Abstract
Spontaneous coronary artery dissection (SCAD) is a non-inflammatory, non-atherosclerotic cause of acute myocardial infarction (AMI) that, by definition, is not iatrogenic or due to trauma. It is a condition that predominantly affects pre- or perimenopausal women without the traditional risk factors for cardiovascular disease.
Purpose of Review
In this review, we will discuss the epidemiology, diagnosis, and management of this condition, with an emphasis on the ongoing research needed to better understand how to care for patients with SCAD.
Recent Findings
There is a paucity of data related to this condition. However, an American Heart Association consensus statement has recently been released that provides helpful insight. There has also been better characterization of pregnancy-associated SCAD.
Summary
We have learned much about SCAD over the last decade and greatly increased the identification of this condition by first responders and physicians through research and patient advocacy. However, there is much we still do not know about this condition, and further research, using larger numbers of patients, is greatly needed to better understand this condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
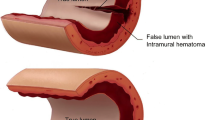
Spontaneous coronary artery dissection (SCAD) is a non-inflammatory, non-atherosclerotic cause of acute myocardial infarction (AMI) that, by definition, is not iatrogenic or due to trauma [1••]. Autopsies and, later, advanced intracoronary imaging with intravascular ultrasound (IVUS) and optical coherence tomography (OCT) have provided insight into the potential pathophysiology of SCAD [2,3,4]. Observations through these modalities suggest that separation of the coronary intima/media and the development of intramural hematoma (IMH) lead to compression of the true lumen. As a result, the overwhelming majority of these patients present with acute coronary syndrome, and in a minority of cases, sudden cardiac death [5,6,7]. While there are notable associations with other conditions, such as fibromuscular dysplasia (FMD), the etiology of SCAD is currently unknown.
SCAD was first described in 1930 by Doctor Harold Pretty in a case of a woman with sudden cardiac death after “partaking in a good meal of fried fish and potato chips” resulting in “severe retching and vomiting.” Autopsy revealed “dissecting aneurysm” of the right coronary artery [8]. After this case report, much of the early descriptions of SCAD were in the peripartum population, leading to initial speculation that this was predominantly a diagnosis of pregnancy. However, more recent data suggest that pregnancy-related SCAD accounts for only a small proportion of SCAD cases [5, 9]. It does highlight the female preponderance of this condition, which has been further demonstrated in subsequent case series [1••]. While we have learned much about SCAD in the last decade, there are still many unanswered questions. This review will summarize what we currently know about this condition and highlight areas in which further research is necessary.
Epidemiology
The true epidemiology of SCAD is largely unknown as, until more recently, it was a generally unrecognized and underdiagnosed condition. This was and still is driven by a low index of suspicion for a cardiac cause of presenting symptoms as well as difficulties in diagnosis by angiography given limitations of the technique itself and lack of operator familiarity with the condition. Much of our published knowledge of the epidemiology of SCAD comes from several single-center registries, small by cardiology standards but growing with improved awareness and, thereby, diagnosis. We do know SCAD disproportionately affects women, who account for > 90% [5, 10, 11, 12•] of cases. The average age of female patients ranges from 45 to 53, but cases of patients in their 7th and 8th decades of life have been described. Men appear to be younger at presentation as compared with women (mean age of 48.6 ± 9.8 vs. 52.3 ± 9.2, p = 0.05) [13]. The majority of patients are classified as white, although this may reflect a component of referral bias [5, 6].
While we know that pregnancy-associated SCAD (PASCAD) accounts for a small proportion of cases overall [14•, 15], SCAD is likely the most common cause of acute coronary syndrome (ACS) in pregnancy, accounting for 56 of 132 (43%) cases of pregnancy-associated ACS in a recent systematic review by Elkayam et al. [16••]. Emerging data also suggest it may be a much more common cause of ACS than previously believed, particularly in younger women. An earlier single-center retrospective analysis of 11,605 angiograms revealed SCAD in 0.2% in the overall population but a much higher prevalence in women under the age of 50 who presented with ACS or, specifically, STEMI (8.7 and 10.8%, respectively) [17]. Several more contemporary series suggest an even higher prevalence of SCAD in younger, female patients. In one series, SCAD was diagnosed in 24.2% of angiograms of women under the age of 50 who presented with ACS and was the most common cause of non-atherosclerotic coronary artery disease in this group of women [18]. In a multi-institutional Japanese study of 20,195 patients, the overall prevalence of SCAD was 0.31%, but the prevalence in women under the age of 50 was 35% (45 of 130 women) [19]. As we continue to grow our current registries of SCAD patients, we will be able to shed greater light on the true epidemiology of this condition.
