Abstract
Purpose of review
This review summarizes the pathophysiology, diagnosis, and treatment of peripartum cardiomyopathy (PPCM), with a focus on recent discoveries of clinical relevance.
Recent findings
An increase in oxidative stress and anti-angiogenic activity play key roles in the pathophysiology of peripartum cardiomyopathy. Therapies that target this dysregulation may have a future role in treatment. Suppression of prolactin release using bromocriptine, a dopamine-receptor antagonist, has been associated with more favorable outcomes in small studies but more research is needed. Similarly, VEGF agonists may prove to be a novel therapy by upregulating angiogenesis.
Summary
Peripartum cardimyopathy typically presents in the third trimester or in first few months postpartum. Both genetic and clinical risk factors for PPCM have been identified. Women with PPCM should be managed by a multidisciplinary team with experience in high risk pregnancy and the treatment of heart failure. These women benefit from the use of standard treatments for heart failure therapy with the exception of avoiding ACE inhibitors and ARBs while pregnant. While the rate of recovery of ventricular function in PPCM is higher than in other forms of dilated cardiomyopathy, mechanical circulatory support and/or cardiac transplantation are required in some cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peripartum cardiomyopathy (PPCM) is an idiopathic cardiomyopathy which develops either during the later stages of pregnancy or in the first months thereafter. Risk factors for PPCM include Black race, multifetal gestation, multiparity, increasing age, and preeclampsia or hypertension [1]. The prevalence of PPCM varies geographically and ethnically. It is as high as 1 in 300 live births in Haiti, compared to 1 in 1000–4000 live births in the USA [2,3,4,5].
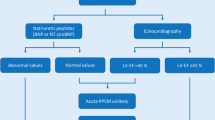
The diagnosis of PPCM should be considered in women presenting with heart failure symptoms such as shortness of breath, lower extremity edema, orthopnea, or paroxysmal nocturnal dyspnea during the peripartum period [6]. This however can be challenging as these are often symptoms of a healthy pregnancy. Alternative causes of acute heart failure and non-cardiac causes of a similar presentation should also be excluded.
The etiology of PPCM is unknown and more research is needed to better understand the genetic underpinnings and pathophysiology. Individuals with PPCM have a high frequency of truncating variants in the gene for the sarcomere protein titan (TTN), similar to patients with idiopathic dilated cardiomyopathy [7•, 8].
The risk of PPCM increases when there is a relative increase in oxidative stress without antioxidant capacity late in pregnancy. Furthermore, an increase in anti-angiogenic activity has been associated with PPCM. Prolactin levels increase early post-partum and abnormal proteolytic cleavage of this hormone can result in an inhibition of angiogenic activity. Suppression of prolactin release using bromocriptine, a dopamine-receptor antagonist, has been associated with more favorable outcome in small studies. Soluble fms-like tyrosine kinase 1 (s Flt1) is a tyrosine kinase protein that is upregulated in preeclampsia and may also be involved in the pathogenesis of PPCM [6, 9, 10].
While PPCM is associated with significant morbidity and mortality, outcomes in women with PPCM are generally better than those in other types of cardiomyopathy. In the multicenter IPAC (Investigations of Pregnancy-Associated Cardiomyopathy) study, 72% of women achieved a LV ejection fraction (LVEF) ≥ 50% at 1 year. Thirteen percent of women in this study had major events (left ventricular assist device (LVAD) placement, cardiac transplantation, or death) or had persistent severe cardiomyopathy by 1 year [11•]. These outcomes are similar to a recent study by Ersboll et al., in which only 14.8% of women had a major adverse event, and almost 2/3 of women had a complete recovery [5]. Other studies have shown poorer outcomes, especially among women of African descent [12, 13]. LV thrombi and embolic events frequently complicate PPCM, occurring in 7–20% of women [12, 14].
Clinical factors associated with a lack of recovery of left ventricular (LV) function include LVEF ≤ 30% at the time of diagnosis, significant LV chamber dilation (LV end-diastolic dimension ≥ 6 cm), reduced RV function on echocardiography calculated by fractional area change, persistent troponin elevation, Black race, and presentation later in the postpartum period [5, 11•, 15, 16]. Women who possess TTN variants associated with dilated cardiomyopathy also appear to be less likely to recover LV function [7•]. Women with hypertensive disorders of pregnancy may have a milder course [5, 17].
