Abstract
Advanced imaging has become essential for recognition of clinically suspected early spondyloarthritis. This report summarizes recent progress towards a data-driven comprehensive definition of a positive sacroiliac joint MRI in axial spondyloarthritis, which incorporates contextual information provided by structural lesions alongside with active changes. A focus is on emerging limitations and challenges with increasing use of imaging in spondyloarthritis. We discuss the ongoing controversy as to whether sacroiliac joint MRI due to its superior reliability and ability to depict both structural and active lesions should be the preferred imaging modality in early disease over the traditional approach with pelvic radiographs. Another challenge is transferring the expanding knowledge about imaging evaluation in spondyloarthritis to the community of rheumatologists and radiologists. Advanced imaging modalities will not become the gold standard for diagnosis of spondyloarthritis, which remains a process of composite deduction based on complementary information obtained from clinical, laboratory, and imaging assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inflammation of the sacroiliac joints (SIJ) is the presenting feature in most patients with early axial spondyloarthritis (SpA). However, symptom overlap between inflammatory and mechanical back pain is considerable, and accessibility of the SIJ to clinical examination is limited. In view of this dilemma in daily routine, imaging of the SIJ has become the most important examination to assist in early recognition of SpA (Fig. 1). While it may take up to 10 years for the first structural lesions to appear on pelvic radiography, magnetic resonance imaging (MRI) has the potential to detect inflammation at the very first manifestation of sacroiliitis. Moreover, MRI has been shown to demonstrate inflammation-related structural SIJ lesions in 60–90 % of SpA patients already in the first 2 1/2 years after symptom onset [1, 2].
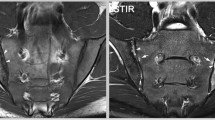
Morphology of the most relevant lesion types on sacroiliac joint MRI in early axial spondyloarthritis. 21-year-old, HLA B27-positive male patient with inflammatory back pain for 3 years. The MRI shows extended bone marrow edema (white arrows) predominantly on the sacral side of both sacroiliac joints on the STIR sequence (right image). The T1SE sequence (left image) displays fat metaplasia (black arrows) mainly in the right sacroiliac joint within and outside areas with bone marrow edema. Erosion (white arrowheads) in the right sacroiliac joint can be demonstrated already at this early disease stage. The evaluation of concomitant lesion types by scrolling simultaneously through synchronized DICOM images of both sequences is the basis for contextual global assessment of a MRI examination. Uniform symbols for annotations. White arrow: bone marrow edema, black arrow: fat metaplasia, white arrowhead: erosion
Imaging and in particular MRI to complement the limited utility of clinical examination for sacroiliitis raised expectations to enhance diagnostic confidence in the challenging setting of clinically suspected early SpA. This is mirrored by the Assessment in Spondyloarthritis International Society (ASAS) classification criteria for axial SpA, which incorporate for the first time MRI of the SIJ as major criterion in the imaging arm [3, 4]. The initial consensus-based ASAS proposal about which imaging features may constitute a positive SIJ MRI indicative of SpA was based on bone marrow edema (BME) alone being “highly suggestive of SpA” [5].
However, several investigations in recent years shed doubt on definitions based on BME alone and suggested data-driven proposals about which SIJ MRI features proved most indicative of early sacroiliitis. Imaging of early sacroiliitis remains a matter of debate, with the recommendation that the definition of a positive MRI in SpA should be highly specific and developed by a data-driven process [6•]. A major concern is limited specificity of SIJ BME alone to discriminate inflammatory from the more common mechanical back pain. Another issue of growing relevance is the role of structural SIJ lesions along with BME not only to facilitate recognition of early disease but also to improve specificity with this diagnosis. In addition, there are several unresolved pivotal issues towards a comprehensive definition of what constitutes a positive MRI in early SpA, such as standardization of the imaging technique, the lack of an “objective” gold standard to calibrate findings on imaging, and an unmet need to disseminate advances in imaging in SpA among both rheumatologists and radiologists. This article reviews the latest advances in the field with a focus on limitations and challenges to using imaging in early SpA.
Controversies Towards a Definition of a Positive SIJ MRI for Spondyloarthritis
Limited Specificity of BME Alone to Define a Positive SIJ MRI for SpA
Since our latest review on the topic in this journal [7], several initiatives provided insight how to proceed towards a data-driven definition of a positive SIJ MRI in SpA. An initial consensual proposal by experts in the field in 2010 was based on SIJ BME alone [5] without taking into account potential concomitant structural lesions on SIJ MRI, which appear already in the first 2 years after symptom onset. However, the characterization of BME in this definition as being “highly suggestive of SpA” remained controversial. Which additional features such as location, extent, demarcation, or intensity of BME would increase specificity to meet this requirement to be highly suggestive of SpA? Does interpretation of fluid-sensitive signal changes alone differ among rheumatologists, radiologists, and primary care providers? An operational amendment to this initial definition required the presence of at least two BME lesions on a single SIJ slice or of a BME lesion observed in the same SIJ quadrant on at least two consecutive slices.
However, subsequent controlled studies, which demonstrated SIJ BME in up to 30 % of mechanical back pain patients and of healthy controls, called into question the specificity of SIJ BME as the only MRI feature to discriminate SpA from Non-SpA back pain patients [7] (Fig. 2). The high prevalence of nonspecific SIJ BME was recently confirmed in a large study from Denmark in patients with back pain of 2–12 months duration recruited from primary care [8•]. Twenty-one percent of 1020 consecutive back pain patients aged 18–40 years showed SIJ BME meeting the ASAS definition of a positive SIJ MRI for SpA, 42 % of whom had low-grade BME not exceeding 25 % of the subcortical bone area. A controlled study in 26 patients with juvenile SpA reported a nearly identical prevalence of mostly subtle non-specific SIJ BME in 20 % of the 35 controls with a mean age of 15.1 years [9•].
