Abstract
Magnetic resonance imaging (MRI) has become an increasingly important imaging technique in osteoarthritis (OA) research, and is widely used in the ongoing endeavor to understand the pathogenesis of OA and to develop structure and disease-modifying OA drugs. MRI offers semiquantitative, quantitative and compositional evaluation of knee OA, and enables visualization of tissues that are not seen by radiography, including but not limited to cartilage, meniscus, bone marrow lesions, synovitis, and muscles. It is now recognized that contrast-enhanced MRI enables more accurate evaluation of synovitis than MRI without contrast. Because of its ability to visualize multiple pain-related tissue pathology in three dimensions, MRI is the best modality for imaging of OA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent advances in magnetic resonance imaging (MRI) techniques have helped osteoarthritis (OA) investigators to understand the OA disease process and identify biomarkers for disease progression. Imaging of OA has heavily relied upon radiography, and radiography-detected joint space narrowing is still the only structural end-point recognized by the United States Food and Drug Administration and the European Medicines Agency as proving the efficacy of disease-modifying osteoarthritis drugs (DMOADs) in phase-III clinical trials. The inherent limitations of radiography have been well documented, however, and are believed by some investigators to be a possible reason for the failure to discover an effective DMOAD to date [1].
MRI enables visualization of several important pathological features of knee OA, including cartilage, meniscus, synovitis, and bone marrow lesions (BML)—although these MRI findings may be present for a large proportion of asymptomatic persons, and MRI-detected lesions may not always have clinical significance [2]. Nevertheless, the OA research community is increasingly using MRI for structural joint assessment in epidemiological and clinical OA studies [3]. Imaging of OA can be broadly classified as “morphological” versus “compositional”. The former includes semiquantitative [4] and quantitative [3] approaches, whereas the latter involves relatively new techniques including dGEMRIC, T2 mapping, T1 rho, sodium imaging, and diffusion imaging [5]. The purpose of this non-systematic narrative review is to present a summary of select original articles, published primarily over the last three years, that describe recent trends and developments from OA research studies using MRI-derived data. This review will focus on OA of the knee, which is the joint most extensively studied in OA research.
Technical Considerations
To enable optimum assessment of MRI-detectable OA features, investigators need to select appropriate pulse sequences. A detailed discussion of these technical challenges has been previously published [4]. In brief, semiquantitative assessment of focal cartilage defects and BML is best achieved by use of a short-tau inversion recovery sequence, or one of the fluid-sensitive (i.e. T2-weighted, intermediate-weighted, or proton density-weighted) fast-spin echo sequences with fat suppression [6, 7]. For longitudinal studies using a semiquantitative scoring of OA features, ‘within-grade’ changes should be recorded to ensure sufficient sensitivity to change [8•]. Moreover, readers of the images need to be fully trained to enable them to distinguish between true signal abnormality arising from pathological change and artifacts that resemble pathological signal changes. For example, susceptibility artifact may have a similar appearance to cartilage damage. Although MRI can be a powerful research tool, if not used correctly it may give misleading or invalid data.
MRI of Cartilage
Semiquantitative Scoring of Cartilage
Several semiquantitative scoring systems have been published, including the two most widely used systems, the whole organ MRI score (WORMS) and Boston Leeds OA knee score (BLOKS), and the newer MRI OA knee score (MOAKS) [4, 9]. A longitudinal study by Laberge et al. revealed that obesity increases the prevalence and severity of cartilage damage over 36 months [10]. Crema et al. revealed that prevalent cartilage damage (i.e. WORMS score of two or greater) and cartilage loss over time are associated with incident BML in the same tibiofemoral compartments. Their findings support the hypothesis that the close interrelation of the osteochondral unit is important to the progression of knee OA [11]. Recent studies provide evidence that focal cartilage defects increase risk of developing knee OA. Roemer et al. revealed that the presence of prevalent cartilage damage and non-cartilaginous pathology at baseline increase the risk of subsequent cartilage loss in the same subregion [12]. A recent population-based study revealed that focal cartilage defects in older adults are common, that most defects remained stable over 2.9 years, and that baseline cartilage defect grade predicted risk of knee arthroplasty over five years [13]. Using data from the Osteoarthritis Initiative (OAI), Virayavanich et al. revealed that frequent knee bending activity was associated with higher prevalence of knee cartilage damage (particularly in the patellofemoral compartment) and with an increased risk of progression of cartilage damage for asymptomatic middle-aged subjects who had risk factors for knee OA [14].