The rate of recurrence of PPCM in subsequent pregnancies is approximately 20% in those who recover normal LV function, with a much higher risk in those who do not have LV functional recovery [18]. Women with a history of PPCM who are considering subsequent pregnancy should receive in-depth counseling regarding the risks of and alternatives to pregnancy at a center experienced in the management of high risk pregnancy.
Treatment
Diet and lifestyle
Diet
-
A low salt diet is recommended for women with PPCM, as in other forms of heart failure [19].
Breastfeeding
-
The recognition that the nursing hormone prolactin may play a pathogenic role in PPCM has led to concerns that breastfeeding may contribute to or worsen the course of PPCM [20].
-
Data regarding the impact of breast feeding on the incidence and outcome of PPCM is very limited.
-
While some have advocated for avoidance of breastfeeding in this population, there is currently no evidence to suggest that this strategy is beneficial.
-
One study has shown that breastfeeding is positively corrected with LV recovery, but these results are difficult to interpret given that the potential for confounding is high [17]. For instance, successful breastfeeding in PPCM may be a marker for less severe disease. At the current moment, there is little data to guide recommendations regarding breastfeeding in PPCM.
-
As breastfeeding is beneficial to the baby and mother, especially in less developed countries, our approach is to recommend continuing breast feeding.
Pharmacological treatment
Conventional heart failure therapies
-
Women with PPCM benefit from the prompt initiation of standard therapies for heart failure with reduced ejection fraction. These include diuretics, beta-blockers, ACE inhibitors or ARBs, and mineralocorticoid receptor blockers (Table 1) [19].
-
Treatment is more limited when PPCM develops during pregnancy. Beta-1 selective drugs like metoprolol are preferred in pregnancy [21]. Ultrasound monitoring for fetal growth restriction is often recommended due to both reported association between some beta-blockers and fetal growth restriction [22], as well as PPCM itself. Hydralazine and nitrates can be considered in place of ACE inhibitor or ARB therapy during pregnancy. The use of ACE inhibitors, ARBs, mineralocorticoid receptor blockers, and renin inhibitors is contraindicated in pregnancy.
-
Most heart failure therapies can be used in breastfeeding women. Benazepril, enalapril, and captopril are generally felt to be safe and are the preferred agents in this population [23].
-
European Society of Cardiology guidelines suggest that agents should be continued for at least 12 months before consideration of withdrawal, and that withdrawal, when it occurs, should be done in a stepwise manner [24•]. Monitoring of ventricular function by echocardiography is recommended.
Beta-blockers
Drug: Metoprolol succinate (extended release)
Standard dose: 12.5–200 mg once daily
Contraindications: Bradycardia or hypotension
Main drug interactions
Main side effects: Bradycardia, hypotension, and fatigue
Inexpensive
Special points
May be associated with fetal growth restriction, avoid atenolol [21, 22].
Considered safe in lactation
Angiotensin converting enzyme inhibitors (ACE inhibitors)
Drug: Captopril or enalapril
Standard dosage:Captopril 3.25–50 mg TID
Enlapril 2.5 mg–20 mg BID
Contraindications: Pregnancy, hypotension, acute renal failure, hyperkalemia
Main drug interactions
Main side effects: hypotension, rise in creatinine, hyperkalemia
Inexpensive
Special points
Contraindicated in pregnancy
Generally considered safe in lactation
Mineralocorticoid receptor blockers
Drug: Spironolactone
Standard dosage: 12.5 to 50 mg once daily
Contraindications: Hyperkalemia, acute renal insufficiency
Main drug interactions: may enhance the hyperkalemic effect of ACE inhibitors.
Main side effects: hypotension
Inexpensive
Special points
Contraindicated in pregnancy
Limited data in lactation, can reduce breastmilk production
Loop diuretics
Drugs: Furosemide or other loop diuretics
Standard dosage:
-
Oral 20 to 40 mg once or twice daily (max 600 mg/day), dosing frequency to be adjusted based on patient specific needs
-
IV: 20 to 40 mg/dose (max 200 mg/dose)
-
IV continuous infusion: Initial 40 mg to 100 mg IV bolus over 1 to 20 min followed by IV infusion rate of 10 to 40 mg/h
Contraindications: Hypersensitivity to sulfonamide-derived products, acute renal failure, uncorrected hypokalemia
Main drug interactions
Main side effects: Hypotension
Special points
Frequently used in pregnancy
PPCM-specific therapy
Bromocriptine:
In the first, randomized controlled trial of 63 patients with PPCM and an LVEF ≤ 35% 2.5 mg of bromocriptine daily for 7 days in addition to standard heart failure therapy was associated with more favorable outcomes compared to those not treated with bromocriptine. Given the small size of this study, more research is needed [25].