Extended bone marrow edema in the sacroiliac joint of a healthy volunteer. 31-year-old female healthy volunteer without back pain and not overweight. The STIR sequence shows bright bone marrow edema signal (white arrows) in the distal right ilium on four consecutive slices meeting the ASAS definition of a positive sacroiliac joint MRI in spondyloarthritis. The corresponding T1SE sequences are pristine. The reason for this lesion remains speculative, ranging from possible vascular signal to putative stress edema. Uniform symbols for annotations, white arrow: bone marrow edema
The reasons for the common finding of SIJ BME also in non-SpA back pain patients and even healthy controls, both in the mature and growing skeleton, remain poorly understood and speculative. Mechanical stress, particularly in the antero-superior and inferior SIJ portion, might induce BME lesions, potentially related to degenerative changes, lumbosacral transitional anomalies, or increased axial load. Ongoing research initiatives focus on a potential association of SIJ MRI features with high impact sports or multiparity (Fig. 3). Potential alternative explanations comprise reactive changes to anatomical SIJ variants [10, 11•], or increased signal from vascular variants mimicking BME on fluid-sensitive MRI sequences. Altered vascularity in the growing skeleton might be a putative reason for SIJ BME lesions in children. This lack of specificity particularly of low-grade SIJ BME, which can be seen both in mechanical and early inflammatory back pain, has shifted the research focus in recent years towards structural lesions of the SIJ.
a, b MRI features mimicking spondyloarthritis—bone marrow edema bordering ileitis condensans. 32-year-old, HLA B27-negative woman with persisting inflammatory back pain starting in the second trimester of her first pregnancy. The upper row shows sacroiliac joint MRI 2 years after giving birth, the lower row 3 years thereafter with milder pain intensity. Initially intense left-sided sacral bone marrow edema (white arrows) bordering extended sclerosis (black arrowheads on T1SE sequence) in the left sacroiliac joint is decreasing over 12 months. The pelvic radiograph (Fig. 3b) shows ileitis condensans (black arrowheads) corresponding to the sclerotic area on sacroiliac joint MRI. Uniform symbols for annotations. White arrow: bone marrow edema, black arrowhead: sclerosis
Pivotal Role of Structural Lesions on SIJ MRI for Recognition of Early SpA
Structural lesions on pelvic radiographs have remained the cornerstone of SIJ assessment over many decades [12, 13]. Tomographic imaging modalities such as MRI offer superior technical standards to recognize structural lesions compared with summation images provided by radiography. Structural changes on SIJ MRI, with erosion showing high specificity in previous systematic controlled studies, are observed in 60–90 % of early SpA patients with a mean symptom duration of 2 ½ years, and may contribute to diagnostic utility of SIJ MRI above SIJ BME alone [1, 2]. Structural SIJ lesions appear early also in juvenile SpA. In a prospective cross-sectional study of 40 children with newly diagnosed juvenile SpA according to the International League of Associations for Rheumatology (ILAR), 7 (88 %) of 8 children showing BME also had evidence of concomitant erosion or sclerosis [14]. A controlled retrospective study in 26 children with juvenile SpA fulfilling the same classification criteria showed erosion in 58 % of the patients after mean symptom duration of 2.2 years [9•].
Moreover, MRI is unique among musculoskeletal imaging modalities to depict concomitant active and structural lesions, and their anatomical distribution by the tomographic technique. Evaluation of SIJ MRI is contextual: the significance of subtle changes on one sequence may be modified by the presence or absence of other lesion types on a complementary sequence, which may impact confidence in a diagnosis of SpA (global assessment). The significance of low-grade bone marrow edema on the STIR sequence often depends on what is seen on the T1SE sequence, especially erosion. Any definition of a positive SIJ MRI restricted to one single lesion (lesion-based assessment) is a priori limited by contextual interpretation of various lesion types occurring concomitantly on SIJ MRI. The incorporation of structural lesions into a definition of a positive MRI in SpA may not only enhance diagnostic utility but also facilitate the notoriously challenging interpretation of subtle BME in the SIJ.
Recent Data on Structural Lesions to Define a Positive SIJ MRI in SpA
A recent report on diagnostic utility of SIJ MRI in two independent back pain cohorts from Canada and Switzerland with different recruitment modes [15•] proposed candidate lesion-based criteria by combining BME and/or erosion for defining a positive SIJ MRI in SpA. Global assessment, which served as reference standard to test various combinations of lesion-based assessment, showed high specificity of 0.95/0.83 in the two cohorts compared to the ASAS definition based on ≥2 BME lesions (specificity 0.76/0.74). An increase in threshold of BME lesions to ≥3 (0.89/0.84) or ≥4 (0.92/0.87) resulted in comparably high specificity to global assessment. Erosion ≥2 lesions and/or BME ≥3 or ≥4 lesions yielded comparably high sensitivity to global assessment without affecting specificity. These combined criteria based on erosion and/or BME showed both higher sensitivity and specificity than the ASAS definition containing BME alone, a finding which reflects the contextual information provided by T1SE and STIR sequences. The Canadian/Swiss study focusing on non-radiographic early SpA patients had three control groups. Positive controls consisted of radiographically confirmed ankylosing spondylitis patients, and healthy volunteers served as negative controls. The third clinically relevant control sample consisted of mechanical back pain patients, which represent the most challenging differential diagnostic condition of early inflammatory back pain. This study design allows for comparison and combination of all possible features on SIJ MRI. Study subjects were classified according to rheumatologist expert opinion based on clinical examination, pelvic radiography, and laboratory values. Studies in other cohorts with early SpA are needed to determine which of the high priority candidate criteria identified in this analysis perform best across the spectrum of SpA seen in clinical practice.
A major unresolved challenge in imaging studies in SpA is the selection of the gold standard with which to compare MRI evaluation. The traditional reference standard used in SpA is rheumatologist expert opinion, which consistently served for deriving the European Spondyloarthropathy Study Group (ESSG), Amor and ASAS classification criteria over the last 2 decades. Rheumatologist expert opinion was deemed to be the most appropriate approach to integrate the results of various examination modalities, as no single clinical, laboratory, or imaging assessment is able to capture the entire spectrum of axial, peripheral, and extraskeletal manifestations in SpA. Predictive validation of assignments based on clinical grounds is needed, but prospective cohort studies are often limited by substantial attrition rates, particularly in slowly evolving conditions such as SpA. Histology of SIJ biopsies as alternative gold standard to evaluate SIJ MRI findings is limited by feasibility issues in daily practice.