Quantitative Cartilage Morphometry
Quantitative cartilage morphometry has been used in several recent OA studies [15–18]. Quantitative measures of articular cartilage structure, for example cartilage thickness loss and denuded areas of subchondral bone, have been reported to be predictive of knee replacement [18]. However, investigators wishing to use the quantitative morphometry approach in a multicenter study should note that data collected from different segmentation teams should be combined only when equivalence is demonstrated for the cartilage metrics of interest: Schneider et al. revealed that segmentation-team differences were a major cause of measurement variability in most cartilage regions for all image series [19].
Eckstein et al. reported weak to moderate correlation and agreement between individual short vs. long-term cartilage loss, and between initial and subsequent observation periods [20]. These results support the theory that longer observation periods are preferable for achieving robust results concerning cartilage loss in individual knees.
McAlindon et al. performed a two-year randomized, placebo-controlled, double-blind, clinical trial, involving 146 participants with symptomatic knee OA, to determine whether vitamin D supplementation reduces symptom and structural progression of knee OA [21]. Compared with placebo, vitamin D supplementation for two years, at a dose sufficient to increase 25-hydroxyvitamin D plasma levels to above 36 ng mL−1, did not reduce knee pain or cartilage volume loss of patients with symptomatic knee OA.
Cao et al. revealed, cross-sectionally, that total body, total hip, spine bone mineral density (BMD), and/or lateral tibial spine BMD were significantly and positively associated with femoral, lateral tibial, and/or patellar cartilage thickness in subjects with ROA, after adjustment for potential confounders [22]. Longitudinally, a high total body BMD was associated with increased femoral cartilage thickness, a high spine BMD was associated with increases in femoral and lateral tibial cartilage thickness, and a high medial tibial spine BMD was associated with increased medial tibial cartilage thickness in subjects with radiographic OA. Thus, both systemic and subchondral BMD are positively associated with increased cartilage thickness in subjects with ROA, suggesting that body BMD might have a protective effect against cartilage thinning in knee OA.
Widmyer et al. reported that high body mass index (BMI) was associated with increased diurnal strain (i.e. change in thickness) in the articular cartilage of the knee [23]. However, it is yet to be determined whether this increase in diurnal strain for high-BMI individuals explains the increased OA risk associated with obesity or instead indicates altered cartilage mechanical properties for these subjects.
Compositional MRI of Cartilage
Compositional MRI enables visualization of the biochemical properties of hyaline cartilage. It may be sensitive to early, pre-morphological changes that are not visible on conventional MRI. Compositional imaging of cartilage matrix changes can be performed by use of advanced MRI techniques, including delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), T1 rho, and T2 mapping. Two of these, dGEMRIC and T1 rho, take advantage of the concentration of highly negatively charged glycosaminoglycans (GAGs) in healthy hyaline cartilage; loss of these GAGs in focal areas affected by possible early disease can be visualized. Both dGEMRIC and T1 rho focus on charge density in cartilage. In contrast, T2 concentrations are affected by a complex combination of the collagen orientation and hydration of cartilage.
Compositional MRI techniques are not routinely used in current clinical practice, and remain research tools that are available only at select academic institutions. Nonetheless, they have been used in clinical trials and observational studies. In a placebo-controlled double-blind pilot study of collagen hydrolysate for mild knee OA, McAlindon et al. [24] revealed that the dGEMRIC score increased (i.e. higher GAG content and better cartilage status) for tibial cartilage regions of interest of patients receiving collagen hydrolysate, and decreased for the placebo group. A significant difference was observed at 24-week follow-up. Future studies may determine whether macroscopic cartilage changes are associated with these early dGEMRIC findings. Souza et al. reported that acute loading of the knee joint resulted in a significant reduction in the T1 rho and T2 relaxation times of the medial tibiofemoral compartment, especially in cartilage regions with small focal defects [25]. These data suggest that changes of T1 rho value under mechanical loading may be related to the biomechanical and structural properties of cartilage. Hovis et al. reported that light exercise was associated with low cartilage T2 values but moderate and strenuous exercise were associated with high T2 values for women, suggesting that activity level can affect cartilage composition [26]. Using a longitudinal study design, Lin et al. revealed that high and very low levels of physical activity were associated with greater progression of cartilage T2 measurements of asymptomatic, middle-aged individuals, suggesting accelerated cartilage matrix biochemical degeneration over time [27]. In an interventional study assessing the effect of weight loss on articular cartilage, Anandacoomarasamy et al. reported that improved articular cartilage quality was indicated by an increase in dGEMRIC index over one year for the medial, but not the lateral, compartment [28]. These findings may reflect the importance of weight loss to possible clinical and structural improvement.
