Abstract
Purpose of Review
Hyperlipidemia is the major cardiovascular morbidity and mortality risk factor. Statins are the first-line treatment for hyperlipidemia. Statin-associated muscle symptoms (SAMS) are the main reason for the discontinuation of statins among patients. The purpose of this review is to guide clinicians to recognize the difference between self-limited and autoimmune statin myopathy in addition to the factors that potentiate them. Finally, treatment strategies will be discussed. This review mostly focuses on new data in the past 3 years.
Recent Findings
Recent findings suggest that SAMS is a complex and multifactorial condition that involves mitochondrial dysfunction, oxidative stress, and immune-mediated mechanisms. Effective management of SAMS requires a thorough evaluation of the patient’s symptoms, risk factors, and medication history, as well as consideration of alternative treatment options.
Summary
While statins are effective in reducing the risk of cardiovascular events, their use is associated with a range of adverse effects, including SAMS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Statins are commonly used for preventing or treating cardiovascular diseases, which are the leading cause of death and disability worldwide [1, 2]. Statins are a class of cholesterol‐lowering drugs and one of the most commonly prescribed medications. They act to inhibit the enzyme 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A reductase [2]. As the most used class of lipid-modifying agents, statins have seen a significant increase in their global use over the last several years, with an annual compound rate of 4.13%, especially in Europe and North America [3, 4]. Statins are both effective and generally safe. The most common side effect of statins is associated muscle symptoms (SAMS). One-third of statin users suffer from SAMS ranging from mild to moderate muscle pain, weakness, or fatigue to life-threatening rhabdomyolysis or immune-mediated necrotizing myopathy (IMNM) (Table 1). SAMS is the main reason for statin discontinuation.
Clinical Phenotypes
Statin-associated muscle symptoms (SAMS) can be categorized into two primary groups. The initial classification encompasses self-limited toxic statin myopathy, presenting a spectrum from myalgia to the severe condition of rhabdomyolysis. The second group comprises statin-induced IMNM, characterized by persistent muscle symptoms that persist even upon discontinuation of statin therapy [6, 7].
Self-Limited Toxic Statin Myopathy
Self-limited statin myopathy includes myalgia with or without mild CK elevation and rhabdomyolysis. Toxic statin myopathy is caused by the direct effect of statins on the muscle cells, leading to muscle damage and inflammation. It usually occurs within the first few months of starting statin therapy. Still, it can also happen later and is more likely to occur with higher doses of statins, or in combination with other drugs or factors that increase the blood levels of statins. Toxic statin myopathy can be prevented or treated by lowering the dose of statins, switching to a different type of statin, avoiding drug interactions, or stopping statin therapy altogether [7,8,9].
Rhabdomyolysis is a severe and potentially life-threatening response to certain medications. It typically manifests with muscle problems such as weakness, muscle pain, and the presence of dark urine. These symptoms may occur with or without an increase in creatine kinase (CK) levels in the blood. The connection between rhabdomyolysis and acute renal failure (ARF) is crucial to understand, as ARF is the most severe and immediately life-threatening consequence of rhabdomyolysis. Approximately 10 to 40% of individuals with rhabdomyolysis end up experiencing ARF, and as many as 15% of all ARF cases can be traced back to rhabdomyolysis as the underlying cause [10].
Statin-Induced IMNM
Many studies demonstrated a clear association between statin exposure and positive anti-HMGCR antibodies. In 2010, the Johns Hopkins Cohort was the first to identify a novel antibody against 100,200-kDa proteins. Subsequent studies confirmed that this autoantibody specifically recognizes HMGCR, which is a 100-kDa protein forming 200-kDa dimers as well. Notably, a significant 83.3% of individuals aged 50 and above who had this condition were found to have been using statins, in contrast to 25% of those with dermatomyositis and 36.8% of those with polymyositis. This suggests a potential link between statin usage and the development of anti-HMGCR myopathy. They demonstrated that 63% of patients with positive HMGCR antibodies had a prior statin prescription [11••, 12]. The differences between self-limited statin myopathy and statin-induced IMNM are shown in Table 2.
Statin Intolerance
Statin intolerance can refer to adverse symptoms that the patient perceives as unacceptable and/or abnormal laboratory tests that suggest undue risk that can be related to statins and lead to discontinuation of statin therapy [13]. Generally, discontinuation of statin utilization due to muscle complaints is more common than discontinuation due to laboratory abnormalities [14, 15]. Statin intolerance (SI) is defined differently by various organizations. The International Lipid Expert Panel (ILEP) defines it as the inability to tolerate a dose of statin required to reduce an individual’s cardiovascular risk sufficiently [15]. The National Lipid Association (NLA) has a broader definition, including any adverse effects that affect the quality of life and lead to the decision to decrease or stop using this drug [13]. The Luso-Latin American Consortium (LLAC) and the Canadian Consensus Working Group (CCWG) have similar definitions, referring to an inability to tolerate ≥ 2 statins at any dose or an inability to tolerate increasing doses, with symptoms not attributable to drug–drug interactions or conditions are known to increase SI [16, 17]. Considering the definitions mentioned above, we suggest the following definition of true statin intolerance: The intolerance of any dose of statin, regardless of dosing schedule or brand specificity, due to intolerable side effects that prevent the use of a statin in doses that effectively lower serum lipids.