Another report in patients with recent-onset chronic back pain from five centers in The Netherlands, Norway, and Italy evaluated the diagnostic utility of structural SIJ lesions only [16•]. An individual lesion was defined according to its presence on two consecutive coronal slices through the SIJ. Performance was best with ≥3 erosions (i.e., potentially up to six slices with an erosion), ≥3 fatty lesions, or a combined cut-off of ≥5 erosions and/or fatty lesions. However, patients were enrolled according to the ASAS definition of a positive SIJ MRI based on BME alone and this information was available to the rheumatologist formulating the gold standard expert opinion as to the diagnosis of SpA. This recruitment strategy raises concerns regarding circularity in identifying optimal lesion definitions for classification and limits the evaluation of candidate definitions incorporating structural lesions at various thresholds or of potential combined definitions containing both structural and/or active changes. Another limitation was the definition of control subjects as having ≤2 SpA items from the ASAS classification criteria set, which was considered to reflect low likelihood for SpA. Evidence is limited whether chronic back pain subjects meeting up to 2 SpA features can be regarded as having non-specific back pain, the most relevant control group in diagnostic utility studies in early SpA. The control group with ≤2 SpA features and the early SpA group defined by positive SIJ MRI according to the ASAS MRI definition showed similar frequency of inflammatory back pain (56 versus 67 %) and good response to non-steroidal anti-inflammatory drugs (29 versus 35 %). Moreover, controls showing up to 2 SpA features are not equivalent to a control group of healthy volunteers, who are essential to assess the clinically relevant “background noise” on MRI of the SIJ.
In 80 consecutive patients aged <16 years who met the ILAR classification criteria for juvenile SpA, the combination of erosion and BME provided the highest diagnostic utility on unenhanced SIJ MRI [17]. These results in the growing skeleton are in accordance with the superior performance of the same lesion combination in adult SpA patients in the Canadian/Swiss study.
Which lesion types should be incorporated into lesion-based criteria to reach an optimal balance between sensitivity and specificity? Relevant factors for selecting certain MRI lesions over others are their reproducibility, specificity, and redundancy of information. In the Canadian and Swiss SpA inception cohorts, mean intraclass correlation over six reader pairs for the number of affected SIJ quadrants was comparably high for BME with 0.75–0.88 and erosion with 0.71–0.82 [15•], compared to only moderate reliability of fat metaplasia of 0.59–0.75 [18]. The limited diagnostic utility of fat metaplasia was mainly due to its strong association with concomitant BME and/or erosion, which highlights the mostly redundant information provided by fat metaplasia compared with BME and/or erosion.
Definition of Active Sacroiliitis on MRI Updated
Based on a systematic literature review, the ASAS MRI working group recently updated the definition of active sacroiliitis on MRI for classification of axial SpA [19•]. The group decided to continue to focus on active sacroiliitis. However, it was deemed essential that readers of a MRI scan simultaneously view sequences designed to identify inflammation and sequences that focus on depiction of structural damage. This new direction takes into account also structural findings to facilitate recognition of active sacroiliitis and thus incorporates the contextual information contained in both T1SE and STIR sequences. The assessment of notoriously challenging subtle SIJ BME lesions may be influenced by the presence of concomitant structural damage, especially erosion. The next step towards a comprehensive definition of a positive SIJ MRI in axial SpA, which incorporates both structural and active lesions, has been assigned top priority on the research agenda.
What Is the Role of Spine MRI in Recognition of SpA?
The Canadian/Swiss inception cohorts examined a potential incremental value of additional spine MRI in the challenging setting of clinically suspected axial SpA but negative or inconclusive SIJ MRI [20•]. Surprisingly, combined spine and SIJ MRI added little incremental value compared with SIJ MRI alone for diagnosing patients with nonradiographic axial SpA. Enhancement in sensitivity by 16–24 % true positive assignments was offset by a loss in specificity of similar degree with 11–27 % false positive assignments. Vertebral corner lesions on spine MRI were the main drivers towards misclassification of controls. Caution is warranted if a classification of SpA is based on spinal MRI alone, where an additional assessment of the SIJ may be advisable.
A separate analysis of the same dataset focused on potential thresholds of spinal MRI to discriminate nonradiographic axial SpA from mechanical back pain patients [21•]. None of the previous candidate spinal thresholds (>2 or >3 corner inflammatory lesions (CIL) or >6 corner fatty lesions (CFL)) [22] for a positive spinal MRI in axial SpA showed clinically relevant diagnostic utility in nonradiographic axial SpA (positive likelihood ratios (LR) of 1.38–2.36). Only a threshold of >6 CILs had moderate to substantial diagnostic utility by positive LR of 6.74–13.26, while CFL did not contribute to diagnostic utility even at a cut-off of ≥10 lesions.
A similar analysis of spine MRI over a broad range of cut-offs separately for inflammatory and fatty lesions was also conducted in the above mentioned Dutch, Norwegian, and Italian multicenter cohort with recent-onset chronic back pain [16•]. Aiming at a specificity of ≥95 % in the control group with ≤2 SpA features, the authors found cut-offs for a positive spinal MRI of ≥5 CIL or CFL. Comparable spinal cut-offs of >6 and ≥5 CIL in the Swiss/Canadian and the Dutch/Norwegian/Italian cohorts despite different design and gold standard suggest that the threshold of ≥3 CIL as proposed by ASAS may be too low. Interestingly, the study by de Hooge et al. showed an age-dependent increase of thresholds (≥3 CIL or CFL <35 years at symptom onset versus ≥6 CIL or ≥7 CFL with onset of back pain between 35 and 45 years), suggesting an age-dependent increase of both lesion types also in non-SpA controls. Two recent reports in patients aged 18–40 and 16–45 years, respectively, with persisting low back pain confirmed a high prevalence of degenerative spinal changes in 87 and 89 % of subjects and consistently showed an age-dependent increase in lesion frequency [23, 24]. Young patients referred to rheumatologic evaluation of suspected inflammatory back pain are likely to have concomitant degenerative spinal changes, which may be challenging to discriminate from inflammatory lesions on spinal MRI. These results question the approach of defining candidate thresholds indicating SpA based on spine MRI alone, without taking into account concomitant lesions on SIJ MRI or in postero-lateral spinal structures.
