Abstract
Purpose of the Review
Erosive hand osteoarthritis (EHOA) is an aggressive form of hand osteoarthritis that leads to significant disability, and recent data suggests that it is increasing in prevalence. This review provides an update of our current understanding of epidemiology, genetic associations, biomarkers, pathogenesis, and treatment of EHOA, with particular focus on studies published within the last 5 years.
Recent Findings
New studies of EHOA have identified new genetic loci associated with disease, including variants in genes involved in inflammation and bone remodeling. Preclinical studies implicate pathways of innate immunity, including some that may be causal in the condition. Recent novel studies showed that inflammatory features identified by ultrasound and MRI are associated with development of erosive lesions over time on conventional radiography. In the future, these imaging modalities may be useful in identifying patients at risk of adverse outcomes.
Summary
Promising new findings in genetics, biomarkers, and treatment targets will hopefully allow for future therapeutic options for this debilitating condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
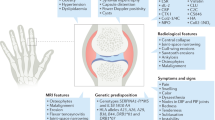
Osteoarthritis (OA) is the most common form of arthritis and a leading cause of musculoskeletal-related disability. The joints of the hand, specifically the proximal and distal interphalangeal joints (PIP and DIPs) and the first carpometacarpal (CMC) joints, are commonly affected by this disease, and hand osteoarthritis (HOA) has a significant impact on the quality of life [1]. Some patients with HOA may suffer from erosive hand osteoarthritis (EHOA), a subtype of disease which tends to be even more disabling than non-erosive HOA [2]. EHOA is an aggressive, inflammatory clinical phenotype of HOA with distinct radiographic findings initially described in 1961 by Crain [3,4,5,6]. His term “interphalangeal arthritis” described 23 women who had inflammatory episodes of OA affecting the DIP and PIP joints, with radiographic findings of bone destruction and digit subluxation. Later in 1966, the term erosive OA was used to describe six women with similar findings [7].
Despite decades of research dedicated to understanding this condition, there are still no disease-modifying treatments that halt or reverse the disease and limited therapeutic choices to address the disability caused by EHOA. This is at least partly due to difficulties in identifying patients with EHOA at early stages and a poor understanding of mechanisms leading to joint destruction, particularly bone erosions. This review will provide an update of our current understanding of the epidemiology, genetic associations, biomarkers, pathogenesis, and treatment of EHOA, with particular focus on studies published within the last 5 years.
Epidemiology
HOA is a very common condition, with prevalence increasing. The most recent estimates of prevalence come from the Global Burden of Disease Study. As of 2019, the worldwide prevalence of OA was estimated to be over 528 million [8]. The hand represents the second most common joint location of OA, after the knee, affecting an estimated 142 million people worldwide, though older studies suggest that radiographic HOA may be more prevalent than clinical HOA [9]. This is significantly more common than RA, which as of 2019 had a global prevalence of 18.56 million. Since 1990, the prevalence of HOA has increased by over 91% [8]. This is in part due to increased recognition of this disease and the aging population.
There have been challenges to studying the epidemiology of EHOA. Namely, there is a lack of clearly defined diagnostic or classification criteria which leads to difficulty in characterizing EHOA in clinical studies [1, 2]. In addition, methods used to assess symptomatic disease or radiographic disease in studies can also lead to variable results, and many studies are done on populations in individual countries which may have disparate genetic backgrounds [10]. Most studies agree that EHOA is common and is a condition that affects older women disproportionately, but the range in prevalence estimates varies widely based on the distinct populations studied. For example, in a UK study of 1076 community-dwelling adults with hand symptoms, the prevalence of EHOA in the study population with symptomatic HOA was 4.8%, which is higher than the prevalence extrapolated from the UK general population based on survey data [11]. In a small Venetian town of 630 people, the prevalence was noted to be 2.7% of the general population studied and 8.5% of the patients with HOA [12]. The EHOA prevalence was noted to be as high as 61.9% in a Belgian study of HOA patients referred to rheumatology clinics [13].
Fortunately, investigators continue to study this condition and expand understanding of how EHOA affects our population. New data from the US-based Osteoarthritis Initiative, a multi-center longitudinal cohort study of adults with or at risk for knee OA, investigated the incidence and characteristics of EHOA in participants [14]. 2.6% of the participants developed EHOA over a 48-month period. Participants who developed EHOA were more likely older, female and white, and were more likely to have greater HOA at baseline. In addition, EHOA patients had lower BMI at baseline, but they were generally less physically active. Erosive changes occurred exclusively in joints which already had changes consistent with HOA. This finding adds to historical data supporting the presence of distinct clinical presentations, serological and radiographic features, and worse progression rates compared with non-erosive HOA, supporting the hypothesis that EHOA is a separate and more severe form of HOA [2, 15, 16].
Disability associated with HOA has also increased. According to the Global Burden of disease registry, the years living with disability (YLD) for individuals with HOA has nearly doubled since 1990 [8]. A recent small study demonstrated that patients with EHOA have a higher clinical burden of pain than those with non-erosive disease [17]. In addition, the QUALYOR study reported the prevalence of HOA and disability associated with this condition in a large cohort of postmenopausal women [18]. The study found that 28% of the population of 1189 patients had radiographic HOA and 40.5% of the total study population fulfilled ACR criteria for symptomatic HOA. The overall prevalence of symptomatic EHOA was 11.8%, and the study noted that 72.7% of patients with erosions were symptomatic. The study also demonstrated that patients with EHOA had lower grip strength and higher AUSCAN score, a patient-reported score measuring pain, stiffness, and function, than symptomatic patients without erosions. This confirms that patients with symptomatic EHOA have a higher level of disability compared with symptomatic, non-erosive HOA patients [18]. Similar to studies of OA affecting other joints, radiographic severity was not strongly correlated with severity of symptoms.