Risk Factors
Clinical risk factors for statin intolerance can include any of the following: pre-existing neuromuscular disorders, advanced age, including those over age 75 in particular [18], female gender [19], obesity, and related metabolic syndrome [20]; vitamin D deficiency [21], uncontrolled hypothyroidism [22], chronic kidney disease, and excessive alcohol consumption; concomitant liver disease, metabolic muscle disorders, and a family history of statin intolerance; and Asian ethnicity [23]. According to recent studies, American Indian ethnicity may also be a risk factor for statin-associated IMNM; therefore, clinicians who serve American Indians need to be familiar with its presentations [24••, 25•].
Drug Interactions
There are several drug interactions that are well-known instigators of statin intolerance in combination with statins. These include CYP3A4 inhibitors/substrate, CYP2C9 inhibitors/substrate, and some other drugs like gemfibrozil, warfarin, P-glycoproteins, digoxin, niacin, and colchicine [8].
Of note, colchicine treats various conditions, including cardiovascular, auto-inflammatory diseases, and gout. In 2019, over 2.7 million prescriptions for colchicine and 112.3 million for statins were filled in the United States. Both medications can cause muscle damage, with colchicine known for its vacuolar myopathy; thus, taken together, the risk of these adverse effects may increase [26].
Diagnostic Approach
When a patient taking statins experiences muscle-related symptoms, it is important to investigate whether the statin therapy has caused these symptoms. This involves measuring CK levels, analyzing risk factors for intolerance or other potential causes of the symptoms, and assessing the impact of temporarily stopping and reintroducing the statin. Therefore, identifying true cases of statin intolerance is of great practical importance to avoid unnecessary discontinuation of statins from patients who would otherwise benefit from them [27]. The American College of Cardiology has recently developed an ACC Statin Intolerance App to aid clinicians in evaluating and managing patients who report muscle symptoms while on a statin (http://www.acc.org/StatinIntoleranceApp). Checking CK at the initiation of statin is not recommended. Most patients on statins with elevated transaminase levels do not have their CK checked in a timely manner; however, finding an elevated CK may prevent further unnecessary testing of liver status, reduce healthcare costs, and allow for earlier treatment of statin-induced muscle damage [28]. Most notably, when the AST is greater than ALT, a pattern typical of statin myopathy suggests that the transaminases are of skeletal muscle origins.
If a patient is on a statin and experiences muscle symptoms, we suggest clinicians use the SAMS-CI (Statin-Associated Muscle Symptoms Clinical Index) to evaluate the likelihood of self-limited (non-autoimmune) statin-related muscle symptoms. SAM-CI is a numeric scale for assessing the likelihood that the patient’s muscle symptom is related to statin use. It focuses on the location and pattern of the muscle symptoms, timing of the symptoms, improvement, and recurrence when physicians rechallenge the patient with a statin regimen. It also depends on how many statin regimens the patient has tried. Different scores lead to different treatment strategies, ranging from statin rechallenge to discontinuation [29]. We advise physicians to monitor CK levels in patients on statin therapy who show any signs of myalgia + / − weakness. We also recommend CK testing if LFTs are incidentally elevated (Fig. 1).
Molecular Mechanism and Pathogenesis
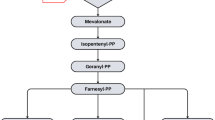
Statin-associated myopathy (SAM) can be implicated in several mechanisms, namely mitochondrial function alteration, oxidative stress, and impaired formation of downstream products of HMG-CoA reductase, including mevalonate, farnesyl pyrophosphate, geranylgeranyl pyrophosphate, exacerbation of the underlying neuromuscular disorders, placebo/nocebo effects, exercise-induced, patient with increased pain sensitivity, and anti-HMGCR antibody-mediated IMNM [30,31,32••].