Backfill—a Unique MRI Lesion as Key Intermediary Between Erosion and Ankylosis
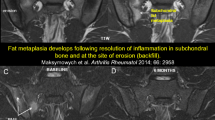
The term “backfill,” characterized by a unique morphological appearance on SIJ MRI, has been coined for repair tissue refilling excavated bone in the SIJ in longstanding SpA [25]. SIJ erosion in SpA is morphologically heterogeneous on MRI. Localized erosion defined by a circumscribed breach of the cortical bone and loss of bone marrow matrix signal on the T1SE sequence represent one end of the spectrum, while confluence of multiple erosions typically on the iliac side can lead to extended erosion along the entire joint extent. In advanced disease, areas of previous erosion may display high signal intensity changes on T1SE sequences in some patients, which may reflect fat metaplasia eventually leading to ankylosis. Backfill on SIJ MRI is clearly demarcated from adjacent normal bone marrow by an irregular dark signal reflecting sclerosis at the border of the eroded bone [26] (Fig. 4).
Backfill—a key intermediary MRI lesion between erosion and ankylosis. 39-year-old, HLA B27-positive male patient with ankylosing spondylitis meeting the modified New York criteria, symptom duration 10 years. The baseline T1SE sequence on the left side shows extended confluent erosion excavating the iliac bone on both sides. A fine irregular black line representing sclerotic tissue (black arrowheads) is separating the original iliac bone (on the left side with fat metaplasia in the distal third) from metaplastic tissue refilling the excavated iliac bone and displaying bright signal on T1SE sequence, which is termed backfill (white asterisks). The T1SE sequence on the follow-up MRI (right image) under anti TNFα treatment 10 years later shows a near complete resolution of the erosive process by integration of previous areas of backfill into iliac bone, leaving but a narrow sclerotic rim of the original joint space (white hash signs) which shows as ankylosis on pelvic radiograph. Uniform symbols for annotations. Black arrowhead: sclerosis, white asterisks: backfill, white hash sign: ankylosis
The evolution of erosion to ankylosis via the repair tissue backfill was previously based on anecdotal evidence in advanced disease with serial MRI examinations over several years. Two recent studies, however, support a disease model whereby ankylosis develops following repair of erosion with backfill as key intermediary step in this pathway. A prospective observational cohort with 147 AS patients, who met the modified NYc, showed that resolution of inflammation and reduction of erosion were each independently associated with the development of new backfill and fat metaplasia at 2 years on multivariate analysis [27•]. In a randomized controlled trial in 52 patients with axial SpA, defined by the ESSG criteria or sacroiliitis on pelvic radiographs or SIJ MRI, tumor necrosis factor inhibitor treatment with adalimumab over 12 weeks was associated with resolution of erosion and development of backfill [28•]. Histopathology from targeted biopsies in areas of MRI-defined backfill is not available. However, such biopsies might provide insight into repair mechanisms in SpA once the erosive process is initiated, and might help in investigating effects of treatment on the development of structural lesions.
Controversies in Technical Protocols for MRI Acquisition
Routine MRI protocols to assess active and structural lesions of the axial skeleton in SpA patients contain by consensus complementary “fat-sensitive” T1SE and “fluid-sensitive” STIR or T2 weighted fat saturated sequences. MRI sequences to facilitate detection of erosion, the key lesion for structural damage in SpA, are candidate amendments to standard MRI protocols. A recent randomized controlled trial comparing sulfasalazine versus etanercept in SpA patients meeting the ASAS classification criteria showed superior reliability of T1 weighted opposed-phase gradient-echo sequences, which are directed at superior detection of structural SIJ damage, versus traditional T1SE sequences resulting in intraclass correlation values for erosion of 0.65 versus 0.18, respectively [29].
Standard MRI protocols in SpA are based on image acquisition in tilted semicoronal planes running parallel to the long axis of the SIJ. This technique allows topographical comparison simultaneously of active and structural lesions. Semicoronal slices readily discriminate the posterior-superior ligamentous from the antero-inferior cartilaginous joint compartment, where inflammation usually starts. A recent study based on semicoronal SIJ slices concluded that the ligamentous compartment provided no incremental diagnostic value for axial SpA, but concomitant BME in both compartments may help discriminate early axial SpA from mechanical back pain patients [30]. Combining semicoronal slices with perpendicular axial slice orientation provides superior anatomical information and may better assist to detect the anatomical site of disease-specific characteristics and to recognize anatomical variants simulating disease such as vascular signals mimicking low-grade BME or inherent joint space irregularities simulating erosion [11•, 31].
Should we apply contrast media for MRI in SpA? A growing body of evidence over the last decade consistently showed that gadolinium-enhanced sequences, which cause discomfort to patients and considerable extra costs, have no incremental value towards detection of sacroiliitis [7]. The additional information was redundant: synovitis, capsulitis, and enthesitis visualized by gadolinium administration were observed only in the presence of BME detected by STIR alone, which allowed a diagnosis of SpA also without contrast application. Complete redundancy of additional findings detected by contrast enhancement compared to STIR sequences alone has recently been confirmed in 68 adult patients with early inflammatory back pain of less than 2 years duration [32], as well as in 51 patients with clinically suspected or diagnosed juvenile SpA [33] and in 80 children meeting the ILAR criteria for juvenile SpA [34]. STIR sequences alone are sufficient to detect axial inflammation in adult and juvenile SpA. The use of contrast medium is not needed, unless for clinically suspected differential diagnostic conditions such as septic sacroiliitis or malignancy.