Genetic Associations
Prior research has suggested that EHOA can be heritable although it does not follow a simple pattern of Mendelian inheritance. A number of genetic variants have been associated with EHOA including gene variants involved in inflammation (i.e., IL1β and HLA alleles), joint development, and chondrocyte signaling (i.e., WNT9A) (reviewed in [2, 19]). Recent studies addressing genetic predisposition further inform our understanding of pathogenesis. A new retrospective population-based study by Kazmers et al. evaluated familial clustering of EHOA in a large statewide database and found a 5.5-fold greater risk of EHOA in first-degree relatives [20•]. It also confirmed risk factors, namely, female sex conferring a 3.48-fold risk of EHOA, with a suggestion that obesity was a risk factor primarily in females and diabetes in males. This study highlighted the heritability of EHOA; additional recent investigations shed more light into specific genetic risk factors for this condition.
Styrkarsdottir et al. recently published the first genome-wide association study of EHOA [21••]. Using a sample size of 1484 patients with EHOA and 550,680 controls from 5 populations, they identified four genetic loci that confer a high risk of EHOA and candidate causal genes that could in the future be potential targets for treatment. Specifically, the study confirmed an association with ALDH1A2 (aldehyde dehydrogenase 1 family member A2) and MGP (member of the osteocalcin/matrix Gla family of proteins) genes, which have been previously identified as risk alleles for HOA [22, 23]. The protein coded for by ALDH1A2 is involved in retinoic acid synthesis, and that of MGP may modulate ectopic calcification, both relevant to OA pathogenesis in other joints. In addition, these genes are expressed by chondrocytes and play a role in expression of nearby genes, so these polymorphisms could have broad impact. In addition, this study identified two new loci within BMP6 and SPP1/MEPE/IBSP, which code for proteins involved in bone and cartilage remodeling. Yet another recent study pointed to novel genetic associations with related inflammatory pathways, adding to the literature highlighting the role of inflammation in EHOA [10]. Jurynec et al. used genomic analysis of families in a statewide database of dominantly inherited OA to identify an association between NOD/RIPK2 polymorphisms and familial OA affecting the hand, shoulder, and foot [24••]. NOD/RIPK2 codes for an innate immune pattern-recognition receptor which controls cellular inflammatory responses. Further studies are needed to supplement our understanding of the mechanistic role that these genes play and whether the pathways they control can be targeted for therapy.
Biomarkers in HOA
Soluble Biomarkers
Several potential serum biomarkers have been suggested in EHOA, but none have been validated to diagnose or monitor the condition. These include erythrocyte sedimentation rate (ESR), soluble IL-2 receptor (sIL-2R), C-reactive protein (CRP), C-telopeptide of type I collagen (CTX I), collagenase cleavage neoepitope (Col2–3/4C), myeloperoxidase (MPO), vistafin, clusterin (CLU), type II collagen cleavage product (C2C), aggrecan epitope (CS846), hyaluronic acid (HA), and nitrated Coll2-1 (Coll2-1NO2) (reviewed in [2, 19]). A recent longitudinal cohort study measured sHA levels at baseline and over 6 years in patients with HOA compared to controls [25]. It showed that patients with HOA had higher baseline levels of serum hyaluronic acid (sHA), which correlated with radiographic severity and higher risk of development of HOA over 6 years. However, this study did not specifically evaluate EHOA. Another recent study by Baloun et al. evaluated a number of microRNAs (miRNAs, small non-coding RNAs that influence many physiological processes) detectable in HOA [26]. They identified three miRNAs, including two previously identified in KOA (miR-23a-3p and miR-146a-5p) and miR-652-3p, which were elevated in the plasma of patients with HOA compared with healthy controls. In this study, there was no significant difference in expression in these biomarkers between EHOA and non-erosive HOA, although these miRNAs also correlated with severity of symptoms and joint pain scores overall.
While synovial fluid (SF) is not easily accessible from IP joints, Oliviero and colleagues found higher levels of inflammatory cytokines (IL1β, IL6) and matrix metalloproteinases (MMP1, MMP3) in knee SF of patients with EHOA compared to non-erosive HOA [27•]. This is interesting in that it suggests that the more aggressive inflammatory nature of EHOA is not just limited to hand IP joint involvement but can be detected in other OA-affected joints in patients with this phenotype. Whether patients with EHOA are at greater risk of more rapid progression of OA at other joint locations is an important question that remains to be studied. In addition, future studies specifically in EHOA are necessary to develop markers that can identify this subset before erosive changes are evident.
Carbamylation-derived products such as homocitrulline and advanced glycation end-products such as carboxymethyllysine accumulate in tissues with aging and disease and have been examined in recent years as potential biomarkers in HOA. Serum homocitrulline and carboxymethyllysine were compared in patients with HOA and EHOA in a cohort of 386 patients. The investigators determined that serum carboxymethyllysine was lower in EHOA than in HOA, and using cartilage samples from cadaveric donors determined that carboxymethyllysine was higher in HOA than in non-HOA cartilage [28]. Further studies are needed to determine whether serum or tissue carboxymethyllysine has utility as a biomarker HOA.