Mitochondrial dysfunction is believed to have a significant implication on SAM because of the enormous amount of energy consumption by the skeletal muscle and their dependence on mitochondria. The proposed mechanisms are depletion of coenzyme Q10 due to inhibition of the mevalonate pathway by statin, presented with mitochondrial dysfunction [30, 33]. Also, statin-induced reactive oxygen species (ROS) accumulation in the fast glycolytic muscle fibers cells, which have relatively lower antioxidant capacity, causes reduced phosphorylation of protein kinase B (Akt) pathway, which leads to disruption of Akt pathway and ultimately leads to mitochondrial depletion. It is worth mentioning that Akt pathway disruption may be caused by transcription and translation rate reduction of peroxisome proliferator-activated receptor gamma coactivator (PGC)-1 alpha and PGC-1 beta [33]. It is reported that statin causes direct inhibition of the electron transport chain (ETC) in mitochondria, which may be directly related to SAM. Besides, ETC inhibition (specifically, complex 1 inhibition) by statins leads to dysregulation of calcium metabolism, which may lead to abnormal excitation–contraction coupling in skeletal muscle cells and may also implicated in SAM. Mitochondrial dysfunction, induced by statin, also correlates with the intrinsic apoptosis pathway’s abnormal activation due to a decreased ratio of the Bcl-2/Bax gene. Though there are many in vitro studies regarding this mechanism, there are no significant in vivo studies about the apoptotic effects of statin. Also, lactone, the inactive prodrug form of statin, has been shown to have a higher concentration in individuals affected by SAM. Lactone is more likely to impair ETC complex III, which may amplify muscle damage caused by statin [33].
Polymorphism of several genes, related to encoding protein for different mitochondrial processes, is also implicated in SAM [30, 33]. Among them, the carnitine palmitoyltransferase (CPT) enzyme system, which is responsible for long-chain fatty acid transport from cytosol to mitochondria, specifically CPT2 deficiency, can cause features of SAM after statin therapy and stress (e.g., infection, severe exercise) [30, 33]. On the other hand, glycine amidinotransferase (GATM), which catalyzes the first step of creatine biosynthesis, following mutations of GATM rs9806699 G > A, rs1719247 C > T and rs1346268 T > C might provide a protective factor for SAM [30, 33, 34].
There are multiple genes involved in statin metabolism. Among them, CYPs genes (involved in phase 1 hydroxylation), UGTs (uridine 5′-diphospho-glucoronosyltransferasees) (involved in phase 2 glucuronidation), SLCO1B1 (solute carrier organic anion transporter family member 1B1) (in influx transporter), and ABCB1(ATP-binding cassette subfamily B member 1) and ABCG2 (efflux transporters) are significant [7, 35]. Among these, SLCO1B1 encodes OATP1B1 (organic anion-transporting polypeptide 1B1). OATP1B1 is a hepatocyte-specific sinusoidal influx transporter. In SLCO1B1, SNP (single-nucleotide polymorphism) rs4149056 (521T > C) is consistently linked with statin-associated myopathy [7, 36,37,38,39,40]. All the other mechanisms stated above may alter statin exposure but are not directly related to statin-associated myopathy [7]. For patients with underlying neuromuscular disorders (myasthenia gravis, motor neuron disease, MELAS, dermatomyositis, polymyositis), untreated hypothyroid myopathy has an increased risk of SAM after statin therapy [30].
Anti-HMGCR antibody-mediated myopathy is strongly related to Class II MHC allele DRB1*11:01 in the case of adults, and in the case of children, it is related to DRB1*07:01 [41]. It was established that most of the initial cases with anti-HMGCR had a previous statin exposure. However, statin exposure was not mentioned in some cases, especially in the Asian population. This may be explained by the presence of natural statin in their foods (oyster mushroom, red yeast rice, etc.), which requires further investigation [31, 41, 42]. It was discovered that anti-HMGCR antibodies target ~ 100-kDa and ~ 200-kDa proteins, where ~ 100 kDa is a monomer and ~ 200 kDa is a dimer [12]. Statin exposure causes excessive expression of the HMGCR, ultimately leading to aberrant protein processing, which may cause neoantigen production. After the beginning of the autoimmune destruction, continuous statin exposure for overexpression of HMGCR is unnecessary as the repaired muscle cells naturally express HMGCR [41]. It requires not only stopping statin but also immunomodulator medication to treat myopathy [31].
A recent study described HMGCR mutation causing limb-girdle muscle disease (LGMD) in humans for the first time [32••]. It showed that LGMD greatly overlaps with the presentation of statin-associated myopathy, including proximal myopathy. As LGMD starts in the fourth decade of life, statin-induced myopathy is also more common and severe in advancing age. Like statin-associated myopathy, female patients are affected more severely and more at younger ages in the LGMD [32••]. Also, just like end-stage statin myopathy and immune-mediated necrotizing myopathy without edema, the MRI picture in the case of LGMD showed mainly large proximal and axial muscle involvement with fatty replacement and atrophy [32••, 41]. The study also exhibits a murine model in which a group of mice previously exposed to statins to develop statin-induced myopathy improved muscle strength when they were given free access to mevalonolactone water [32••]. This finding may indicate that the pathogenesis of SAM may be associated with mutation of the HMGCR gene itself, which may cause accumulative defects. Further studies/research is needed to reach a final conclusion.