Should nonsteroidal anti-inflammatory drugs (NSAID), the standard treatment in SpA, be temporarily discontinued before an axial MRI, under the assumption that NSAID might alter BME lesions? A pilot study in 2009 showed no significant reduction of SIJ and thoracolumbar BME after an open-label 6-week trial with etoricoxib in 20 AS patients [35]. Two recent trials confirmed these results. An uncontrolled trial explored the impact of a full-dose NSAID treatment over 6 weeks on SIJ BME after a washout period of 14–20 days prior to baseline MRI [36]. There was no clinically relevant decrease in extent of SIJ BME according to the SPARCC score, but minimal loss in BME intensity in 20 patients with clinically suspected axial SpA, who met the ASAS definition of a positive SIJ MRI. A double-blind, randomized controlled trial in 50 patients with chronic nonspecific low back pain compared celecoxib versus acetaminophen over 4 weeks with a washout period of 14 days prior to baseline MRI [37]. In accordance with previous reports, SIJ and spine inflammation according to SPARCC scores did not change with either of the two compounds. These data support the current practice to continue NSAID treatment when ordering axial MRI in patients suspected to have SpA.
Should SIJ MRI Replace Pelvic Radiographs in Recognition of Early SpA?
The EULAR recommendations for the use of imaging in the diagnosis and management of SpA in clinical practice state that conventional radiography of the SIJ is recommended as the first imaging method to diagnose sacroiliitis [38•]. If the diagnosis of axial SpA cannot be established based on clinical features and conventional radiography, and axial SpA is still suspected, MRI of the SIJ is recommended. This statement is supported by the European Society of Musculoskeletal Radiology [39]. Radiographic evaluation of SIJ according to the modified NYc [13] is the gold standard in classification of axial SpA. However, several studies applying these criteria have consistently shown limited agreement among trained readers with kappa values around 0.5 [40]. A post hoc analysis of pelvic radiographs by trained central readers in two interventional trials in non-radiographic axial SpA resulted in re-classification of 36 and 37 % of the patients regarding fulfillment of the radiographic modified NYc [41, 42], which raised concerns in a public hearing of the Food and Drug Administration in 2013, whether radiographic classification is appropriate for clinical trials in early axial SpA [43].
Concerns about limited reliability of radiographic modified NYc in suspected early SpA were fuelled by a recent report highlighting only moderate agreement (kappa 0.54) of SIJ evaluation on pelvic radiographs by central rheumatologist and radiologist readers in a SpA inception cohort [44•]. Conversely, assessment of SIJ MRI in the same cohort according to the ASAS definition based on active inflammatory lesions showed substantial agreement among central readers with a kappa value of 0.73 [45•]. Superior performance of SIJ MRI has also been reported in juvenile SpA [9•]. In a controlled retrospective study of 26 patients with juvenile SpA, global assessment was much more reliably made on SIJ MRI (kappa 0.80) compared to pelvic radiography (kappa 0.30).
A potential source for disagreement in the modified NYc is the lack of standardized and validated definitions for the five radiographic lesion types contained in the original description [12, 13]. Furthermore, there is only limited data regarding their frequency and morphology in mechanical back pain patients, in subjects with high physical activity, or in healthy controls. The modified NYc derived from a cohort of 183 HLA-B27 positive AS patients, their HLA-B27 positive or negative first-degree relatives and population controls [13] may not be directly applicable to back pain patients clinically suspected to have early axial SpA. Concordance rates in radiographic mNYc may depend on the proportion of AS patients in a given study sample. Substantial reliability with kappas of 0.66–0.69 between two trained rheumatologist readers was recorded in a study of 217 AS patients who all met the modified NYc [46]. However, a recent report on a SpA inception cohort with only 15.7 % modified NYc positive back pain patients found an only fair reproducibility (kappa 0.39) among seven radiology and rheumatology readers with varying experience in imaging in SpA [40]. The limited reliability of the modified NYc may contribute to substantial measurement error when assessing sacroiliac radiographic progression in early SpA [47•]. Blinded evaluation of baseline and 2 years follow-up pelvic radiographs in a cohort of 449 clinically defined recent onset SpA patients showed a progression rate from modified NYc negative to positive subjects of 4.9 % after 2 years, but unexpectedly also a regression rate of comparable magnitude with 5.7 % switching back from modified NYc positive to negative.
A paradigmatic shift of the traditional approach to use pelvic radiography as first-line imaging assessment for classification of SpA seems premature. Radiography is readily available nearly worldwide and can be performed with minimal time loss and at relatively low costs. These feasibility issues do not apply to the potential alternative, MRI of the SIJ, which has limited or no access in many parts of the world. However, a critical re-appraisal of using pelvic radiographs in the clinical setting of suspected early SpA is warranted, and in health care settings, where SIJ MRI is readily available, it may be the preferred imaging modality in early axial SpA.
Future Directions
There is international consensus that the definition of a positive SIJ MRI in SpA is based on simultaneous assessment of concomitant structural and active lesions on T1SE and STIR sequences. This global assessment of SIJ MRI consistently showed the highest specificity compared to lesion-based assessment. The most important next step is to establish a comprehensive lesion-based definition containing both structural and active lesion types, erosion being the most reliable candidate to represent structural changes. Candidate definitions of a positive SIJ MRI in SpA should be tested in longitudinal and unselected cohorts of chronic back pain patients without applying classification criteria containing MRI features to avoid circularity issues.
A major limitation to interpretation of MRI of the axial skeleton is lack of data in clinically relevant control groups. Important steps towards a comprehensive lesion-based definition of a positive SIJ MRI in SpA are data from large cohorts of healthy controls to assess the “background noise” in SIJ and spine and their evolution over time, of mechanical back pain patients to refine the thresholds to discriminate this relevant differential diagnostic condition from early axial SpA, and of clinically relevant groups enduring higher than average axial mechanical load such as heavy labor workers, endurance sports athletes, or multiparous women.