A variety of newer compositional techniques have also been investigated. Raya et al. found that in-vivo diffusion tensor imaging based on a 7 T MR system was better able than T2 mapping to distinguish OA knees from non-OA knees [29]. Other work on 7 T systems reported on the reproducibility of the method in vivo [30]. Other compositional techniques that might reward further investigation are T2* mapping [31] and sodium imaging [30] of cartilage. These techniques seem promising, but they will need to be rendered practical and compatible with standard MR imaging systems before they can be used for research or for making clinical diagnoses. A recent study by Newbould et al. revealed that a clinically feasible sodium MRI, with a 3 T field strength, moderate imaging time, and fluid attenuation by T1 weighting, successfully differentiated between healthy controls and OA subjects.
MRI of Meniscus
Meniscal pathology is believed to be linked to the knee OA disease process, because structural alterations (i.e. meniscal tear, maceration, or extrusion) can lead to a loss of normal function regarding buffering the mechanical load at the tibiofemoral joint [32]. Englund et al. studied multiple risk factors for medial meniscal pathology in 791 knees from the MOST study, with normal medial meniscal status at baseline, and found that knee trauma and generalized OA, expressed as multiple bony enlargements of finger joints, varus alignment, and obesity, were risk factors for medial meniscal pathology [33].
In their population-based study in South Korea, Kim et al. reported the frequency of meniscal damage for their subjects (mean age 72 years) to be 49.7 % for men and 71.2 % for women [34]. The severity of knee pain correlated significantly with medial meniscal damage grade. In a study by Stehling et al., when the knee was subjected to an axial mechanical load subjects with degenerative knee abnormality had significantly increased meniscus extrusion compared with normal subjects [35]. In a longitudinal controlled follow-up study of subjects receiving arthroscopic partial medial meniscectomy (APMM), Wang et al. described an increased risk for APMM patients of subsequent cartilage damage in the tibio and patello-femoral joints [36]. Using the BML and the data from the Multicenter Osteoarthritis Study, Crema et al. revealed an association of medial meniscal extrusion with medial meniscal tears, medial cartilage damage, and varus alignment, whereas lateral meniscal extrusion was associated with lateral meniscal tears, lateral cartilage damage, and valgus alignment [37]. In a two-year clinical trial involving 161 knee OA patients, Raynauld et al. revealed that severe medial meniscal tearing and medial meniscal extrusion detected by MRI at baseline were strong long-term predictors of total knee replacement [38]. Using data from the OAI, Badlani et al. revealed that knees with meniscus tears with greater radial involvement and extrusion are at greater risk of later development of radiographic OA [39]. Fig. 1.
The first paper from the meniscal tear with osteoarthritis research (MeTeOR) was recently published [40]. The MeTeOR trial is a multicenter, randomized, controlled trial involving symptomatic patients 45 years of age or older with an MRI-detected meniscal tear and evidence of mild-to-moderate OA, as determined by the presence of MRI-detected cartilage defects. Investigators randomly assigned 351 patients to surgery and postoperative physical therapy or to a standardized physical-therapy regimen (with the option to cross over to surgery at the discretion of the patient and surgeon). The patients were evaluated at six and 12 months. The primary outcome was the difference between the groups with respect to change in the Western Ontario and McMaster Universities osteoarthritis index (WOMAC) physical-function score (ranging from 0 to 100, with higher scores indicating more severe symptoms) six months after randomization. In the intention-to-treat analysis, no significant differences to functional improvement were found between the study groups six months after randomization. However, 30 % of patients assigned to the physical-therapy group crossed over to surgery during the first six months.