Pathophysiology
Determination of the true pathophysiology of SCAD is difficult in an era where postmortem analyses are not routinely requested. This is compounded by the difficulties of studying this rare condition on a basic science level. We do know that IMH is the likely cause of ACS in SCAD based on histopathological results [20] and OCT/IVUS [21, 22]. There are two hypotheses for pathophysiology that results in the IMH: (1) an intimal dissection results in blood flow between in the intima and media, causing a false lumen and (2) rupture of the vaso-vasorum leads to accumulation of hematoma within the medial space [23]. In a recent analysis of 240 patients by Waterbury et al., only 123 (51%) had an identified intimal tear, suggesting that both hypotheses may be in play. There is also some question of whether the increased pressure in the false lumen as a result of the IMH causes intimal disruption into the true lumen, suggesting intimal tear is not the inciting event. Given that the vast majority of SCAD patients are women, attention has been turned to the potential role of female sex hormones in vessel fragility. There are data to suggest multiparity as a risk factor for SCAD, which has led to a hypothesis that progressive progesterone and estrogen mediated weakening of the vessel wall over the course of multiple pregnancies may, at least in part, explain this finding [24]. It is proposed that this hormone-mediated weakening leads to cystic medial necrosis and vaso-vasorum instability [16••, 25]. There is a similar proposed pathway for aortic dissection in pregnancy [26]. To what extent these hormonal factors contribute to both PASCAD and SCAD outside of pregnancy has yet to be elucidated.
Associated Conditions
Fibromuscular Dysplasia
FMD is a non-atherosclerotic, non-inflammatory vascular condition that most often results in a classic “string-of-beads” pattern and can cause arterial stenosis, aneurysm, and/or dissection in any arterial bed, with the renal and carotid arteries being the most common [27]. Like SCAD, the etiology and pathophysiology of FMD are not well understood and, to date, there has been no genetic mutation identified. The first case series to describe an association between FMD and SCAD was by Pate et al. in 2005. They described seven women with ACS who also had angiographic features consistent with renal FMD and suggested FMD may be the cause of the identified coronary abnormalities seen on angiogram [28]. Since this initial description, subsequent cohort studies have reported SCAD and concomitant FMD in anywhere from 17 to 86% of patients [1••]. It is unclear why there is such a substantial variation in this association from cohort to cohort. Demographic differences, variation in imaging modalities, rigor in identifying FMD, and other factors are likely contributing and deserve further investigation. Diagnosis and management of FMD are beyond the scope of this review, but given the strong association with SCAD, a firm understanding of this condition is crucial to the management of SCAD patients.
Other Conditions
There have been numerous case reports of autoimmune and inflammatory conditions, including rheumatoid arthritis, systemic lupus erythematosus, and inflammatory bowel disease, associated with SCAD. There are also good data to suggest that these conditions increase the overall risk of myocardial infarction from all causes [29]. There has been little utility in screening all patients for these conditions. However, patients should be screened for clinical signs and symptoms and consideration should be made for further testing (and possible rheumatology referral) if there is suspicion for an underlying autoimmune disease. Similarly, a very small minority of patients have a previously diagnosed genetic connective tissue disease, such as Marfan Syndrome, or suspicion for one of these conditions. The utility of genetic testing for all SCAD patients to identify one of these conditions is very low yield. It should also be noted that there has been no causal gene identified for SCAD. However, if there is clinical suspicion for a connective tissue disease, consideration should be made for referral to a cardiovascular geneticist with expertise in these conditions.
Pregnancy Associated SCAD
As previously mentioned, PASCAD is the most common mechanism of myocardial infarction in this population. The majority of these patients present in the postpartum period or the third trimester [16••]. We have also observed cases several months to a year or more postpartum, particularly in women who are still breastfeeding [14•]. The etiology of PASCAD is largely unknown. Clearly, SCAD is not an occurrence in all pregnancies, so there is some predisposition to dissection that has yet to be elucidated. It is likely that an underlying abnormality of the vessel wall or superimposed inflammation in combination with the hormonal and hemodynamic changes of pregnancy may predispose these women to SCAD, but we certainly need further research in this area.