Imaging Biomarkers
Central erosions at IP joints are the hallmark radiographic changes that distinguish EHOA from non-erosive HOA (Fig. 1A), and radiographic scoring systems have been developed to track the phasic changes specifically seen in EHOA. The most well-known of these is the Verbruggen-Veys (VV) score [29] which assesses progression of HOA as defined by five anatomical phases: normal (N phase), stationary (S phase), disappeared joint space (J phase), erosive lesions (E phase) and remodeled joint (R phase). A study by Neuprez et al. evaluated a cohort of 203 patients with HOA. Radiographs were recorded at baseline and clinical and radiographic progression assessed over 2 years, using both the well-known Kellgren-Lawrence (KL) score (which does not evaluate erosive changes) and the Verbruggen-Veys (VV) score [29]. The authors found that there was significant radiographic progression in the entire cohort and > 4 swollen joints and EHOA at baseline predicted new erosions over 2 years [30]. Despite more radiographic progression in the participants with EHOA at baseline, there was no significant difference in pain, function, or stiffness in this population over time. However, patients with at least four erosive joints and better function at baseline were more likely to have a decline in function at 2 years [30]. An analysis of 84 patients in the Netherlands showed that radiographic KL scores correlated very poorly with degree of pain, though patients did report high levels of pain on visual analogue scale (VAS, a score of pain from 0 to 10) [31]. Both of these studies confirm that degree of pain and radiographic severity are often discordant in OA. Another recent study of patients with both EHOA and non-erosive HOA comparing degree of pain and disability found that there was a weakly positive correlation between pain scores and erosive disease on radiography [17]. This study used the radiographic Kallman score, which is a validated scale that measures HOA structural damage specifically by measuring osteophytes, joint space narrowing, subchondral cysts, subchondral sclerosis, lateral deformity, and cortical collapse and thus is more detailed than the commonly used KL scale. Although results should be interpreted with caution from this very small study, across studies, there is general agreement that the degree of radiographic damage seen at one point in time does not correlate strongly with degree of pain in an individual patient. However, recent and historical studies point to a greater rate of radiographic progression and disability over time in patients with EHOA vs non-erosive disease [30, 32].
Erosive hand OA imaging. A X-ray demonstrating central erosions with “gull-wing” appearance (red asterisks), joint space narrowing with osteophytes (yellow asterisks), and lateral PIP subluxation of the 3rd and 4th fingers. B STIR MRI showing 2nd and 3rd PIP mild synovial thickening and fluid effusion with central subchondral erosions (red asterisks) and 3rd and 4th PIP joint space narrowing (yellow asterisks). C Ultrasound imaging of the 3rd IFP joint showing bone irregularity (green line), osteophytes (blue asterisk), bone erosion (white arrow), and capsule distention (red arrow)
Ultrasound (US) and MRI can detect a broader range of pathologic features of arthritis in the IP joints and detect them earlier, than conventional radiographs (Fig. 1B, C). These imaging modalities have helped to identify associations between synovitis and bone marrow lesions (BML) with clinical symptoms in both HOA and EHOA [15, 33,34,35]. A recent cross-sectional study evaluated the relationship between MRI features of disease and structural damage identified on X-rays using the previously mentioned VV radiographic score. As expected, MRI was able to detect more erosions and abnormal joint pathology than conventional radiography. These authors additionally found correlations between erosions/structural damage on X-ray or MRI and synovitis and BML [36]. A large systematic review and meta-analysis of 32 studies evaluating features of ultrasound and MRI on signs, symptoms, and radiographic progression of HOA was also recently conducted [37•]. The analysis found that imaging features of joint inflammation and symptoms were sometimes associated but also suggested that inflammatory features detected by US or MRI portend a higher risk of worsening or new radiographic abnormalities. These recent publications support MRI and US as more sensitive imaging modalities and thus invaluable in research studies investigating early detection methods and risk factors for progression. But until their importance is established as diagnostic or management tools, the cost and availability may supersede the immediate clinical utility. In the future, if effective treatments are identified to stop the progression of EHOA, early detection with MRI or US imaging could be very useful.
Pathogenesis
Few mechanistic studies specifically addressing HOA exist, and the underlying mechanism of the development of erosive disease is not clear, in part due to limited access to tissues from affected hand joints at early stages of disease and lack of availability of a good animal model. However, mechanisms driving HOA in general are thought to be similar to those driving OA in other joints. OA is now thought to involve a complex interplay between abnormal mechanical forces acting on cartilage and bone and a chronic inflammatory reaction within the joint that promotes pathologic joint tissue crosstalk, resulting in progressive cartilage loss, abnormal bone remodeling, synovitis, and joint pain. Although the hands are not weight bearing, they are load bearing, and reports of patients with hemiparesis who do not develop structural damage consistent with HOA side affected by paralysis do suggest that mechanical forces play a role in the development of HOA, as they do in OA in other joints [38]. In addition, the importance of inflammation in HOA is supported by prior studies, as well as the recent work highlighting genetic associations in innate immune pathways [10, 24••]. Thus, the interplay between mechanics and inflammation seems to apply equally to HOA.