Anticoagulation
-
Thrombotic events are common in PPCM [14•].
-
Anticoagulation is indicated in the setting of LV thrombus or thrombotic complications.
-
There are no data regarding the specific efficacy of different anticoagulants in PPCM; therefore, the choice of anticoagulant is at the discretion of the provider.
Interventional procedures
Obstetrical management
-
When PPCM develops in pregnancy, preterm delivery is often required.
-
Decision-making regarding the mode and timing of delivery is complex and should be made in consultation with a multidisciplinary team including cardiology, high-risk pregnancy specialists, anesthesia, and neonatology.
-
If possible, delivery should occur at a tertiary medical center with specific experience in the management of cardiac disease in pregnancy.
-
Vaginal delivery is tolerated in many cases. Minimization of the hemodynamic stress of delivery is recommended, typically by the use of early epidural anesthesia and an assisted second stage of labor if appropriate [23].
-
In woman with hemodynamic instability or significant hemodynamic compromise, cesarean section may be required.
-
Close attention to the potential hemodynamic effects of pain, hemorrhage, and autotranfusion from the uteroplacental bed to the maternal circulation is advised [23]. In addition, the rise in systemic vascular resistance post delivery causes an abrupt increase in afterload.
-
Hemodynamic monitoring may be required during delivery, and many women with PPCM benefit from admission to an intensive care unit or cardiology unit in the early postpartum period to monitor hemodynamics as often the highest risk time for CHF decompensation
-
Arrhythmia monitoring is indicated, given that arrhythmias are common in this population and are associated with adverse outcomes [26].
-
If timing and patient stability allow, optimization of the woman’s volume status with diuretics is recommended before delivery.
Implantable cardioverter defibrillator and cardiac resynchronization
-
There is an association between PPCM and malignant ventricular arrhythmias [26].
-
There are no PPCM-specific guidelines regarding the use of ICDs and cardiac resynchronization therapy.
-
In other forms of non-ischemic cardiomyopathy, ICD implantation is not recommended unless the left ventricular ejection fraction fails to improve after a period of goal directed medical therapy, usually at least 3 months [19].
-
The high rate of recovery of LV function in PPCM means that many women will not go on to need ICD implantation.
-
Some studies have documented continued improvement in LV function late after the development of PPCM [27]. The benefit of waiting longer to potentially avoid an ICD needs to be weighed against the risk of death from ventricular arrhythmia. The European Society of Cardiology PPCM guidelines state that waiting more than 6–12 months for myocardial recovery is unlikely to provide additional benefit, and exposes patients to excess harm [24•].
-
A wearable defibrillator may be a good option for high-risk women during the waiting period.
Mechanical circulatory support
-
A variety of mechanical support options exist for women with decompensated PPCM. Patients who are not responding to medical therapy should be cared for at a tertiary care institution with mechanical circulatory support capability.
-
A full discussion of the mechanical circulatory support options is beyond the scope of this review.
Cardiac transplantation
-
Cardiac transplantation may also be an option for select women with decompensated PPCM. Consultation with a transplant center is recommended.
Emerging therapies
As discussed above, bromocriptine has been associated with more favorable outcomes in smaller studies but larger studies are needed [25]. Novel therapeutics for PPCM include targeting molecular pathways such as with VEGF agonists to promote angiogenesis, or with neutralizers of the micro-RNA, mIR-146a, which is upregulated in PPCM and implicated in the pathogenesis [9].
Counseling on future pregnancy
Women with a history of PPCM should receive in depth counseling regarding the risks of and alternatives to subsequent pregnancy. Patients with a history of PPCM who do not recover LV function are at highest risk for complications and a new pregnancy is discouraged. In these women, contraception with a non-estrogen-based therapy (IUD) or partner sterilization should be considered.