In the largest series to date of PASCAD by Havakuk et al., the mean age was 34 years, with 40% of women over the age of 35 [30]. The vast majority of cases presented in the 3rd trimester or postpartum period (17 and 72.5% respectively), which is consistent with the general finding that cardiovascular conditions of pregnancy occur largely in the peripartum and postpartum period. PASCAD patients appear to have a higher rate of pre-eclampsia as compared with pregnancy patients overall, but like SCAD patients as a whole, a small percentage of these patients have conventional cardiovascular risk factors [31]. PASCAD patients generally present with higher risk features than SCAD patients overall, with a lower ejection fraction and a higher percentage of left main and LAD artery dissections [31]. As with all SCAD, a conservative approach, when possible, is recommended in these patients.
Triggers
While the underlying pathophysiology of SCAD is not fully understood, a number of precipitants or triggers for the event have been observed and are outlined below. These events are thought to increase cardiocirculatory stress and may provoke a dissection, particularly in the presence of pre-existing arterial fragility from underlying disorders.
Emotional Stressors
An early observation in the management of SCAD patients was that the acute event was often preceded by an extreme emotional stressor. In one single-center series of 168 cases, 40% of patients reported an emotional stressor prior to their event [5]. These stressors ranged from death in the family, to marriage issues, to job stress. Emotional stressors were reported to be much more common precipitants of SCAD in women than in men [32]. It is postulated that stress catecholamine surge during these events leads to coronary artery shear stress that at least in part contributes to the pathophysiology of SCAD in these cases. While this hypothesis has not been specifically tested in SCAD patients, a similar mechanism has been proposed in other stress-induced cardiovascular conditions such as stress-induced cardiomyopathy (Takotsubo syndrome) [33]. Interestingly, there is evidence from a small retrospective series that SCAD may be misdiagnosed as Takotsubo syndrome, indicating there may be some overlap between the two conditions [34, 35].
Physical Stressors
Physical stressors are also considered important potential precipitants of SCAD. In the same 168-patient series, 24% reported intense physical activity prior to their SCAD event, with about 12% reporting intense isometric exercises or weight lifting [5]. In another series, it was observed that patients were typically fit and engaged in activities beyond light cardiovascular exercises (e.g., skiing, triathlons, cycling, marathons, etc.) [36]. Although the pathophysiology of SCAD associated with physical activity is poorly understood, it is presumed that exposure to increased shear stress as a result of high-intensity exercise may predispose to dissection, especially in those with underlying arteriopathy [37]. An analysis of predisposing factors for men vs. women found that isometric exercise and heavy lifting were more common precipitants of SCAD in men compared with women [32]. Less-frequent precipitants involving valsalva-like maneuvers such as retching/vomiting, severe coughing, and intense bearing down have also been cited [4, 38, 39]. With such activities, the sudden increase in intrathoracic pressure and, thereby, systemic blood pressure may play a role, although this is extrapolated from aortic dissection data [40].
Hormonal Triggers
Unlike hormonal triggers related to pregnancy, other potential hormone-mediated SCAD triggers such as the perimenopausal state, use of oral contraceptives or hormone replacement therapy, and infertility treatments have less supportive data, although these associations have been reported. There have been case reports of SCAD as a result of beta HCG therapy [41, 42], the use of oral contraceptive therapy, and during menstruation [43]. There have also been observations that breastfeeding/lactation may be associated a precipitant [5, 14•]. SCAD related to high-dose steroid administration has also been noted [44]. More research is needed to elucidate if these hormonal influences have causal associations with SCAD.
Illicit Drug Use
There are reports of SCAD related to recreational drug use, such as cocaine and amphetamines. The use of recreational drugs in men presenting with SCAD was reported to be more common than women, although the patient sample size was small [32]. Cocaine prevents reuptake of norepinephrine and other catecholamines, which can lead to vasoconstriction, hypertension, tachycardia, and thrombogenesis. These changes can provide a milieu that predisposes patients to vascular damage, including SCAD [45, 46].
