outcomes in pregnancy related vs. non-pregnancy related spontaneous coronary artery dissection: an international multi-center collaborative study. Circulation. 2019;140:10590.
Codsi E, Tweet MS, Rose CH, Arendt KW, Best PJM, Hayes SN. Spontaneous coronary artery dissection in pregnancy: what every obstetrician should know. Obstet Gynecol. 2016;128(4):731–8. https://doi.org/10.1097/AOG.0000000000001630.
•• Kok SN, Tweet MS. Recurrent spontaneous coronary artery dissection. Expert review of cardiovascular therapy. https://doi.org/10.1080/14779072.2021.1877538. Reviews what we currently understand about recurrence of SCAD.
Kronzer VL, Tarabochia AD, Lobo Romero AS, Tan NY, O’Byrne TJ, Crowson CS, et al. Lack of association of spontaneous coronary artery dissection with autoimmune disease. J Am Coll Cardiol. 2020;76(19):2226–34. https://doi.org/10.1016/j.jacc.2020.09.533.
Franke KB, Nerlekar N, Marshall H, Psaltis PJ. Systematic review and meta-analysis of the clinical characteristics and outcomes of spontaneous coronary artery dissection. Int J Cardiol. 2021;322:34–9. https://doi.org/10.1016/j.ijcard.2020.08.076.
Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol. 2017;70(9):1148–58. https://doi.org/10.1016/j.jacc.2017.06.053.
Rigatelli G, Dell’Avvocata F, Picariello C, Zuin M, Giordan M, Roncon L. Characterization of single vs. recurrent spontaneous coronary artery dissection. Asian Cardiovasc Thorac Ann. 2018;26(2):89–93. https://doi.org/10.1177/0218492318757041.
Tweet MS, Codsi E, Best PJM, Gulati R, Rose CH, Hayes SN. Menstrual chest pain in women with history of spontaneous coronary artery dissection. J Am Coll Cardiol. 2017;70(18):2308–9. https://doi.org/10.1016/j.jacc.2017.08.071.
Tweet MS, Young KA, Rose CH, Best PJ, Gulati R, Hayes SN. Abstract 13,043: pregnancy after spontaneous coronary artery dissection: a case series of 22 women and 31 pregnancies. Circulation. 2019;140:A13043.
•• Tweet MS, Young KA, Best PJM, Hyun M, Gulati R, Rose CH, et al. Association of pregnancy with recurrence of spontaneous coronary artery dissection among women with prior coronary artery dissection. JAMA Netw Open. 2020;3(9):e2018170. https://doi.org/10.1001/jamanetworkopen.2020.18170. Provides insight into a common and challenging clinical scenario, i.e., how to counsel women who desire pregnancy after SCAD.
Henkin S, Negrotto SM, Tweet MS, Kirmani S, Deyle DR, Gulati R, et al. Spontaneous coronary artery dissection and its association with heritable connective tissue disorders. Heart. 2016;102(11):876–81. https://doi.org/10.1136/heartjnl-2015-308,645.
Nakamura M, Yajima J, Oikawa Y, Ogasawara K, Uejima T, Abe K, et al. Vascular Ehlers-Danlos syndrome--all three coronary artery spontaneous dissections. J Cardiol. 2009;53(3):458–62. https://doi.org/10.1016/j.jjcc.2008.09.007.
• Kaadan MI, MacDonald C, Ponzini F, Duran J, Newell K, Pitler L, et al. Prospective cardiovascular genetics evaluation in spontaneous coronary artery dissection. Circ Genom Precis Med. 2018;11(4):e001933. https://doi.org/10.1161/CIRCGENETICS.117.001933. Valuable and clinically helpful information on their own findings as well as summary of current understanding of genetics and SCAD.
Kiando SR, Tucker NR, Castro-Vega LJ, Katz A, D’Escamard V, Tréard C, et al. PHACTR1 is a genetic susceptibility locus for fibromuscular dysplasia supporting its complex genetic pattern of inheritance. PLoS Genetics. 2016;12(10):e1006367. https://doi.org/10.1371/journal.pgen.1006367.
•• Adlam D, et al. Association of the PHACTR1/EDN1 genetic locus with spontaneous coronary artery dissection. J Am Coll Cardiol. 2019;73(1):58–66. https://doi.org/10.1016/j.jacc.2018.09.085. Novel paper that helps us better understand the association of FMD, migraines, cervicocerebral dissection and SCAD.
• Turley TN, O’Byrne MM, Kosel ML, de Andrade M, Gulati R, Hayes SN, et al. Identification of susceptibility loci for spontaneous coronary artery dissection. JAMA Cardiol. 2020;5(8):1–10. https://doi.org/10.1001/jamacardio.2020.0872. Cutting edge GWAS in SCAD that opens up doors for future research inquiry.