Self-limited toxic statin myopathy usually does not show the characteristic anti-HMGCR antibody. However, biopsy shows necrotizing and regenerating muscle fibers and expression of MHC I protein on the regenerating muscle fibers [32••, 43]. This finding makes distinguishing it from IMNM difficult and usually requires negative anti-HMGCR antibody testing.
Risk of SAMS with Different Statins
Muscle injury is thought to be affected by the type and amount of statin used [44]. The incidence of myopathy appears to be lowest with fluvastatin, pravastatin, and pitavastatin, possibly due to the lack of metabolism by cytochrome P450 3A4 (CYP3A4) and are thus less likely to be involved with drug interactions [45, 46]. The lipophilicity of statins is also controversial. It is believed that hydrophilic statins (pravastatin, rosuvastatin) may cause fewer muscular events than lipophilic statins (e.g., simvastatin, atorvastatin) due to lower passive diffusion into muscle cells. A recent observational cohort study showed no significant difference between hydrophilic and lipophilic statins regarding muscular events. However, more studies are needed before implications for clinical use [47].
Natural Statins
In addition to medications, statin is present in mushrooms [48], red yeast rice [49], and Pu-erh tea [50]. A recent study reported the exacerbation of adult anti-HMGCR myopathy after mushroom intake [51]. Another case report showed that one out of six patients with positive anti-HMGCR myopathy, which presumed limb-girdle muscular dystrophy (LGMD), had a history of mushroom supplement intake. These studies indicate that mushroom supplements can trigger IMNM [52••]. Therefore, food-derived statins may be a risk factor for anti-HMGCR myopathy in patients who do not receive statin-based medication. Red yeast rice is known for containing monacolin K, similar to lovastatin in terms of pharmaceutical features. Some supplements also contain statins. A recent case report showed that the Bacopa supplement, frequently used in Ayurvedic medicine regimens, may trigger IMNM as it has the ability to inhibit HMGCR [53].
Management
Possible Strategies to Reduce SAMS Prevalence
Coenzyme Q10 (Ubiquinone)
Statins not only decrease cholesterol synthesis but also significantly decrease the synthesis of CoQ10. While there is a lack of evidence demonstrating the effectiveness of coenzyme Q10 in reducing statin-associated muscle symptoms, it is still commonly prescribed in clinical practice to alleviate such symptoms. A recent meta-analysis indicates that coenzyme Q10 (CoQ10) supplementation does not significantly affect statin-induced myopathy, as measured by creatine kinase (CK) activity and muscle pain scores. The study analyzed eight randomized controlled trials with a total of 427 participants and found no evidence of publication bias [54]. Thus, we do not recommend CoQ10 routinely. However, if patients insist on trying this supplement, we do not disagree as it is unlikely to cause harm.
Vitamin D Supplement
A correlation between vitamin D deficiency and SAMS has been suggested, but the impact of statin on vitamin D levels is still uncertain. According to a recent meta-analysis of cohort studies, patients with muscle complaints related to statin use had lower levels of 25OHD in their blood than patients who did not have such side effects. Most patients with muscle intolerance to statins and vitamin D deficiency could resume statin treatment after taking vitamin D supplements. However, the exact link between muscle problems and vitamin D deficiency due to statins remains unclear. It might be helpful to check the blood levels of vitamin D before giving statins to patients and to provide vitamin D supplements to patients with insufficient blood vitamin D levels to reduce the risk of SAMS [55,56,57].
Ascorbic Acid
A recent study on mice suggests ascorbic acid can reduce creatine kinase (CK) levels in statin-induced muscle injuries. Moreover, this study observed that supplementation with ascorbic acid increased the swimming tolerance time of the mice. These findings suggest that vitamin C may positively impact muscle endurance and overall physical performance, particularly in the context of statin-induced muscle injuries [58].
Alternative Drugs for Statin-Intolerant Patients
Mevalonolactone
A recent article described an autosomal recessive progressive limb-girdle muscle disease caused by a partial loss of function missense mutation in HMGCR that encodes a key enzyme of the mevalonate pathway in six patients. Treatment with mevalonolactone showed improved muscle strength and increased peak expiratory flow, forced vital capacity, and FEV1 in patients. Improvement in the function of activities in daily living was noticed as well. They also tested mevalonolactone on a murine model of severe statin-induced myopathy. This study showed that oral mevalonolactone can protect mice from myotoxicity induced by high-dose statin [32••]. This may prove to have efficacy for statin-related myotoxicity in humans.