Clinical Presentation and Diagnosis
We know that the overwhelming majority of SCAD patients present either with ST elevation myocardial infarction or non-ST elevation myocardial infarction (26–87 and 13–69%, respectively, depending on the series) [1••]. Ventricular arrhythmias or sudden cardiac death have been reported in 3–11% of patients and cardiogenic shock in 2–5% of patients [1••]. While we often cite evidence that women with ACS present differently from men, the vast majority of SCAD patients (greater than 95%) present with chest pain [47]. Nausea/vomiting, diaphoresis, and dyspnea have also been commonly reported, both with and without chest pain [47]. Given that SCAD often occurs in younger women who appear otherwise healthy, it is not uncommon to find the diagnosis was initially missed by first responders and physicians alike. Since, as noted above, the vast majority of patients present with an NSTEMI or STEMI, an EKG and a troponin are vital tools for ruling out ACS and potential SCAD.
The gold standard for diagnosis of SCAD is coronary angiogram. Saw et al. [48] have developed a SCAD angiographic classification consisting of three types: type 1 represents the classic appearance with contrast staining of the arterial wall and multiple lumens; type 2 represents a diffuse narrowing, varying in severity and length, with type 2A being a narrowing bordered by a normal proximal and distal lumen and type 2B being narrowing that extends to the distal tip of the artery; type 3 represents a focal or tubular stenosis that is difficult to distinguish from atherosclerosis. In general, type 1 and, in most cases, type 2 SCAD are diagnosed without the need for intracoronary imaging. For type 3 lesions, optical coherence tomography (OCT) and intravascular ultrasound (IVUS) can be considered given it is often difficult to distinguish from an atherosclerotic lesion [1••]. However, these imaging modalities require technical and interpretive expertise that may not be available in all cases. Furthermore, there is reluctance to further instrument an artery that may be prone to dissection propagation and other complications. There are pros and cons to both IVUS and OCT that are beyond the scope of this review. It should also be noted that coronary computed tomography angiography (CCTA) can be considered useful in some cases. It has become a tool used to triage intermediate risk patients with acute coronary syndrome at many institutions. In the case of SCAD, it is not an ideal initial imaging modality for a number of reasons including: lower spatial and temporal resolution as compared with angiography, difficulty of identifying IMH (particularly of smaller caliber mid to distal vessels), and variable availability of properly gated and protocolled imaging [1••]. However, CCTA can be used to confirm IMH of proximal lesions after angiography or for evaluation of SCAD patients with symptoms or question of recurrence their initial presentation.
Management
As a general rule, a conservative approach is advocated for those patients who are hemodynamically stable, not having refractory angina, and free of critical left main or proximal coronary SCAD. This is largely due to technical difficulties in the treatment of these lesions with percutaneous intervention (PCI) or coronary artery bypass grafting (CABG). Attempts at PCI can lead to extension of the dissection or IMH, which can result in placement of long/multiple stents or further disruption of the vessel making PCI difficult or not possible. We also know that the vast majority of vessels, when studied in the weeks to months after the initial angiographic diagnosis, have healed on subsequent angiography [15]. IMH resorption can result in stent malapposition that increases the risk of stent thrombosis. When PCI is necessary, there are means by which to optimize the success of the procedure [1••]. In terms of CABG, there is suggestion that both venous and arterial conduits can fail once the native vessel has healed [15]. Therefore, CABG is reserved for extreme cases in which multivessel dissection and hemodynamically instability are present. Even in this scenario, the evidence for CABG is extremely limited. There has also been discussion of the use of temporary ventricular support devices, such as the Impella, extracorporeal membrane oxygenation, or intraortic balloon pump counterpulsation in cases of SCAD with cardiogenic shock, but this has not been systematically investigated in substantial numbers. There have been case reports using the Impella CP device in a limited number of cases of peripartum SCAD that warrants further review.
While in-hospital mortality for SCAD is low, up to 14% of patients require in-hospital revascularization, largely due to propagation of dissection. Furthermore, although not quantified, there is a risk of sudden cardiac death in this setting. Therefore, we generally recommend a more prolonged period of monitoring (48 to 72 h, with some experts advocating a longer duration of observation) after SCAD is diagnosed. There is a paucity of data for medical management of SCAD but suggestions, based on my personal practice, are outlined below:
-
1.