As mentioned previously, one inflammatory signaling pathway recently of interest in HOA is the NOD/RIPK2 pathway [24••]. This pathway is critical in control of inflammation and defense against bacterial infections and cellular damage [39]. After discovering an allele of the RIPK2 gene associated with familial HOA, Jurynec et al. used genome editing to introduce the HOA-associated RIPK2 allele into mice. Since there is no well-established HOA mouse model, the authors used a common model of knee OA. The authors found that while in the absence of injury the mice expressing the risk allele had normal knees histologically, the mice had a magnified response to injury and increased risk of developing KOA [24••]. This study calls attention to this pathway both as a potential biomarker for early identification of OA risk and a target for treatment and prevention in patients at risk.
What distinguishes EHOA from non-erosive HOA is the development of central bone “erosions” (Fig. 1A). The mechanism of bone erosion in EHOA has also been difficult to study. However, prior work utilizing tissue from IP joints in EHOA undergoing joint replacement suggests mechanisms distinct from the invasive synovial pannus known to drive marginal erosions seen in RA. Histologic findings in the resected IP joints showed complete erosion of articular cartilage, with the exposed bone undergoing extensive osteoclastic resorption and the presence of fibrocartilaginous resurfacing [40]. Thus, the central erosions seen in EHOA seem to occur at sites of excessive bone remodeling and osteoclast activity, which may lead to weakness and collapse of the cortical plate resulting in characteristic bone deformities and “erosions” seen on X-rays as well as gull-wing and sawtooth erosions. More studies are needed to fully understand the mechanisms leading to bone erosions in EHOA.
The microbiome has been of recent interest in relation to rheumatology and systemic inflammatory diseases [41], and a recent study suggests a link between symptomatic HOA and the gut microbiome [42]. Investigators used the DIGICOD cohort, a French-based cohort of patients with HOA with a high proportion of patients (45.8%) with EHOA to study the gut-joint axis [43]. Using this cohort enriched for EHOA, four serum tryptophan metabolites, eight metabolite ratios, and one metabolic pathway were identified to be associated with EHOA compared with non-erosive HOA [44].Tryptophan is an essential amino acid which is transformed by microbes into metabolites that are subsequently used in the indole pathway, which functions in mucosal immunity [45]. Higher tryptophan levels were associated with decreased odds of EHOA, and certain other tryptophan metabolites such as 3-HAA and 5-HTP were associated with increased odds of EHOA. These metabolites were also found to be associated with pain, with increased 3-HAA and 5-HTP levels associated with the number of patient-reported painful joints. The investigators concluded that there are significant variations of tryptophan metabolism in HOA suggesting that gut dysbiosis may have a role in the pathogenesis of this condition, particularly in the evolution of erosive disease and pain.
New and Emerging Treatments
Our search of the https://clinicaltrials.gov/ website revealed that in the last 10 years, the number of new trials of interventions to treat HOA has doubled, with 32 trials initiated between 2004 and 2013 and 64 from 2014 to present [46]. There is a growing interest in developing treatment for all forms of HOA given the prevalence of the condition and its impact on hand-related disability globally. Since the 2018 EULAR and 2019 ACR guidelines for the treatment of OA were published, several new treatments for the management of EHOA are being studied [47, 48].
Given the aggressive and inflammatory nature of EHOA, many conventional and biologic DMARDS have been trialed in EHOA in attempts to halt the progression on joint destruction and relieve symptoms. However, according to the ACR/AF 2019 guideline for OA management, methotrexate, hydroxychloroquine, TNF inhibitors, and interleukin receptor antagonists are not recommended due to the absence of benefit noted in those trials [48,49,50]. A recent randomized controlled trial of 0.5 mg colchicine twice a day vs placebo in HOA showed no effect on the primary outcome of hand pain or any of the secondary outcomes such as tender and swollen joints, grip strength, CRP, or scores from the Michigan Hand Questionnaire [51]. This study included a high proportion (60%) of patients with EHOA, though a comparison with non-erosive disease was not reported. Although these recent negative trials are somewhat discouraging, it is important to recognize that mechanisms of bone erosion may be different in RA and OA, and the role of crystal deposition in EHOA is not yet clear. Furthermore, trials in HOA are often limited by use of radiographic criteria for inclusion which may exclude patients with early stages of disease when disease modification may be more achievable.
Methotrexate, a medication commonly used as the backbone of therapy for RA, has been evaluated in two recent trials. Ferrero et al. recently randomized 64 patients with symptomatic EHOA to receive 10 mg weekly methotrexate or placebo for 3 months. Unfortunately, the study failed to meet the primary outcome of decreased pain from baseline to 3 months [52]. Another study is currently underway using a higher dose (methotrexate 20 mg weekly) specifically in HOA patients with synovitis detected by MRI, although the full report is not yet published [53]. Whether this study can be extrapolated to the EHOA population remains to be tested.
The ACR/AF 2019 guideline also strongly recommends against bisphosphonates as a treatment for OA including HOA due to lack of supporting data [48], though small previous trials have suggested that clodronate may be effective in reducing pain in EHOA [54]. Given the importance of bone remodeling in EHOA, there are ongoing studies evaluating other treatments targeting bone remodeling including denosumab. Denosumab is a monoclonal antibody that binds RANKL to inhibit osteoclast maturation, ultimately reducing bone turnover [55]. Preliminary results from a recent trial of 100 patients with EHOA comparing denosumab to placebo were reported in conference abstracts, with promising results [56]. While the full trial has not been published, these data merit further investigation of this strategy, especially in patients with comorbid osteoporosis. The risks of rapid bone loss with discontinuation of this agent may limit its utility and need to be considered in this patient population before its use can be recommended.