Promising technical amendments to MRI acquisition protocols such as novel sequences to enhance recognition of structural lesions or axial planes to better discriminate SpA from non-SpA-related lesions need to be evaluated.
A major challenge of imaging evaluation in SpA barely addressed at present is the transfer of this rapidly expanding knowledge to the broad community of rheumatologists and radiologists. Imaging in SpA is usually not part of the standard training of rheumatologists, while radiologists spend much of their time in the evaluation of degenerative spinal disorders, and SpA constitutes only a small minority of routine practice.
Conclusions
Advanced imaging has become essential for recognition of disease in clinically suspected early SpA. BME alone, particularly if subtle in appearance, lacks specificity and may be misleading, as it is seen in about every fourth mechanical back pain patient and healthy volunteer. The reasons for the widespread prevalence of non-specific low-grade BME are poorly understood. The contextual information provided by structural lesions alongside with active changes by simultaneous assessment of complementary T1SE and STIR sequences has become pivotal to assist in timely recognition of clinically suspected axial SpA. Erosion proved to be the key lesion among structural damage changes due to high specificity and reproducibility and its presence in the majority of early SpA patients shortly after symptom onset, both in adult and juvenile SpA patients. The next step towards a comprehensive definition of a positive SIJ MRI in axial SpA, which incorporates both structural and active lesions, has been assigned top priority on the research agenda.
The increasing use of MRI in SpA revealed several limitations, such as differences in specificity between global versus lesion-based assessment, the selection of the most appropriate gold standard with which to compare MRI evaluation, and lack of data in clinically relevant control groups. There is an ongoing controversy as to whether SIJ MRI due to its superior reliability and ability to depict also active lesions should be the preferred imaging modality in early disease over the traditional approach with pelvic radiographs.
Despite manifold limitations, MRI has become an indispensable tool for recognition of early SpA, but is not able to capture all facets of the entire spectrum of disease. Thus, diagnosis of SpA remains a process of pattern recognition taking into account contextual information provided by clinical, laboratory, and imaging evaluation.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Weber U, Lambert RGW, Østergaard M, Hodler J, Pedersen SJ, Maksymowych WP. The diagnostic utility of magnetic resonance imaging in spondylarthritis. An international multicenter evaluation of one hundred eighty-seven subjects. Arthritis Rheum. 2010;62:3048–58.
Althoff CE, Sieper J, Song IH, Haibel H, Weiss A, Diekhoff T, et al. Active inflammation and structural change in early active axial spondyloarthritis as detected by whole-body MRI. Ann Rheum Dis. 2013;72:967–73.
Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–83.
Sepriano A, Landewé R, van der Heijde D, Sieper J, Akkoc N, Brandt J, et al. Predictive validity of the ASAS classification criteria for axial and peripheral spondyloarthritis after follow-up in the ASAS-cohort: a final analysis. Ann Rheum Dis. 2016;75:1034–42.
Rudwaleit M, Jurik AG, Hermann KGA, Landewé R, van der Heijde D, Baraliakos X, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI Group. Ann Rheum Dis. 2009;68:1520–7.
Deodhar A. Sacroiliac joint magnetic resonance imaging in the diagnosis of axial spondyloarthritis: “a tiny bit of white on two consecutive slices” may be objective, but not specific. Arthritis Rheum. 2016;68:775–8. This topical editorial reviews controversies in imaging of early SpA and recommends that the definition of a positive MRI in SpA should be highly specific and developed by a data-driven process.
Weber U, Maksymowych WP. Advances and challenges in spondyloarthritis imaging for diagnosis and assessment of disease. Curr Rheumatol Rep. 2013;15:345.
Arnbak B, Jurik AG, Hørslev-Petersen K, Hendricks O, Hermansen LT, Loft AG, et al. Associations between spondyloarthritis and MRI findings: a cross-sectional analysis of 1020 patients with persistent low back pain. Arthritis Rheum. 2016;68:892–900. In this Danish cohort study, 21% of 1020 consecutive young back pain patients showed SIJ BME meeting the ASAS definition of a positive MRI for SpA, 42% of whom had low grade BME.
Jaremko JL, Liu L, Winn NJ, Ellsworth JE, Lambert RGW. Diagnostic utility of magnetic resonance imaging and radiography in juvenile spondyloarthritis: evaluation of the sacroiliac joints in controls and affected subjects. J Rheumatol. 2014;41:963–70. This retrospective controlled cohort study in juvenile SpA showed high diagnostic utility of SIJ MRI and a prevalence of non-specific SIJ BME in 20% of the controls having a mean age of 15.1 years.
Prassopoulos PK, Faflia CP, Voloudaki AE, Gourtsoyiannis NC. Sacroiliac joints: anatomical variants on CT. J Comput Assist Tomogr. 1999;23:323–7.
Egund N, Jurik AG. Anatomy and histology of the sacroiliac joints. Semin Musculoskelet Radiol. 2014;18:332–40. This review describes the normal anatomy and histology of the SIJ to facilitate detection of the anatomical site of disease-specific lesions and of normal variants simulating disease.
Kellgren JH, Jeffrey MR. The epidemiology of chronic rheumatism. Volume 2: Atlas of Standard Radiographs of Arthritis. Oxford, UK: Blackwell Scientific Publications;1963:36–40.
Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–8.
Weiss PF, Xiao R, Biko DM, Chauvin NA. Assessment of sacroiliitis at diagnosis of juvenile spondyloarthritis by radiography, magnetic resonance imaging, and clinical examination. Arthritis Care Res. 2016;68:187–94.
Weber U, Østergaard M, Lambert RGW, Pedersen SJ, Chan SM, Zubler V, et al. Candidate lesion-based criteria for defining a positive sacroiliac joint MRI in two cohorts of patients with axial spondyloarthritis. Ann Rheum Dis. 2015;74:1976–82. In this study in 2 independent SpA inception cohorts, lesion-based criteria for a positive SIJ MRI based on both BME and/or erosion performed best for classification of axial SpA, reflecting the contextual information provided by T1SE and STIR sequences.