Antiplatelet therapy: I often recommend lifelong aspirin 81 mg, but there is variation in this practice. In theory, a degree of thrombus burden at the site of the dissection flap can develop, and aspirin may provide some protection in this scenario should there be a recurrence. Certainly, the patient’s bleeding risk (including in those patients with menorrhagia) should be taken into account. In the case of PCI, the recommendations for dual antiplatelet therapy (DAPT) would be the same as for atherosclerotic lesions. In the case of conservatively managed SCAD, consideration can be made for 1–3 months of DAPT. Again, the patient’s bleeding risk should be taken into account. In theory, there is concern for propagation of intramural hematoma, so some experts advise aspirin alone or no DAPT altogether.
-
2.
Beta blockers: There is evidence to suggest that beta blockers may reduce the risk of recurrence. In a review of 327 SCAD patients by Saw et al., the risk of recurrence was reduced in those patients on a beta blocker (HR, 0.36; 95% CI, 0.18–0.73) [49]. While many patients express an intolerance to beta blockers, it is important, if at all possible, to remain on some dose of beta blocker. Often, a change in the type of beta blocker can improve patient symptoms.
-
3.
Calcium channel blockers: For those with ongoing chest pain, calcium channel blockers can be a very useful (and well tolerated) drug in this patient population.
-
4.
Anticoagulation: As a general rule, heparin and other anticoagulation therapy is stopped at the time of diagnosis of SCAD given concern for propagation of intramural hematoma.
-
5.
Nitrates: Nitrates can be used as an anti-anginal in select patients with ongoing pain. However, anecdotally, there are SCAD patients that appear to have increased angina with nitrates. Side effects, including hypotension and headaches, can prohibit its use, particularly in this patient population.
-
6.
Statins: Statins are not generally recommended in SCAD given some evidence of increased recurrence in those using statins [6]. However, if the patient meets criteria for initiation for primary prevention, then a statin should be initiated.
-
7.
Cardiac rehabilitation: There is evidence that the rate of referral and participation to cardiac rehabilitation is low in this patient population. A study by the Vancouver General Hospital SCAD program showed that an initially conservative exercise regimen is safe in these patients. They generally recommended a blood pressure no higher than 130/80 mmHg; heart rate of 50–70% of maximal predicated; light weights of 2–12 lb as an initial starting point [50]. We will generally increase the exercise regimen as tolerated beyond this initial conservative approach based on patient symptoms and level of endurance. In general, an eventual return to or gradual build to moderate intensity exercise is ultimately encouraged, as there is no evidence of harm related to this level of exercise. For patients with ongoing angina after SCAD, a deferment of an exercise regimen until symptoms are under control should be considered.
Prognosis and Recurrence
The long-term prognosis and recurrence risk of SCAD is largely unknown at this time. Within the first 10 years after SCAD, the risk of major adverse cardiovascular events (MACE), predominantly due to recurrent SCAD, is felt to be somewhere around 30%. At this point, there are no clear markers available to predict recurrence.
Conclusion
While our understanding of SCAD, from pathophysiology to management, has greatly increased over time, there is still little evidence to support much of how we manage these patients. As recognition of this condition has increased, so has our ability to develop larger cohorts of SCAD patients. This will hopefully allow us to better understand SCAD and allow more tailored management of this condition.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Hayes SN, ESH K, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, et al. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. 2018;137:e523–57 This is the first US based consensus statement discussing SCAD. Very in-depth overview of the condition and its current management by experts in the field.
Paulo M, Sandoval J, Lennie V, Dutary J, Medina M, Gonzalo N, et al. Combined use of OCT and IVUS in spontaneous coronary artery dissection. JACC Cardiovasc Imaging. 2013;6:830–2.
Asuncion CM, Hyun J. Dissecting intramural hematoma of the coronary artery in pregnancy and the puerperium. Obstet Gynecol. 1972;40:202–10.
Sivam S, Yozghatlian V, Dentice R, McGrady M, Moriarty C, Di Michiel J, et al. Spontaneous coronary artery dissection associated with coughing. J Cyst Fibros. 2014;13:235–7.
Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7:645–55.
Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579–88.
Lettieri C, Zavalloni D, Rossini R, Morici N, Ettori F, Leonzi O, et al. Management and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 2015;116:66–73.
Pretty H. Dissecting aneurysm of coronary artery in a woman aged 42: rupture. Br Med J. 1931;667.
Tweet MS, Hayes SN, Codsi E, Gulati R, Rose CH, Best PJM. Spontaneous coronary artery dissection associated with pregnancy. J Am Coll Cardiol. 2017;70:426–35.