Bempedoic Acid
A recent meta-analysis demonstrated the significant efficacy of bempedoic acid in reducing major adverse cardiac events [59]. In a double-masked, randomized, placebo-controlled trial involving 13,970 patients aged 18 to 85 across 32 counties, it was observed that statin-intolerant patients using bempedoic acid experienced a significantly lower risk of major cardiovascular adverse events, including cardiovascular-related death, nonfatal stroke, nonfatal myocardial infarction, and coronary revascularization compared to the control group. Furthermore, the bempedoic acid group demonstrated a more pronounced reduction in high-sensitivity C-reactive protein (hs-CRP) levels than the placebo group. However, patients on bempedoic acid showed a higher incidence of hyperuricemia, gout, and cholelithiasis than the placebo group. Notably, the two groups had no significant difference concerning myalgia and rhabdomyolysis [60]. Another trial has shown that monotherapy with 180 mg daily bempedoic acid in patients with hyperlipidemia who cannot tolerate statins due to muscular adverse effects can lower the LDL-C levels by approximately 24.5% to placebo [61].
Ezetimibe and PCSK9 Inhibitors
Ezetimibe is recommended as the second line of therapy for statin-intolerant patients by the American College of Cardiology. If the effect of ezetimibe is not enough to lower LDL-C, adding the PCSK9 inhibitors is recommended [62]. In particular, PCSK9 inhibitors are generally considered safe and well tolerated in patients with positive anti-HMGCR statin myopathy where statins are absolutely contraindicated. In a small study, 8 out of 122 patients with anti-HMGCR IMNM evaluated at the Johns Hopkins Myositis Center with severe cardiovascular disease began treatment with PCSK9 inhibitors. During the follow-up period, no decrease in hip flexion or arm abductor muscle strength was observed [63••].
Exercise
Previous studies indicate that statin exacerbates muscle injury with eccentric or high-intensity exercise, as evidenced by higher post-exercise CK levels compared to a control group. They suggested clinicians consider statin discontinuation for several days before endurance physical events like marathons [64, 65]. In contrast, another study found that statin-induced myopathy is neither aggravated by physical activity nor prevented by fitness training in mice with high cholesterol levels [66]. A recent study showed that statin users can have a physically active lifestyle and perform moderate-intensity exercise without having skeletal muscle injury exacerbation and elevation in muscle enzymes such as LDH and CK. This approach could be crucial in mitigating the risk of cardiovascular disease. They also measured cardiac muscle markers, such as cTnI and NT-proBNP, to assess damage to the heart muscle and showed no significant differences between those experiencing symptoms and those without symptoms among statin users, as well as the control group [67].
Conclusion
Statins are widely used for the management of hyperlipidemia and have been shown to reduce the risk of cardiovascular events. However, they are associated with a range of adverse effects, including self-limited statin toxicity, and statin-induced IMNM. The mechanisms underlying these adverse effects are complex and multifactorial, involving mitochondrial dysfunction, oxidative stress, and immune-mediated mechanisms. Effective management of SAMS requires a thorough evaluation of the patient’s symptoms, risk factors, and medication history, as well as consideration of alternative treatment options. Ongoing research is needed to elucidate the mechanisms of statin-associated muscle involvement further and develop more effective strategies for preventing and managing these adverse effects.
Data Availability
No datasets were generated or analyzed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cardiovascular diseases (CVDs) [Internet]. [cited 2023 Oct 12]. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292(5519):1160–4.
Blais JE, Wei Y, Yap KKW, Alwafi H, Ma TT, Brauer R, et al. Trends in lipid-modifying agent use in 83 countries. Atherosclerosis. 2021;328:44–51.
Guadamuz JS, Shooshtari A, Qato DM. Global, regional and national trends in statin utilisation in high-income and low/middle-income countries, 2015–2020. BMJ Open. 2022;12(9):e061350.
Rosenson RS, Baker SK, Jacobson TA, Kopecky SL, Parker BA, The National Lipid Association’s Muscle Safety Expert Panel null. An assessment by the Statin Muscle Safety Task Force: 2014 update. J Clin Lipidol. 2014;8(3 Suppl):S58-71.
Mohassel P, Mammen AL. The spectrum of statin myopathy. Curr Opin Rheumatol. 2013;25(6):747–52.
Turner RM, Pirmohamed M. Statin-related myotoxicity: a comprehensive review of pharmacokinetic, pharmacogenomic and muscle components. J Clin Med. 2019;9(1):22.
Norata GD, Tibolla G, Catapano AL. Statins and skeletal muscles toxicity: from clinical trials to everyday practice. Pharmacol Res. 2014;88:107–13.
Li JJ, Liu HH, Wu NQ, Yeo KK, Tan K, Ako J, et al. Statin intolerance: an updated, narrative review mainly focusing on muscle adverse effects. Expert Opin Drug Metab Toxicol. 2020;16(9):837–51.