• Saw J, Yang ML, Trinder M, Tcheandjieu C, Xu C, Starovoytov A, et al. Chromosome 1q21.2 and additional loci influence risk of spontaneous coronary artery dissection and myocardial infarction. Nat Commun. 2020;11(1):4432. https://doi.org/10.1038/s41467-020-17,558-x. Cutting edge GWAS in SCAD that opens up doors for future research inquiry.
Kok SN, Hayes SN, Cutrer FM, Raphael CE, Gulati R, Best PJM, et al. Prevalence and clinical factors of migraine in patients with spontaneous coronary artery dissection. J Am Heart Assoc. 2018;7(24):e010140. https://doi.org/10.1161/JAHA.118.010140.
Kaumann AJ, Frenken M, Posival H, Brown AM. Variable participation of 5-HT1-like receptors and 5-HT2 receptors in serotonin-induced contraction of human isolated coronary arteries. 5-HT1-like receptors resemble cloned 5-HT1D beta receptors. Circulation. 1994;90(3):1141–53. https://doi.org/10.1161/01.cir.90.3.1141.
Alzubi J, Jabri A, Hedrick D. Abstract 15,755: spontaneous coronary artery dissection following exposure to triptan. Circulation. 2019;140:A15755.
Abu-Haniyeh A, Goyal A, Faulx M. Abstract 12,091: -vessel spontaneous coronary artery dissection after triptan exposure. Circulation. 2018;138:A12091.
• Oakes TM, Kovacs R, Rosen N, Doty E, Kemmer P, Aurora SK, et al. Evaluation of cardiovascular outcomes in adult patients with episodic or chronic migraine treated with galcanezumab: data from three phase 3, randomized, double-blind, placebo-controlled EVOLVE-1, EVOLVE-2, and REGAIN studies. Headache. 2020;60(1):110–23. https://doi.org/10.1111/head.13684. Clinically helpful in counseling patients with migraine and SCAD.
Krittanawong C, Tweet MS, Hayes SE, Bowman MJ, Gulati R, Squires RW, et al. Usefulness of cardiac rehabilitation after spontaneous coronary artery dissection. Am J Cardiol. 2016;117(10):1604–9. https://doi.org/10.1016/j.amjcard.2016.02.034.
Chou AY, Prakash R, Rajala J, Birnie T, Isserow S, Taylor CM, et al. The first dedicated cardiac rehabilitation program for patients with spontaneous coronary artery dissection: description and initial results. Can J Cardiol. 2016;32(4):554–60. https://doi.org/10.1016/j.cjca.2016.01.009.
•• Johnson AK, Hayes SN, Sawchuk C, Johnson MP, Best PJ, Gulati R, et al. Analysis of posttraumatic stress disorder, depression, anxiety, and resiliency within the unique population of spontaneous coronary artery dissection survivors. J Am Heart Assoc. 2020;9(9):e014372. https://doi.org/10.1161/JAHA.119.014372. Highlights important mental health considerations in the care of SCAD patients.
Edwards KS, Vaca KC, Naderi S, Tremmel JA. Patient-reported psychological distress after spontaneous coronary artery dissection: evidence for post-traumatic stress. J Cardiopulm Rehabil Prev. 2019;39(5):E20–3. https://doi.org/10.1097/HCR.0000000000000460.
Wagers TP, Stevens CJ, Ross KV, Leon KK, Masters KS. Spontaneous coronary artery dissection (SCAD): female survivors’ experiences of stress and support. J Cardiopulm Rehabil Prev. 2018;38(6):374–9. https://doi.org/10.1097/HCR.0000000000000330.
Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76–82. https://doi.org/10.1002/da.10113.
Loprinzi CE, Prasad K, Schroeder DR, Sood A. Stress Management and Resilience Training (SMART) program to decrease stress and enhance resilience among breast cancer survivors: a pilot randomized clinical trial. Clin Breast Cancer. 2011;11(6):364–8. https://doi.org/10.1016/j.clbc.2011.06.008.
Bhagra A, Medina-Inojosa JR, Vinnakota S, Arciniegas MC, Garcia M, Sood A, et al. Stress management and resilience intervention in a women’s heart clinic: a pilot study. J Womens Health (Larchmt). 2019;28:1705–10. https://doi.org/10.1089/jwh.2018.7216.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Shimoli Shah declares that she has no conflict of interest. Marysia Tweet declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Reproductive Health and Cardiovascular Disease
Rights and permissions
About this article
Cite this article
Shah, S., Tweet, M. Imaging of Spontaneous Coronary Artery Dissection and Counseling Patients of Reproductive Age. Curr Treat Options Cardio Med 23, 52 (2021). https://doi.org/10.1007/s11936-021-00927-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s11936-021-00927-0