The HOPE trial published in 2019 showed potential short-term benefit of oral prednisolone (10 mg daily for 6 weeks) for pain in HOA but has not been studied specifically in EHOA [57]. Intra-articular corticosteroid injections can minimize glucocorticoid toxicity given lower cumulative doses used on infrequent occasions. The ACR/AF 2019 guidelines conditionally recommend the use of ultrasound guided intra-articular glucocorticoid injection in HOA [48], whereas the 2018 EULAR HOA guideline does not recommend their routine use except in the case of a painful flare [47]. One small study evaluated twelve female patients with EHOA who underwent ultrasound guided intra-articular glucocorticoid injection. A total of 31 joints, with active clinical disease (VAS pain score > 7) and with US-detected synovial thickening, were injected with small doses of triamcinolone acetonide. At 1, 3, and 6 months after injection, VAS pain score was decreased significantly, as was US evidence of effusion, capsule distension, and synovial hypertrophy [58]. The interventions were well tolerated without significant side effects. While placebo-controlled studies are needed to confirm efficacy, it is exciting that this intervention was able to effectively relieve pain and inflammation with minimal side effects. This may represent a promising treatment option for some patients with HOA and only intermittent symptoms; however, no disease-modifying effect is expected.
The 2019 ACR/AF guideline for treatment of OA strongly recommends against glucosamine for hand OA but conditionally recommends chondroitin [48]. These supplements are nevertheless popular other-the-counter remedies utilized by patients with OA in the USA. In Europe, prescription-grade crystalline glucosamine sulfate (pCGS) is widely used as therapy for knee OA, and this treatment is considered a SYSADOA (symptomatic, slow-acting drug for OA). A retrospective case control study in patients with knee OA and symptomatic EHOA was stratified into groups who were exposed to or not exposed to pCGS as a treatment for knee OA. After 6 months, there was a significant improvement in VAS pain and FIHOA score, a 10-item questionnaire that evaluates difficulty performing tasks of hand function, in the pCGS group, as well as decreased NSAID and acetaminophen use [59]. While this is a promising trend, this was not a placebo-controlled trial, and so results should be interpreted with caution. Previous well-controlled trials did not find convincing evidence of benefit of glucosamine or chondroitin in knee OA [60]. Many other supplements have been studied in patients with HOA as well. The recent RADIANT study evaluated patients with symptomatic and radiographic HOA in an Internet-based, randomized, placebo-controlled trial of a supplement containing Boswellia serrata, pine bark extract, methylsulfonylmethane, and curcumin. The study had a large population (37%) of EHOA, but there was no improvement in VAS pain score over the course of the 12-week trial [61]. It is important to note that in the US, these supplements can represent a significant financial cost to the patient.
New and emerging non-pharmacologic treatments for EHOA are also being studied. A pilot study of transauricular vagus nerve stimulation in 20 patients with EHOA led to significantly decreased pain in 16/18 of the patients who completed the study [62]. Enrollment is ongoing for a trial of 156 patients with EHOA who will be randomized to receive 20 min of transcutaneous vagal nerve stimulation vs placebo daily for 12 weeks. The primary outcome will be self-reported hand pain, and secondary outcomes include function, quality of life, serum biomarker levels, compliance, and tolerance [63]. Although results are not yet in, addressing the neurological axis of HOA pain via interventions like this one represents an innovative approach, and innovations are desperately needed for our patients with EHOA.
Summary and Conclusion
Recent studies have improved our understanding of HOA and EHOA, especially as it pertains to biomarkers, genetic associations, pathogenesis, and risk factors for radiographic progression. For now, management guidelines generally suggest a focus on providing patients with non-pharmacologic support through physical interventions to maintain and improve hand function, in conjunction with appropriate/intermittent use of existing pharmacologic therapies to treat flares of pain and inflammation (i.e., NSAIDs and intra-articular glucocorticoids). While for many years treatment strategies have yielded generally disappointing results, there are glimmers of hope in the recent literature as innovative approaches are tested. In particular, we need to better understand the mechanisms underlying development and progression of the central “erosions” that characterize EHOA, given the increased disability associated with this subtype of HOA. As we better understand the pathogenesis, biomarkers, and treatment targets of this condition through ongoing research, new treatment strategies will emerge to address this debilitating condition.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Marshall M, Watt FE, Vincent TL, Dziedzic K. Hand osteoarthritis: clinical phenotypes, molecular mechanisms and disease management. Nat Rev Rheumatol. 2018;14:641–56.
Favero M, Belluzzi E, Ortolan A, Lorenzin M, Oliviero F, Doria A, et al. Erosive hand osteoarthritis: latest findings and outlook. Nat Rev Rheumatol. 2022;18:171–83.
Zhang W, Doherty M, Leeb BF, Alekseeva L, Arden NK, Bijlsma JW, et al. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: report of a task force of ESCISIT. Ann Rheum Dis. 2009;68:8–17.
Punzi L, Favero M, Frallonardo P, Ramonda R. Time to redefine erosive osteoarthritis. RMD Open. 2015;1:e000105.
Haugen IK, Felson DT, Abhishek A, Berenbaum F, Bierma-Zeinstra S, Borgen T, et al. Development of classification criteria for hand osteoarthritis: comparative analyses of persons with and without hand osteoarthritis. RMD Open. 2020;6:e001265.