Rashid HN, Wong DT, Wijesekera H, Gutman SJ, Shanmugam VB, Gulati R, et al. Incidence and characterisation of spontaneous coronary artery dissection as a cause of acute coronary syndrome—a single-centre Australian experience. Int J Cardiol. 2016;202:336–8.
Eleid MF, Guddeti RR, Tweet MS, Lerman A, Singh M, Best PJ, et al. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ Cardiovasc Interv. 2014;7:656–62.
• Rogowski S, Maeder MT, Weilenmann D, Haager PK, Ammann P, Rohner F, et al. Spontaneous coronary artery dissection: angiographic follow-up and long-term clinical outcome in a predominantly medically treated population. Catheter Cardiovasc Interv. 2017;89:59–68 One of the papers that systematically describes the healing of SCAD in follow-up angiography.
Fahmy P, Prakash R, Starovoytov A, Boone R, Saw J. Pre-disposing and precipitating factors in men with spontaneous coronary artery dissection. JACC Cardiovasc Interv. 2016;9:866–8.
• Saw J, Ricci D, Starovoytov A, Fox R, Buller CE. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv. 2013;6:44–52 A review of the characteristics of patients with SCAD, with an emphasis on the association with fibromusuclar dysplasia.
Tweet MS, Eleid MF, Best PJ, Lennon RJ, Lerman A, Rihal CS, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7:777–86.
•• Elkayam U, Jalnapurkar S, Barakkat MN, Khatri N, Kealey AJ, Mehra A, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation. 2014;129:1695–702 The most in-depth review available regarding pregnancy-associated SCAD.
Vanzetto G, Berger-Coz E, Barone-Rochette G, Chavanon O, Bouvaist H, Hacini R, et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg. 2009;35:250–4.
Saw J, Aymong E, Mancini GB, Sedlak T, Starovoytov A, Ricci D. Nonatherosclerotic coronary artery disease in young women. Can J Cardiol. 2014;30:814–9.
Nakashima T, Noguchi T, Haruta S, Yamamoto Y, Oshima S, Nakao K, et al. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: a report from the angina pectoris-myocardial infarction multicenter investigators in Japan. Int J Cardiol. 2016;207:341–8.
Lunebourg A, Letovanec I, Eggenberger P, Lehr HA. Images in cardiovascular medicine. Sudden cardiac death due to triple vessel coronary dissection. Circulation. 2008;117:2038–40.
Arnold JR, West NE, van Gaal WJ, Karamitsos TD, Banning AP. The role of intravascular ultrasound in the management of spontaneous coronary artery dissection. Cardiovasc Ultrasound. 2008;6:24.
Poon K, Bell B, Raffel OC, Walters DL, Jang IK. Spontaneous coronary artery dissection: utility of intravascular ultrasound and optical coherence tomography during percutaneous coronary intervention. Circ Cardiovasc Interv. 2011;4:e5–7.
Waterbury TM, Tweet MS, Hayes SN, Prasad A, Lerman A, Gulati R. Coronary endothelial function and spontaneous coronary artery dissection. Eur Heart J Acute Cardiovasc Care. 2018;20:2048872618795255.
Koller PT, Cliffe CM, Ridley DJ. Immunosuppressive therapy for peripartum-type spontaneous coronary artery dissection: case report and review. Clin Cardiol. 1998;21:40–6.
Briguori C, Bellevicine C, Visconti G, Focaccio A, Aprile V, Troncone G. In vivo histological assessment of a spontaneous coronary artery dissection. Circulation. 2010;122:1044–6.
Kamel H, Roman MJ, Pitcher A, Devereux RB. Pregnancy and the risk of aortic dissection or rupture: a cohort-crossover analysis. Circulation. 2016;134:527–33.
Kadian-Dodov D, Gornik HL, Gu X, Froehlich J, Bacharach JM, Chi YW, et al. Dissection and aneurysm in patients with fibromuscular dysplasia: findings from the US Registry for FMD. J Am Coll Cardiol. 2016;68:176–85.
Pate GE, Lowe R, Buller CE. Fibromuscular dysplasia of the coronary and renal arteries? Catheter Cardiovasc Interv. 2005;64:138–45.
Teague H, Mehta NN. The link between inflammatory disorders and coronary heart disease: a look at recent studies and novel drugs in development. Curr Atheroscler Rep. 2016;18:3.