It should be noted that tearing of meniscal roots is a distinctly different entity from tearing of menici themselves. A study by Guermazi et al. revealed that isolated medial posterior meniscal root tearing (i.e. without tearing of the body or anterior and/or posterior horns of the medial meniscus) is associated with incident and progressive medial tibiofemoral cartilage loss [41•]. Meniscal root tears can lead to meniscal extrusion, because the meniscus loses a ligament that anchors it to the tibial plateau (Fig. 1). Thus, meniscal root tears should not be neglected by OA research studies.
In addition to morphological imaging of the meniscus by use of the conventional MRI sequences described above, publications have reported on use of different MRI techniques. Wenger et al. used a within-person comparative approach, and revealed that painful knees had reduced meniscal coverage of the tibial plateau and greater extrusion of the meniscal body when compared with knees without pain from a subcohort of the OAI [42]. The authors used a 3D meniscal segmentation technique, which provides quantitative measures of meniscal size, position, and other variables. Using the data from the OAI, a more recent study by the same group of researchers revealed altered meniscal position and shape (i.e. more bulging) in both compartments of knees with medial compartment OA compared with control knees without OA [43], implying that these findings may be related to OA pathogenesis and/or disease consequences. Using the dGEMRIC technique to assess the meniscus substance in knees of OA patients and normal control knees of younger subjects, Li et al. revealed significant differences between the groups when using both ionic and non-ionic contrast agents [44]. The authors concluded that the difference in meniscal T1Gd between OA patients and normal subjects was not determined by charge distribution, but might be related to changes in wash-in and wash-out kinetics. Wang et al. revealed that T1 rho values are higher in specific subregions of the meniscus and tibiofemoral cartilage, suggesting that regional damage of both femorotibial hyaline cartilage and menisci may be associated with osteoarthritis [45]. Williams et al. used the ultrashort echo time-enhanced (UTE) T2* mapping technique to detect human meniscus degeneration in vitro and in vivo in subjects at risk of developing OA [46]. In this cadaver and human study, significant elevations of UTE-T2* values were observed in the menisci of subjects with anterior cruciate ligament injury but without clinical evidence of subsurface meniscal abnormality. This finding suggests that UTE-T2* mapping is sensitive to subclinical meniscus degeneration, but it is not yet known whether altered intrameniscal biochemical values can predict progression to either meniscal degeneration and tearing or the development of OA.
MRI of Synovitis
Synovitis is an important pathological feature of knee OA because it is known to be associated with pain [47, 48•, 49, 50], and could therefore potentially be a target for a DMOAD. Synovitis can be evaluated by use of MRI with or without gadolinium contrast injection. Without contrast injection it is not possible to discriminate between synovial fluid (i.e. joint effusion) and the synovium, because both of them have the same hyperintensity on fat-suppressed fluid-sensitive sequences, including short tau inversion recovery (STIR) and fat-suppressed T2-weighted fast spin echo (FSE) sequences. Therefore, for synovitis evaluation by use of non-enhanced MRI (NEMRI), T2 hyperintensity within the Hoffa fat pad is used as a surrogate marker. Using NEMRI and a semiquantitative approach, Knoop et al. revealed that quadriceps weakness was associated with synovitis for patients with knee OA [51]. Zhang et al. revealed that change in synovitis is associated with fluctuations of knee pain for patients with knee OA [49].
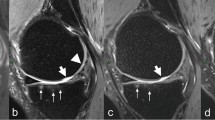
Importantly, a study by Loeuille et al. correlated MRI findings with findings from histology based on synovial membrane biopsy, and revealed that contrast-enhanced MRI (CEMRI) could detect biopsy-proved synovitis, whereas NEMRI could not [52••]. Crema et al. revealed that CEMRI is more specific than NEMRI for synovitis and is better correlated with pain [50]. Using semiquantitative scoring on CEMRI as the reference for synovitis, NEMRI has a high sensitivity (0.71–0.88) but a relatively low specificity (0.30–0.55) [50]. Although one study found that NEMRI-based semiquantitative assessment of synovitis is associated with measurements of synovitis based on CEMRI [53], increasing literature evidence suggests that CEMRI is a better option for MRI-based synovitis assessment (Fig. 2). Identification of the location at which synovitis occurs seems to be an important part of the evaluation. Damage of the posterior horns of the meniscus is associated with adjacent perimeniscal synovitis (adjusted odds ratio 2.5, 95 % CI 1.3–4.8) but not with synovitis of the posterior cruciate recess, indicating that synovitis at these two locations may have different pathomechanisms [54]. Baker et al. revealed a positive association between plasma mediators of inflammation and synovitis evaluated by use of CEMRI [55]. For future clinical and epidemiological studies focusing on synovitis, more than one CEMRI-based semiquantitative scoring system is available [48•, 55]. The most recently published scoring system by Guermazi et al. enables comprehensive evaluation of whole knee synovitis, including evaluation of 11 anatomical locations [48•], although this system needs to be validated by future studies. NEMRI-based scoring systems are also available [9, 56–58] and are currently much more widely used than CEMRI-based scoring systems. Where use of CEMRI is not possible, NEMRI is still a valid alternative for synovitis assessment, provided that investigators are aware of the limitations of NEMRI as described above.