The risk of recurrence in women who have recovered LV function can be as high as 20% but this is based on small retrospective studies. Women taking ACEi or ARB should discontinue these medications at least 3 months prior to conception. Hydralazine and isosorbide dinitrate together may be used instead. Data on safety of aldosterone antagonists (spironolactone) in pregnancy is limited and the general recommendation is to discontinue it. Beta 1 selective blockers can be continued during pregnancy and while the data is limited, metoprolol is felt to be generally safe. There are no guidelines to direct frequency of echocardiographic monitoring in pregnancy. Based on available literature and expert opinion, a baseline study and repeat echocardiogram after the first and second trimesters, 2–4 weeks prior to delivery, before discharge from the hospital, and 3 months after delivery are recommended. The clinician should have a low threshold for repeating an echocardiogram sooner when any concerning symptoms are reported by the patient.
Conclusion
Peripartum cardiomyopathy affects one in a thousand births worldwide. Both genetic and clinical risk factors for PPCM have been identified. The rate of recovery of ventricular function in PPCM is higher than in other forms of dilated cardiomyopathy. Women with PPCM should be managed by a multidisciplinary team with experience in high-risk pregnancy and the treatment of heart failure.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133(14):1397–409. https://doi.org/10.1161/CIRCULATIONAHA.115.020491.
Fett JD, Christie LG, Carraway RD, Ansari AA, Sundstrom JB, Murphy JG. Unrecognized peripartum cardiomyopathy in Haitian women. Int J Gynaecol Obstet. 2005;90(2):161–6. https://doi.org/10.1016/j.ijgo.2005.05.004.
Desai D, Moodley J, Naidoo D. Peripartum cardiomyopathy: experiences at King Edward VIII Hospital, Durban, South Africa and a review of the literature. Trop Dr. 1995;25(3):118–23. https://doi.org/10.1177/004947559502500310.
Brar SS, Khan SS, Sandhu GK, Jorgensen MB, Parikh N, Hsu JW, et al. Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol. 2007;100(2):302–4. https://doi.org/10.1016/j.amjcard.2007.02.092.
Ersboll AS, Johansen M, Damm P, Rasmussen S, Vejlstrup NG, Gustafsson F. Peripartum cardiomyopathy in Denmark: a retrospective, population-based study of incidence, management and outcome. Eur J Heart Fail. 2017;19(12):1712–20. https://doi.org/10.1002/ejhf.882.
Hilfiker-Kleiner D, Sliwa K. Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat Rev Cardiol. 2014;11(6):364–70. https://doi.org/10.1038/nrcardio.2014.37.
• Ware JS, Li J, Mazaika E, Yasso CM, DeSouza T, Cappola TP, et al. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N Engl J Med. 2016;374(3):233–41. https://doi.org/10.1056/NEJMoa1505517. The authors sequenced 43 genes which are known to be associated with dilated cardiomyopathy in a population of women with PPCM. They found a similar distribution of truncating variants, suggesting that a common genetic predisposition may underlie both disorders.
van Spaendonck-Zwarts KY, Posafalvi A, van den Berg MP, Hilfiker-Kleiner D, Bollen IA, Sliwa K, et al. Titin gene mutations are common in families with both peripartum cardiomyopathy and dilated cardiomyopathy. Eur Heart J. 2014;35(32):2165–73. https://doi.org/10.1093/eurheartj/ehu050.
Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest. 2013;123(5):2143–54. https://doi.org/10.1172/JCI64365.
Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485(7398):333–8. https://doi.org/10.1038/nature11040.
• McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G, et al. Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol. 2015;66(8):905–14. https://doi.org/10.1016/j.jacc.2015.06.1309.Multicenter US-based study which that most women with PPCM recover LV function. Thisteen percent of women in this study had a major event or persistent severe cardiomyopathy at 1 year.
Sliwa K, Skudicky D, Bergemann A, Candy G, Puren A, Sareli P. Peripartum cardiomyopathy: analysis of clinical outcome, left ventricular function, plasma levels of cytokines and Fas/APO-1. J Am Coll Cardiol. 2000;35(3):701–5.
Irizarry OC, Levine LD, Lewey J, Boyer T, Riis V, Elovitz MA, et al. Comparison of clinical characteristics and outcomes of peripartum cardiomyopathy between African American and non-African American women. JAMA Cardiol. 2017;2(11):1256–60. https://doi.org/10.1001/jamacardio.2017.3574.