Crain DC. Interphalangeal osteoarthritis: characterized by painful, inflammatory episodes resulting in deformity of the proximal and distal articulations. JAMA. 1961;175:1049–53.
Belhorn LR, Hess EV. Erosive osteoarthritis. Semin Arthritis Rheum. 1993;22:298–306.
VizHub - GBD Results - Institute for Health Metrics and Evaluation. In: GBD Restuls. https://vizhub.healthdata.org/gbd-results/. Accessed 22 Jun 2023
van Saase JL, van Romunde LK, Cats A, Vandenbroucke JP, Valkenburg HA. Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann Rheum Dis. 1989;48:271–80.
Ramonda R, Musacchio E, Campana C, Frigato M, Frallonardo P, Barbieri V, et al. Immunogenetic aspects of erosive osteoarthritis of the hand in patients from northern Italy. Scand J Rheumatol. 2011;40:139–44.
Marshall M, Peat G, Nicholls E, van der Windt D, Myers H, Dziedzic K. Subsets of symptomatic hand osteoarthritis in community-dwelling older adults in the United Kingdom: prevalence, inter-relationships, risk factor profiles and clinical characteristics at baseline and 3-years. Osteoarthritis Cartilage. 2013;21:1674–84.
Cavasin F, Punzi L, Ramonda R, Pianon M, Oliviero F, Sfriso P, et al. Prevalence of erosive osteoarthritis of the hand in a population from Venetian area. Reumatismo. 2004;56:46–50.
Wittoek R, Cruyssen BV, Verbruggen G. Predictors of functional impairment and pain in erosive osteoarthritis of the interphalangeal joints: comparison with controlled inflammatory arthritis. Arthritis Rheum. 2012;64:1430–6.
McAlindon TE, Driban JB, Roberts MB, Duryea J, Haugen IK, Schaefer LF, et al. Erosive hand osteoarthritis: incidence and predictive characteristics among participants in the osteoarthritis initiative. Arthritis Rheumatol Hoboken NJ. 2021;73:2015–24.
Haugen IK, Christensen BS, Bøyesen P, Sesseng S, van der Heijde D, Kvien TK. Increasing synovitis and bone marrow lesions are associated with incident joint tenderness in hand osteoarthritis. Ann Rheum Dis. 2016;75:702–8.
Ramonda R, Frallonardo P, Musacchio E, Vio S, Punzi L. Joint and bone assessment in hand osteoarthritis. Clin Rheumatol. 2014;33:11–9.
Duarte-Salazar C, Marín-Arriaga N, Miranda-Duarte A. The high clinical burden of erosive hand osteoarthritis is associated with clinical findings, pain, and radiographic severity. Reumatol Clínica. 2022;18:338–42.
Auroux M, Merle B, Fontanges E, Duvert F, Lespessailles E, Chapurlat R. The disability associated with hand osteoarthritis is substantial in a cohort of post-menopausal women: the QUALYOR study. Osteoarthritis Cartilage. 2022;30:1526–35.
Plotz B, Bomfim F, Sohail MA, Samuels J. Current epidemiology and risk factors for the development of hand osteoarthritis. Curr Rheumatol Rep. 2021;23:61.
• Kazmers NH, Meeks HD, Novak KA, Yu Z, Fulde GL, Thomas JL, et al. Familial clustering of erosive hand osteoarthritis in a large statewide cohort. Arthritis Rheumatol Hoboken NJ. 2021;73:440–7. This study of familial clustering of EHOA in a statewide database suggesting that there was a 5.5-fold greater risk in first-degree relatives of EHOA cases compared to controls.
•• Styrkarsdottir U, Stefansdottir L, Thorleifsson G, Stefansson OA, Saevarsdottir S, Lund SH, et al. Meta-analysis of erosive hand osteoarthritis identifies four common variants that associate with relatively large effect. Ann Rheum Dis. 2023;82:873–80. This is the first genome-wide association study of EHOA. Four common sequence variants associated with EHOA were identified and the associated genes could be causal.
Styrkarsdottir U, Thorleifsson G, Helgadottir HT, Bomer N, Metrustry S, Bierma-Zeinstra S, et al. Severe osteoarthritis of the hand associates with common variants within the ALDH1A2 gene and with rare variants at 1p31. Nat Genet. 2014;46:498–502.
den Hollander W, Boer CG, Hart DJ, Yau MS, Ramos YFM, Metrustry S, et al. Genome-wide association and functional studies identify a role for matrix Gla protein in osteoarthritis of the hand. Ann Rheum Dis. 2017;76:2046–53.
•• Jurynec MJ, Gavile CM, Honeggar M, Ma Y, Veerabhadraiah SR, Novak KA, et al. The NOD/RIPK2 signaling pathway contributes to osteoarthritis susceptibility. Ann Rheum Dis. 2022;81:1465–73. This study identified an allele of the RIPK gene as a risk factor for familial hand OA. Mice expressing the allele developed more severe knee OA after an inciting injury. This study sheds light on genetic component of HOA, the importance of inflammatory pathways, and potential future treatment targets.
Saruga T, Sasaki E, Inoue R, Chiba D, Ota S, Iwasaki H, et al. Usefulness of serum hyaluronic acid levels as a predictor of incidence of hand osteoarthritis analyzed by longitudinal analysis from the Iwaki cohort. Sci Rep. 2021;11:4074.