De Hooge M, van den Berg R, Navarro-Compán V, Reijnierse M, van Gaalen F, Fagerli K, et al. Patients with chronic back pain of short duration from the SPACE cohort: which MRI structural lesions in the sacroiliac joints and inflammatory and structural lesions in the spine are most specific for axial spondyloarthritis? Ann Rheum Dis. 2016;75:1308–14. This report from a recent-onset back pain cohort focussed on diagnostic utility of structural SIJ lesions only. Patients were enrolled according to the ASAS definition of a positive SIJ MRI, which was available to the rheumatologist formulating the gold standard expert opinion as to the diagnosis of SpA. Control subjects had up to 2 SpA items from the ASAS classification criteria set.
Herregods N, Dehoorne J, Joos R, Jaremko JL, Baraliakos X, Leus A, et al. Diagnostic value of MRI features of sacroiliitis in juvenile spondyloarthritis. Clin Radiol. 2015;70:1428–38.
Weber U, Pedersen SJ, Zubler V, Rufibach K, Chan SM, Lambert RGW, et al. Fat infiltration on magnetic resonance imaging of the sacroiliac joints has limited diagnostic utility in nonradiographic axial spondyloarthritis. J Rheumatol. 2014;41:75–83.
Lambert RGW, Bakker PAC, van der Heijde D, Weber U, Rudwaleit M, Hermann KGA, et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis. 2016. doi:10.1136/annrheumdis-2015-208642. This update on the definition of active sacroiliitis on MRI for classification of axial SpA incorporates the simultaneous assessment of MRI sequences designed to identify active and structural lesions taking into account the contextual information contained in both lesion types.
Weber U, Zubler V, Zhao Z, Lambert RGW, Chan SM, Pedersen SJ, et al. Does spinal MRI add incremental diagnostic value to MRI of the sacroiliac joints alone in patients with non-radiographic axial spondyloarthritis? Ann Rheum Dis. 2015;74:985–92. The combination of spine and SIJ MRI in 2 independent SpA inception cohorts added little incremental value compared with SIJ MRI alone for diagnosing patients with early axial SpA. Vertebral corner lesions were the main drivers towards misclassification.
Weber U, Zhao Z, Rufibach K, Zubler V, Lambert RGW, Chan SM, et al. Diagnostic utility of candidate definitions for demonstrating axial spondyloarthritis on magnetic resonance imaging of the spine. Arthritis Rheum. 2015;67:924–33. None of the previous candidate spinal thresholds for a positive spinal MRI in axial SpA showed clinically relevant diagnostic utility in 2 independent SpA inception cohorts.
Hermann KGA, Baraliakos X, van der Heijde DMFM, Jurik AG, Landewé R, Marzo-Ortega H, et al. Descriptions of spinal MR lesions and definition of a positive MRI of the spine in axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI study group. Ann Rheum Dis. 2012;71:1278–88.
Arnbak B, Jensen TS, Egund N, Zejden A, Hørslev-Petersen K, Manniche C, et al. Prevalence of degenerative and spondyloarthritis-related magnetic resonance imaging findings in the spine and sacroiliac joints in patients with persistent low back pain. Eur Radiol. 2016;26:1191–203.
De Bruin F, ter Horst S, Bloem HL, van den Berg R, de Hooge M, van Gaalen F, et al. Prevalence of degenerative changes of the spine on magnetic resonance images and radiographs in patients aged 16–45 years with chronic back pain of short duration in the Spondyloarthritis Caught Early (SPACE) cohort. Rheumatology. 2016;55:56–65.
Weber U, Pedersen SJ, Østergaard M, Rufibach K, Lambert RGW, Maksymowych WP. Can erosions on MRI of the sacroiliac joints be reliably detected in patients with ankylosing spondylitis—a cross-sectional study. Arthritis Res Ther. 2012;14:R124.
Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert RG, Pedersen SJ. Development and preliminary validation of the spondyloarthritis research consortium of Canada magnetic resonance imaging sacroiliac joint structural score. J Rheumatol. 2015;42:79–86.
Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert RG, Pedersen SJ. Fat metaplasia and backfill are key intermediaries in the development of sacroiliac joint ankylosis in patients with ankylosing spondylitis. Arthritis Rheum. 2014;66:2958–67. The findings in this prospective observational cohort of 147 AS patients support a disease model whereby ankylosis develops following repair of SIJ erosion with backfill as key intermediary step.
Pedersen SJ, Poddubnyy D, Sørensen IJ, Loft AG, Hindrup JS, Thamsborg G, et al. Course of magnetic resonance imaging-detected inflammation and structural lesions in the sacroiliac joints of patients in the randomized, double-blind, placebo-controlled Danish multicenter study of adalimumab in spondyloarthritis, as assessed by the Berlin and Spondyloarthritis Research Consortium of Canada methods. Arthritis Rheum. 2016;68:418–29. In this randomized controlled trial in axial SpA, treatment with a tumour necrosis factor inhibitor was associated with resolution of erosion and development of backfill.
Krohn M, Braum LS, Sieper J, Song IH, Weiss A, Callhoff J, et al. Erosions and fatty lesions of sacroiliac joints in patients with axial spondyloarthritis: evaluation of different MRI techniques and two scoring methods. J Rheumatol. 2014;41:473–80.
Weber U, Maksymowych WP, Chan SM, Rufibach K, Pedersen SJ, Zhao Z, et al. Does evaluation of the ligamentous compartment enhance diagnostic utility of sacroiliac joint MRI in axial spondyloarthritis? Arthritis Res Ther. 2015;17:246.
Jans L, van Praet L, Elewaut D, van den Bosch F, Carron P, Jaremko JL, et al. MRI of the SI joints commonly shows non-inflammatory disease in patients clinically suspected of sacroiliitis. Eur J Radiol. 2014;83:179–84.