Kasaoka S, Todani M, Kaneko T, Kawamura Y, Oda Y, Tsuruta R, et al. Peak value of blood myoglobin predicts acute renal failure induced by rhabdomyolysis. J Crit Care. 2010;25(4):601–4.
Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200 and 100 kDa proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum. 2010;62(9):2757–66 This study defined the first description of the anti-HMGCR autoantibody.
Mammen AL, Chung T, Christopher-Stine L, Rosen P, Rosen A, Doering KR, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63(3):713–21.
Guyton JR, Bays HE, Grundy SM, Jacobson TA, The National Lipid Association Statin Intolerance Panel null. An assessment by the Statin Intolerance Panel: 2014 update. J Clin Lipidol. 2014;8(3 Suppl):S72-81.
Wei MY, Ito MK, Cohen JD, Brinton EA, Jacobson TA. Predictors of statin adherence, switching, and discontinuation in the USAGE survey: understanding the use of statins in America and gaps in patient education. J Clin Lipidol. 2013;7(5):472–83.
Banach M, Rizzo M, Toth PP, Farnier M, Davidson MH, Al-Rasadi K, et al. Statin intolerance - an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch Med Sci. 2015;11(1):1–23.
Mancini GBJ, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Consensus Working Group Update (2016). Can J Cardiol. 2016;32(7 Suppl):S35-65.
Sposito AC, Faria Neto JR, de Carvalho LSF, Lorenzatti A, Cafferata A, Elikir G, et al. Statin-associated muscle symptoms: position paper from the Luso-Latin American Consortium. Curr Med Res Opin. 2017;33(2):239–51.
Roberts CGP, Guallar E, Rodriguez A. Efficacy and safety of statin monotherapy in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2007;62(8):879–87.
Karalis DG, Wild RA, Maki KC, Gaskins R, Jacobson TA, Sponseller CA, et al. Gender differences in side effects and attitudes regarding statin use in the Understanding Statin Use in America and Gaps in Patient Education (USAGE) study. J Clin Lipidol. 2016;10(4):833–41.
Brinton EA, Maki KC, Jacobson TA, Sponseller CA, Cohen JD. Metabolic syndrome is associated with muscle symptoms among statin users. J Clin Lipidol. 2016;10(4):1022–9.
Michalska-Kasiczak M, Sahebkar A, Mikhailidis DP, Rysz J, Muntner P, Toth PP, et al. Analysis of vitamin D levels in patients with and without statin-associated myalgia - a systematic review and meta-analysis of 7 studies with 2420 patients. Int J Cardiol. 2015;15(178):111–6.
Robison CD, Bair TL, Horne BD, McCubrey RO, Lappe DL, Muhlestein JB, et al. Hypothyroidism as a risk factor for statin intolerance. J Clin Lipidol. 2014;8(4):401–7.
Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med. 2009;150(12):858–68.
Muruganandam M, Iqbal A, Akpan EB, Dolomisiewicz AC, Waters YM, Emil NS, et al. Statin-associated immune-mediated necrotizing myositis in Native Americans. Rheumatology (Oxford). 2022;61(12):4855–62 This study is one of several publications highlighting the increased risk of autoimmune statin myopathy in Native American patients.
Close RM, Close LM, Galdun P, Gerstberger S, Rydberg M, Christopher-Stine L. Potential implications of six American Indian patients with myopathy, statin exposure and anti-HMGCR antibodies. Rheumatology (Oxford). 2021;60(2):692–8 This study is the first report in the medical literature about the potential increased risk of anti-HMGCR myopathy in Native Americans with elevated lipids.
Schwier NC, Cornelio CK, Boylan PM. A systematic review of the drug-drug interaction between statins and colchicine: patient characteristics, etiologies, and clinical management strategies. Pharmacotherapy. 2022;42(4):320–33.
Stulc T, Ceška R, Gotto AM. Statin intolerance: the clinician’s perspective. Curr Atheroscler Rep. 2015;17(12):69.
Nidhaan A, Hastings A, Gonzalez D, Joshi R, Gilbert N, O’connor C. Frequency of checking creatine kinase in patients on statins with elevated transaminase for early detection of statin induced myopathy [abstract]. Arthritis Rheumatol. 2022;74 (suppl 9). https://acrabstracts.org/abstract/frequency-of-checking-creatine-kinase-in-patients-on-statins-with-elevated-transaminase-for-early-detection-of-statin-induced-myopathy/. Accessed Nov 8, 2023
Rosenson RS, Miller K, Bayliss M, Sanchez RJ, Baccara-Dinet MT, Chibedi-De-Roche D, et al. The Statin-Associated Muscle Symptom Clinical Index (SAMS-CI): revision for clinical use, content validation, and inter-rater reliability. Cardiovasc Drugs Ther. 2017;31(2):179–86.