Havakuk O, Goland S, Mehra A and Elkayam U. Pregnancy and the risk of spontaneous coronary artery dissection: an analysis of 120 contemporary cases. Circ Cardiovasc Interv. 2017;10.
Naderi S. Spontaneous coronary artery dissection and pregnancy. Curr Treat Options Cardiovasc Med. 2017;19:69.
Fahmy P, Prakash R, Starovoytov A, Saw J. TCT-180 predisposing and precipitating factors in men with spontaneous coronary artery dissection. J Am Coll Cardiol. 2015;66:B67.
Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–48.
Vidula M, Duran J, Partida R, Coppolino W, Naderi S, Wood M. Patients with spontaneous coronary artery dissection demonstrate wall motion abnormalities consistent with TAKOTSUBO cardiomyopathy. J Am Coll Cardiol. 2016;67:318.
Chou AY, Sedlak T, Aymong E, Sheth T, Starovoytov A, Humphries KH, et al. Spontaneous coronary artery dissection misdiagnosed as Takotsubo cardiomyopathy: a case series. Can J Cardiol. 2015;31:1073e5–8.
Naderi S, Weinberg I, Lindsay M, Wood M. Spontaneous coronary artery dissection patients significantly more fit THAN the average patient referred for exercise stress testing. J Am Coll Cardiol. 2015;65:A1303.
Padilla J, Harris RA, Rink LD, Wallace JP. Characterization of the brachial artery shear stress following walking exercise. Vasc Med. 2008;13:105–11.
Velusamy M, Fisherkeller M, Keenan ME, Kiernan FJ, Fram DB. Spontaneous coronary artery dissection in a young woman precipitated by retching. J Invasive Cardiol. 2002;14:198–201.
Lin AH, Shutt BJ, Dendall RT and Bennett W. Multivessel spontaneous coronary artery dissection treated with staged percutaneous coronary intervention in a non-postpartum female. BMJ Case Rep. 2012;2012.
de Virgilio C, Nelson RJ, Milliken J, Snyder R, Chiang F, MacDonald WD, et al. Ascending aortic dissection in weight lifters with cystic medial degeneration. Ann Thorac Surg. 1990;49:638–42.
Hardegree EL, Tweet MS, Hayes SN, Gulati R, Kane GC. Multivessel spontaneous coronary artery dissection associated with hormonal infertility therapy in a 39-year-old female. J Cardiol Cases. 2012;5:e69–72.
Lempereur M, Grewal J, Saw J. Spontaneous coronary artery dissection associated with beta-HCG injections and fibromuscular dysplasia. Can J Cardiol. 2014;30:464e1–3.
Nakamoto K, Matsuda M, Kanno K, Segawa T, Nishimoto O, Nishiyama H, et al. A case of a young, healthy woman with spontaneous coronary artery dissection associated with oral contraceptive use: long-term residual dissection of the coronary artery. J Cardiol Cases. 2013;8:179–82.
Keir ML, Dehghani P. Corticosteroids and spontaneous coronary artery dissection: a new predisposing factor? Can J Cardiol. 2016;32:395e7–8.
Singh A, Khaja A, Alpert MA. Cocaine and aortic dissection. Vasc Med. 2010;15:127–33.
Steinhauer JR, Caulfield JB. Spontaneous coronary artery dissection associated with cocaine use: a case report and brief review. Cardiovasc Pathol. 2001;10:141–5.
Luong C, Starovoytov A, Heydari M, Sedlak T, Aymong E, Saw J. Clinical presentation of patients with spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2017;89:1149–54.
Saw J, Mancini GB, Humphries K, Fung A, Boone R, Starovoytov A, et al. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter Cardiovasc Interv. 2016;87:E54–61.
Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol. 2017;70:1148–58.
Chou AY, Prakash R, Rajala J, Birnie T, Isserow S, Taylor CM, et al. The first dedicated cardiac rehabilitation program for patients with spontaneous coronary artery dissection: description and initial results. Can J Cardiol. 2016;32:554–60.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sahar Naderi declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Women and Ischemic Heart Disease
Rights and permissions
About this article
Cite this article
Naderi, S. Spontaneous Coronary Artery Dissection: an Overview. Curr Atheroscler Rep 20, 58 (2018). https://doi.org/10.1007/s11883-018-0761-7
Published:
DOI: https://doi.org/10.1007/s11883-018-0761-7