Van Onna M, van Tubergen A, van der Heijde D, Jurik AG, Landewé R. Gadolinium contrast-enhanced MRI sequence does not have an incremental value in the assessment of sacroiliitis in patients with early inflammatory back pain by using MRI in combination with pelvic radiographs: a 2-year follow-up study. Clin Exp Rheumatol. 2014;32:225–30.
Weiss PF, Xiao R, Biko DM, Johnson AM, Chauvin NA. Detection of inflammatory sacroiliitis in children with magnetic resonance imaging. Is gadolinium contrast enhancement necessary? Arthritis Rheum. 2015;67:2250–6.
Herregods N, Jaremko JL, Baraliakos X, Dehoorne J, Leus A, Verstraete K, et al. Limited role of gadolinium to detect active sacroiliitis on MRI in juvenile spondyloarthritis. Skeletal Radiol. 2015;44:1637–46.
Jarrett SJ, Sivera F, Cawkwell LS, Marzo-Ortega H, McGonagle D, Hensor E, et al. MRI and clinical findings in patients with ankylosing spondylitis eligible for anti-tumour necrosis factor therapy after a short course of etoricoxib. Ann Rheum Dis. 2009;68:1466–9.
Varkas G, Jans L, Cypers H, van Praet L, Carron P, Elewaut D, et al. Six-week treatment of axial spondyloarthritis patients with an optimal dose of nonsteroidal antiinflammatory drugs: early response to treatment in signal intensity on magnetic resonance imaging of the sacroiliac joints. Arthritis Rheum. 2016;68:672–8.
Bedaiwi MK, Sari I, Wallis D, O'Shea FD, Salonen D, Haroon N, et al. Clinical efficacy of celecoxib compared to acetaminophen in chronic nonspecific low back pain: results of a randomized controlled trial. Arthritis Care Res. 2016;68:845–52.
Mandl P, Navarro-Compán V, Terslev L, Aegerter P, van der Heijde D, D’Agostino MA, et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis. 2015;74:1327–39. A set of 10 evidence-based recommendations on the use of imaging in the clinical management of axial and peripheral SpA was developed by systematic literature review and expert consensus.
Sudol-Szopińska I, Jurik AG, Eshed I, Lennart J, Grainger A, Østergaard M, et al. Recommendations of the ESSR arthritis subcommittee for the use of magnetic resonance imaging in musculoskeletal rheumatic diseases. Semin Musculoskelet Radiol. 2015;19:396–411.
Christiansen AA, Hendricks O, Kuettel D, Hørslev-Petersen K, Jurik AG, Nielsen S, et al. Evaluation of sacroiliac joint radiographs in patients with chronic low back pain: is erosion the main driver of interreader disagreement? Arthritis Rheum. 2015;67:S3852 [abstract 3210].
U.S. Food and Drug Administration, Department of Health & Human Services. Arthritis Advisory Committee Meeting: Adalimumab (Humira®) for active non-radiographic axial spondyloarthritis (nr-axSpA) in adults with objective signs of inflammation by elevated CRP or MRI, who have had inadequate response or are intolerant to NSAIDs; July 23, 2013; Available from: www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM361563.pdf.
U.S. Food and Drug Administration, Department of Health & Human Services. Arthritis Advisory Committee Meeting: Certolizumab (Cimzia®) for active axial spondyloarthritis, including ankylosing spondylitis; July 23, 2013; Available from: www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM361565.pdf.
Deodhar A, Reveille JD, van den Bosch F, Braun J, Burgos-Vargas R, Caplan L, et al. The concept of axial spondyloarthritis: joint statement of the spondyloarthritis research and treatment network and the Assessment of SpondyloArthritis international Society in response to the US Food and Drug Administration’s comments and concerns. Arthritis Rheum. 2014;66:2649–56.
Van den Berg R, Lenczner G, Feydy A, van der Heijde D, Reijnierse M, Saraux A, et al. Agreement between clinical practice and trained central reading in reading of sacroiliac joints on plain pelvic radiographs. Results from the DESIR cohort. Arthritis Rheum. 2014;66:2403–11. This report in a SpA inception cohort demonstrated only moderate agreement (kappa 0.54) of radiographic SIJ evaluation by central readers raising concerns about reliability of radiographic modified NYc in suspected early SpA.
Van den Berg R, Lenczner G, Thévenin F, Claudepierre P, Feydy A, Reijnierse M, et al. Classification of axial SpA based on positive imaging (radiographs and/or MRI of the sacroiliac joints) by local rheumatologists or radiologists versus central trained readers in the DESIR cohort. Ann Rheum Dis. 2015;74:2016–21. Assessment of SIJ MRI in the same SpA inception cohort as above showed substantial agreement among central readers (kappa 0.73) for the ASAS definition of a positive MRI based on active inflammatory lesions.
Spoorenberg A, de Vlam K, van der Linden S, Dougados M, Mielants H, van de Tempel H, et al. Radiological scoring methods in ankylosing spondylitis. Reliability and change over 1 and 2 years. J Rheumatol. 2004;31:125–32.
Dougados M, Demattei C, van den Berg R, Vo Hoang V, Thevenin F, Reijnierse M, et al. Rate and predisposing factors of sacroiliac radiographic progression after a 2 years follow-up period in recent onset spondyloarthritis. Arthritis Rheum. 2016. doi:10.1002/art.39666. Blinded evaluation of sacroiliac radiographic progression in early SpA over 2 years showed progression from modified NYc negative to positive in 4.9%, but vice versa also regression in 5.7% switching back from positive to negative.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
UW, AGJ, RGWL, and WPM declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Spondyloarthritis
Rights and permissions
About this article
Cite this article
Weber, U., Jurik, A.G., Lambert, R.G.W. et al. Imaging in Spondyloarthritis: Controversies in Recognition of Early Disease. Curr Rheumatol Rep 18, 58 (2016). https://doi.org/10.1007/s11926-016-0607-7
Published:
DOI: https://doi.org/10.1007/s11926-016-0607-7