Baloun J, Pekáčová A, Švec X, Kropáčková T, Horvathová V, Hulejová H, et al. Circulating miRNAs in hand osteoarthritis. Osteoarthritis Cartilage. 2023;31:228–37.
• Oliviero F, Ramonda R, Scanu A, Galozzi P, Favero M, Punzi L. Levels of inflammatory cytokines and metalloproteinases are increased in knee synovial fluid of patients with concomitant erosive hand osteoarthritis. Clin Exp Rheumatol. 2020;38:800. This study is the first to show that levels of inflammatory biomarkers are elevated in the knees of patients with EHOA, suggesting that the more aggressive inflammatory nature of EHOA is not just limited to hand joints.
Cambon-Binder A, Jaisson S, Tuffet S, Courties A, Eymard F, Okwieka A, et al. Serum carboxymethyllysine concentration is associated with erosive hand osteoarthritis. Osteoarthritis Cartilage. 2023;31:976–84.
Verbruggen G, Veys EM. Erosive and non-erosive hand osteoarthritis. Use and limitations of two scoring systems. Osteoarthritis Cartilage. 2000;8:S45–54.
Neuprez A, Kaux J-F, Locquet M, Beaudart C, Reginster J-Y. The presence of erosive joints is a strong predictor of radiological progression in hand osteoarthritis: results of a 2-year prospective follow-up of the Liège Hand Osteoarthritis Cohort (LIHOC). Arthritis Res Ther. 2021;23:12.
Giesen T, Sanduleanu S, Jansen TLTA. Erosive hand osteoarthritis (EHOA): analysis of consecutive patients presenting with EHOA in a hospital-based rheumatology practice and its implications for an upcoming interventional study. Clin Rheumatol. 2022;41:1833–41.
Haugen IK, Mathiessen A, Slatkowsky-Christensen B, Magnusson K, Bøyesen P, Sesseng S, et al. Synovitis and radiographic progression in non-erosive and erosive hand osteoarthritis: is erosive hand osteoarthritis a separate inflammatory phenotype? Osteoarthritis Cartilage. 2016;24:647–54.
Ramonda R, Favero M, Vio S, Lacognata C, Frallonardo P, Belluzzi E, et al. A recently developed MRI scoring system for hand osteoarthritis: its application in a clinical setting. Clin Rheumatol. 2016;35:2079–86.
Eymard F, Foltz V, Chemla C, Gandjbakhch F, Etchepare F, Fautrel B, et al. MRI and ultrasonography for detection of early interphalangeal osteoarthritis. Joint Bone Spine. 2022;89:105370.
Obotiba AD, Swain S, Kaur J, Doherty M, Zhang W, Abhishek A. Reliability of detection of ultrasound and MRI features of hand osteoarthritis: a systematic review and meta-analysis. Rheumatol Oxf Engl. 2022;61:542–53.
Allado E, Wittoek R, Ferrero S, Albuisson E, Chary-Valckenaere I, Roux C, et al. Assessment of structural lesions, synovitis and bone marrow lesions in erosive hand osteoarthritis on MRI (0.3T) compared to the radiographic anatomical Verbruggen-Veys score. PLoS ONE. 2020;15:e0234972.
• Obotiba AD, Swain S, Kaur J, Yaseen K, Doherty M, Zhang W, et al. Synovitis and bone marrow lesions associate with symptoms and radiographic progression in hand osteoarthritis: a systematic review and meta-analysis of observational studies. Osteoarthritis Cartilage. 2021;29:946–55. This study showed that inflammatory features identified by ultrasound and MRI are associated with development of erosive lesions. These advanced imaging techniques could help identify patients at risk of adverse outcomes.
Droz-Bartholet F, Verhoeven F, Prati C, Wendling D. Prevention of hand osteoarthritis by hemiparesis. Arthritis Rheumatol Hoboken NJ. 2016;68:647.
Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014;14:9–23.
Favero M, Perino G, Valente ML, Tiengo C, Ramonda R. Radiological and histological analysis of two replaced interphalangeal joints with active subchondral bone resorption in erosive hand osteoarthritis: a novel mechanism? Skeletal Radiol. 2017;46:385–91.
Clemente JC, Manasson J, Scher JU. The role of the gut microbiome in systemic inflammatory disease. BMJ. 2018;360:j5145.
Wei J, Zhang C, Zhang Y, Zhang W, Doherty M, Yang T, et al. Association between gut microbiota and symptomatic hand osteoarthritis: data from the Xiangya Osteoarthritis Study. Arthritis Rheumatol. 2021;73:1656–62.
Sellam J, Maheu E, Crema MD, Touati A, Courties A, Tuffet S, et al. The DIGICOD cohort: a hospital-based observational prospective cohort of patients with hand osteoarthritis – methodology and baseline characteristics of the population. Joint Bone Spine. 2021;88:105171.
Binvignat M, Emond P, Mifsud F, Miao B, Courties A, Lefèvre A, et al. Serum tryptophan metabolites are associated with erosive hand osteoarthritis and pain: results from the DIGICOD cohort. Osteoarthritis Cartilage. 2023;31:1132–43.
Lamas B, Natividad JM, Sokol H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018;11:1024–38.
Hand Osteoarthritis. In: ClinicalTrials.gov. https://classic.clinicaltrials.gov/. Accessed 3 Jul 2023
Kloppenburg M, Kroon FP, Blanco FJ, Doherty M, Dziedzic KS, Greibrokk E, et al. 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann Rheum Dis. 2019;78:16–24.
Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020;72:149–62.
Kloppenburg M, Ramonda R, Bobacz K, Kwok W-Y, Elewaut D, Huizinga TWJ, et al. Etanercept in patients with inflammatory hand osteoarthritis (EHOA): a multicentre, randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2018;77:1757–64.
Aitken D, Laslett LL, Pan F, Haugen IK, Otahal P, Bellamy N, et al. A randomised double-blind placebo-controlled crossover trial of HUMira (adalimumab) for erosive hand OsteoaRthritis - the HUMOR trial. Osteoarthritis Cartilage. 2018;26:880–7.
Davis CR, Ruediger CD, Dyer KA, Lester S, Graf SW, Kroon FPB, et al. Colchicine is not effective for reducing osteoarthritic hand pain compared to placebo: a randomised, placebo-controlled trial (COLAH). Osteoarthritis Cartilage. 2021;29:208–14.
Ferrero S, Wittoek R, Allado E, Cruzel C, Fontas E, Breuil V, et al. Methotrexate treatment in hand osteoarthritis refractory to usual treatments: a randomised, double-blind, placebo-controlled trial. Semin Arthritis Rheum. 2021;51:831–8.
Wang Y, Jones G, Keen H, Hill C, Wluka A, Kasza J, et al. Op0070 methods - a randomized controlled trial of methotrexate to treat hand osteoarthritis with synovitis. Ann Rheum Dis. 2023;82:48–8.
Saviola G, Abdi-Ali L, Povino MR, Campostrini L, Sacco S, Dalle Carbonare L, et al. Intramuscular clodronate in erosive osteoarthritis of the hand is effective on pain and reduces serum COMP: a randomized pilot trial-The ER.O.D.E. study (ERosive Osteoarthritis and Disodium-clodronate Evaluation). Clin Rheumatol. 2017;36:2343–50.
Hanley DA, Adachi JD, Bell A, Brown V. Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract. 2012;66:1139–46.
Wittoek R, Verbruggen G, Vanhaverbeke T, Elewaut D. Op0071 Effect of denosumab on structure modification in erosive hand osteoarthritis: results of a 48-weeks, monocentric, randomized, placebo-controlled, double-blind phase 2 study and open label extension phase. Ann Rheum Dis. 2023;82:48–9.
Kroon FPB, Kortekaas MC, Boonen A, Böhringer S, Reijnierse M, Rosendaal FR, et al. Results of a 6-week treatment with 10 mg prednisolone in patients with hand osteoarthritis (HOPE): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;394:1993–2001.
Favero M, Hoxha A, Frallonardo P, Ortolan A, Lorenzin M, Felicetti M, et al. Efficacy and safety of ultrasound-guided intra-articular glucocorticoid injection in erosive hand osteoarthritis. Pain Med. 2021;22:1229–32.
Tenti S, Veronese N, Cheleschi S, Seccafico I, Bruyère O, Reginster J-Y, et al. Prescription-grade crystalline glucosamine sulfate as an add-on therapy to conventional treatments in erosive osteoarthritis of the hand: results from a 6-month observational retrospective study. Aging Clin Exp Res. 2022;34:1613–25.
Clegg DO, Reda DJ, Harris CL, Klein MA, O’Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795–808.
Liu X, Robbins S, Eyles J, Fedorova T, Virk S, Deveza LA, et al. Efficacy and safety of a supplement combination on hand pain among people with symptomatic hand osteoarthritis an Internet-based, randomised clinical trial the RADIANT study. Osteoarthritis Cartilage. 2021;29:667–77.
Courties A, Deprouw C, Maheu E, Gibert E, Gottenberg J-E, Champey J, et al. Effect of transcutaneous vagus nerve stimulation in erosive hand osteoarthritis: results from a pilot trial. J Clin Med. 2022;11:1087.
Courties A, Deprouw C, Rousseau A, Berard L, Touati A, Kalsch J, et al. Transcutaneous vagus nerve stimulation in erosive hand osteoarthritis: protocol for the randomised, double-blind, sham-controlled ESTIVAL trial. BMJ Open. 2022;12:e056169.
Acknowledgments
We thank Dr. S. Vio, radiologist at the University Hospital of Padova, for the radiological imaging.
Funding
We gratefully acknowledge the support from the National Institutes of Health R01-AR075737, the VA BLR&D Program I01-BX004912, and the VA RR&D Program I21-RX003854 and I01-RX003988 (C. R. S.).
Author information
Authors and Affiliations
Contributions
M. B. and C. S. wrote the main manuscript with additional critical contributions from M. F. and R. R. M. F. and R. R. created the figure. M. B., C. S., M. F., and R. R. reviewed and edited the manuscript, and all authors approve the final submitted version.
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Scanzello serves on the editorial boards for Arthritis & Rheumatology and Osteoarthritis & Cartilage and is an inventor on a provisional patent application describing a novel method for treating osteoarthritis. Dr. Bean, Prof. Ramonda, and Dr. Favero have nothing to declare.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bean, M.B., Favero, M., Ramonda, R. et al. Erosive Hand Osteoarthritis: Recent Advances and Future Treatments. Curr Rheumatol Rep 26, 103–111 (2024). https://doi.org/10.1007/s11926-023-01130-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-023-01130-9