Vinci P, Panizon E, Tosoni LM, Cerrato C, Pellicori F, Mearelli F, et al. Statin-associated myopathy: emphasis on mechanisms and targeted therapy. Int J Mol Sci. 2021;22(21):11687.
Selva-O’Callaghan A, Alvarado-Cardenas M, Pinal-Fernández I, Trallero-Araguás E, Milisenda JC, Martínez MÁ, et al. Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Expert Rev Clin Immunol. 2018;14(3):215–24.
Yogev Y, Shorer Z, Koifman A, Wormser O, Drabkin M, Halperin D, et al. Limb girdle muscular disease caused by HMGCR mutation and statin myopathy treatable with mevalonolactone. Proc Natl Acad Sci. 2023;120(7):e2217831120 This study shows that mevalonolactone can protect mice from myotoxicity induced by high-dose statin. This could be applicable in some anti-HMGCR autoantibody positive patients.
Mollazadeh H, Tavana E, Fanni G, Bo S, Banach M, Pirro M, et al. Effects of statins on mitochondrial pathways. J Cachexia Sarcopenia Muscle. 2021;12(2):237–51.
Liu M, Fan F, Zhang Y, Li J. The association of GATM polymorphism with statin-induced myopathy: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2021;77(3):349–57.
Davies JT, Delfino SF, Feinberg CE, Johnson MF, Nappi VL, Olinger JT, et al. Current and emerging uses of statins in clinical therapeutics: a review. Lipid Insights. 2016;14(9):13–29.
Xiang Q, Chen SQ, Ma LY, Hu K, Zhang Z, Mu GY, et al. Association between SLCO1B1 T521C polymorphism and risk of statin-induced myopathy: a meta-analysis. Pharmacogenomics J. 2018;18(6):721–9.
Balasubramanian R, Maideen NMP. HMG-CoA reductase inhibitors (statins) and their drug interactions involving CYP enzymes, P-glycoprotein and OATP transporters-an overview. Curr Drug Metab. 2021;22(5):328–41.
Shahrure ZM, Irshaid YM, Mustafa KN, Abujbara MA, Al Shhab M, El-Khateeb MS, et al. SLCO1B1 gene polymorphisms (rs2306283 and rs4149056) and statin-induced myopathy in Jordanian diabetics. Curr Rev Clin Exp Pharmacol. 2021;16(3):281–8.
Xiang Q, Zhang XD, Mu GY, Wang Z, Liu ZY, Xie QF, et al. Correlation between single-nucleotide polymorphisms and statin-induced myopathy: a mixed-effects model meta-analysis. Eur J Clin Pharmacol. 2021;77(4):569–81.
Turongkaravee S, Jittikoon J, Lukkunaprasit T, Sangroongruangsri S, Chaikledkaew U, Thakkinstian A. A systematic review and meta-analysis of genotype-based and individualized data analysis of SLCO1B1 gene and statin-induced myopathy. Pharmacogenomics J. 2021;21(3):296–307.
Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Immune-mediated necrotizing myopathy. Curr Rheumatol Rep. 2018;20(4):21.
Selva-O’Callaghan A, Alvarado-Cardenas M, Marin A, Pinal-Fernandez I. Statins and myositis: the role of anti-HMGCR antibodies. Expert Rev Clin Immunol. 2015;11(12):1277–9.
Chung T, Christopher-Stine L, Paik JJ, Corse A, Mammen AL. The composition of cellular infiltrates in anti-HMG-CoA reductase-associated myopathy. Muscle Nerve. 2015;52(2):189–95.
Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289(13):1681–90.
Wiggins BS, Saseen JJ, Page RL, Reed BN, Sneed K, Kostis JB, et al. Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2016;134(21):e468–95.
Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients–the PRIMO study. Cardiovasc Drugs Ther. 2005;19(6):403–14.
Mueller AM, Liakoni E, Schneider C, Burkard T, Jick SS, Krähenbühl S, et al. The risk of muscular events among new users of hydrophilic and lipophilic statins: an observational cohort study. J Gen Intern Med. 2021;36(9):2639–47.
Lo YC, Lin SY, Ulziijargal E, Chen SY, Chien RC, Tzou YJ, et al. Comparative study of contents of several bioactive components in fruiting bodies and mycelia of culinary-medicinal mushrooms. Int J Med Mushrooms. 2012;14(4):357–63.
Klimek M, Wang S, Ogunkanmi A. Safety and efficacy of red yeast rice (Monascus purpureus) as an alternative therapy for hyperlipidemia. P T. 2009;34(6):313–27.
Jeng KC, Chen CS, Fang YP, Hou RCW, Chen YS. Effect of microbial fermentation on content of statin, GABA, and polyphenols in Pu-Erh tea. J Agric Food Chem. 2007;55(21):8787–92.