Sliwa K, Mebazaa A, Hilfiker-Kleiner D, Petrie MC, Maggioni AP, Laroche C et al. Clinical characteristics of patients from the worldwide registry on peripartum cardiomyopathy (PPCM): EURObservational Research Programme in conjunction with the Heart Failure Association of the European Society of Cardiology Study Group on PPCM. Eur J Heart Fail. 2017;19(9):1131–1141. doi:https://doi.org/10.1002/ejhf.780. Description of the baseline clinical and echocardiographic characteristics of women enrolled in this multicenter registry.
Hu CL, Li YB, Zou YG, Zhang JM, Chen JB, Liu J, et al. Troponin T measurement can predict persistent left ventricular dysfunction in peripartum cardiomyopathy. Heart. 2007;93(4):488–90. https://doi.org/10.1136/hrt.2006.087387.
Blauwet LA, Delgado-Montero A, Ryo K, Marek JJ, Alharethi R, Mather PJ et al. Right ventricular function in peripartum cardiomyopathy at presentation is associated with subsequent left ventricular recovery and clinical outcomes. Circ Heart Fail. 2016;9(5). doi:https://doi.org/10.1161/CIRCHEARTFAILURE.115.002756.
Safirstein JG, Ro AS, Grandhi S, Wang L, Fett JD, Staniloae C. Predictors of left ventricular recovery in a cohort of peripartum cardiomyopathy patients recruited via the internet. Int J Cardiol. 2012;154(1):27–31. https://doi.org/10.1016/j.ijcard.2010.08.065.
Elkayam U. Risk of subsequent pregnancy in women with a history of peripartum cardiomyopathy. J Am Coll Cardiol. 2014;64(15):1629–36. https://doi.org/10.1016/j.jacc.2014.07.961.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. https://doi.org/10.1016/j.jacc.2013.05.019.
Triebel J, Clapp C, de la Martinez EG, Bertsch T. Remarks on the prolactin hypothesis of peripartum cardiomyopathy. Front Endocrinol (Lausanne). 2017;8:77. https://doi.org/10.3389/fendo.2017.00077.
Lydakis C, Lip GY, Beevers M, Beevers DG. Atenolol and fetal growth in pregnancies complicated by hypertension. Am J Hypertens. 1999;12(6):541–7.
Tanaka K, Tanaka H, Kamiya C, Katsuragi S, Sawada M, Tsuritani M, et al. Beta-blockers and fetal growth restriction in pregnant women with cardiovascular disease. Circ J. 2016;80(10):2221–6. https://doi.org/10.1253/circj.CJ-15-0617.
Taylor J. The first ESC Guidelines on the management of cardiovascular diseases during pregnancy. Eur Heart J. 2011;32(24):3055–6. https://doi.org/10.1093/eurheartj/ehr235.
• Bauersachs J, Arrigo M, Hilfiker-Kleiner D, Veltmann C, Coats AJ, Crespo-Leiro MG, et al. Current management of patients with severe acute peripartum cardiomyopathy: practical guidance from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail. 2016;18(9):1096–105. https://doi.org/10.1002/ejhf.586. Comprehensive guidelines regarding the management of women with PPCM, including critical care management.
Hilfiker-Kleiner D, Haghikia A, Berliner D, Vogel-Claussen J, Schwab J, Franke A, et al. Bromocriptine for the treatment of peripartum cardiomyopathy: a multicentre randomized study. Eur Heart J. 2017;38(35):2671–9. https://doi.org/10.1093/eurheartj/ehx355.
Mallikethi-Reddy S, Akintoye E, Trehan N, Sharma S, Briasoulis A, Jagadeesh K, et al. Burden of arrhythmias in peripartum cardiomyopathy: analysis of 9841 hospitalizations. Int J Cardiol. 2017;235:114–7. https://doi.org/10.1016/j.ijcard.2017.02.084.
Pillarisetti J, Kondur A, Alani A, Reddy M, Reddy M, Vacek J, et al. Peripartum cardiomyopathy: predictors of recovery and current state of implantable cardioverter-defibrillator use. J Am Coll Cardiol. 2014;63(25 Pt A):2831–9. https://doi.org/10.1016/j.jacc.2014.04.014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pregnancy and Cardiovascular Disease
Rights and permissions
About this article
Cite this article
Khan, A., Paré, E. & Shah, S. Peripartum Cardiomyopathy: a Review for the Clinician. Curr Treat Options Cardio Med 20, 91 (2018). https://doi.org/10.1007/s11936-018-0690-3
Published:
DOI: https://doi.org/10.1007/s11936-018-0690-3