Adler B, Christopher-Stine L, Tiniakou E. Mushroom supplements triggering a flare of HMGCR immune mediated necrotising myopathy. BMJ Case Rep. 2022;15(5):e248880.
Mohassel P, Landon-Cardinal O, Foley AR, Donkervoort S, Pak KS, Wahl C, et al. Anti-HMGCR myopathy may resemble limb-girdle muscular dystrophy. Neurol Neuroimmunol Neuroinflamm. 2018;6(1):e523 This study reminds clinicians that children (and possibly some young adults) with suspected muscular dystrophy and very high CK levels should be screened for anti-HMGCR autoantibodies.
Yaworski AM, Blyumin M, Chang T, Mammen AL, Greene M. Necrotizing myopathy with elevated anti-HMGCR antibodies following exposure to the supplement Bacopa. Muscle Nerve. 2023;67(2):E1-3.
Wei H, Xin X, Zhang J, Xie Q, Naveed M, Kaiyan C, et al. Effects of coenzyme Q10 supplementation on statin-induced myopathy: a meta-analysis of randomized controlled trials. Ir J Med Sci. 2022;191(2):719–25.
Hou Q, Pang C, Chen Y. Association between vitamin D and statin-related myopathy: a meta-analysis. Am J Cardiovasc Drugs. 2022;22(2):183–93.
Gupta A, Thompson PD. The relationship of vitamin D deficiency to statin myopathy. Atherosclerosis. 2011;215(1):23–9.
Hlatky MA, Gonzalez PE, Manson JE, Buring JE, Lee IM, Cook NR, et al. Statin-associated muscle symptoms among new statin users randomly assigned to vitamin D or placebo. JAMA Cardiol. 2023;8(1):74–80.
Zabihi M, Askarian F, Hekmatimoghaddam S, Rashidi Nooshabadi M, Zabihi MS, Mousavinasab SR. Ascorbic acid significantly decreases creatine kinase plasma levels in an animal model of statin/fibrate-induced myopathy. Adv Pharmacol Pharm Sci. 2021;2021:5539595.
Krishna Mohan GV, Chenna VSH, Tirumandyam G, Mian AR, Rashid A, Saleem F. Efficacy and safety of bempedoic acid to prevent cardiovascular events in individuals at risk of cardiovascular diseases: a meta-analysis of randomized-control trials. Cureus. 15(5):e38662
Nissen SE, Lincoff AM, Brennan D, Ray KK, Mason D, Kastelein JJP, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. 2023;388(15):1353–64.
Laufs U, Banach M, Mancini GBJ, Gaudet D, Bloedon LT, Sterling LR, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7):e011662.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):3168–209.
Tiniakou E, Rivera E, Mammen AL, Christopher-Stine L. Use of proprotein convertase subtilisin/kexin type 9 inhibitors in statin-associated immune-mediated necrotizing myopathy: a case series. Arthritis Rheumatol. 2019;71(10):1723–6 PCSK9 inhibitors appear to be a safe non-statin alternative treatment for lipid lowering in patients with anti-HMGCR autoantibodies and persistent hypercholesterolemia.
Parker BA, Augeri AL, Capizzi JA, Ballard KD, Troyanos C, Baggish AL, et al. Effect of statins on creatine kinase levels before and after a marathon run. Am J Cardiol. 2012;109(2):282–7.
Thompson PD, Zmuda JM, Domalik LJ, Zimet RJ, Staggers J, Guyton JR. Lovastatin increases exercise-induced skeletal muscle injury. Metabolism. 1997;46(10):1206–10.
Chung HR, Vakil M, Munroe M, Parikh A, Meador BM, Wu PT, et al. The impact of exercise on statin-associated skeletal muscle myopathy. PLoS ONE. 2016;11(12):e0168065.
Allard NAE, Janssen L, Lagerwaard B, Nuijten MAH, Bongers CCWG, Rodenburg RJ, et al. Prolonged moderate-intensity exercise does not increase muscle injury markers in symptomatic or asymptomatic statin users. J Am Coll Cardiol. 2023;81(14):1353–64.
Author information
Authors and Affiliations
Contributions
TF and IK wrote the main manuscript text, and TF and LCS prepared fall tables and figures. LCS critically reviewed the manuscript, edited for content and clarity, and provided additional editorial support and writing. All authors reviewed the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Christopher-Stine has an intellectual property interest in the anti-HMGCR autoantibody testing and received royalties from Inova Diagnostics/Werfen for licensing of the anti-HMGCR antibody testing.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rastegar, T.F., Khan, I.A. & Christopher-Stine, L. Decoding the Intricacies of Statin-Associated Muscle Symptoms. Curr Rheumatol Rep 26, 260–268 (2024). https://doi.org/10.1007/s11926-024-01143-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-024-01143-y