Comparison of non-enhanced (NE–MRI) and contrast-enhanced MRI (CE–MRI) of synovitis in knee osteoarthritis. Axial NE–MRI shows a fluid-equivalent signal within the joint cavity, suggestive of joint effusion in the peripatellar recesses (arrows). It is not possible to distinguish between effusion and inflamed synovium because both are depicted as hyperintensity. In contrast, axial CE–MRI shows marked synovial enhancement (arrowheads), which can be clearly differentiated from the effusion depicted as hypointensity (arrow)
MRI of Bone Marrow Lesions (BML)
Although MRI-detected BML in and of themselves can be a non-specific finding and may indicate pathological processes other than OA [59], in the context of knee OA they are an important feature and are extensively studied by the OA research community. Recent MRI-driven research studies indicate that BML might be a potential imaging biomarker for quantifying structural change in knee joints affected by OA [60].
To better understand the biological and mechanical pathways linking cartilage, bone, and marrow changes in the progression of OA, Kazakia et al. used micro-computed tomography (μCT), high-resolution peripheral quantitative computed tomography (HR-pQCT), and Fourier transform infrared (FTIR) spectroscopy to evaluate the bone structure and composition of BML associated with knee OA, [61]. Trabecular bone within BML was higher in volume fraction, with more and thicker trabeculae that were more plate-like in structure compared with those in unaffected regions. BML trabecular tissue composition had lower phosphate and carbonate content. Marrow infiltration by a fibrous collagen network and evidence of increased bone remodeling were present. These structural and compositional changes were specifically localized to regions underlying cartilage degradation, supporting the paradigm of focal interactions among bone, marrow, and cartilage in the progression of knee OA.
In a population-based study of subjects with knee pain, Ip et al. revealed that BML were present in 11 % of subjects with no OA, 38 % of subjects with pre-radiographic OA (Kellgren-Lawrence grade <2 on radiography, but with notable cartilage damage on MRI), and 71 % of subjects with radiographic OA [62]. BML were significantly associated with pain on climbing stairs, but not with pain on walking or with total WOMAC score. In another population-based study, the Framingham osteoarthritis study, Guermazi et al. reported that 52 % (371/710) of persons without radiographic OA had BML, irrespective of pain status [2]. Stein et al. studied 160 participants from the progression subcohort of the OAI and revealed that knees with an anterior cruciate ligament (ACL) tear had significantly more BML in the lateral femur than those without ACL tearing. Using the data from the MOST study, Hayashi et al. reported that knee malalignment is associated with increased risk of incident and enlarging BML in the more loaded compartments of the tibiofemoral joint [63]. These findings emphasize the effect of joint biomechanics on structural integrity of the whole joint, including subchondral bone [64]. Also using data from the MOST study, Zhang et al. [49] revealed that changes to MRI-detected BML were associated with corresponding fluctuations in knee pain. A reduction in BML was associated with pain resolution, suggesting that subchondral bone might be a potential target for individualized therapy.
A report by Dore et al. described a possible association of systemic and nutritional risk factors with consequent BML development, supporting the hypothesis that subchondral bone metabolism is not affected only by the biomechanical properties of localized loading [65]. A randomized clinical trial reported by Laslett et al. revealed that a single infusion of zoledronic acid reduces knee pain and areal BML size and increases the proportion improving over six months [66].
Recently, two novel methods were proposed to enable quantification of the volume of BML. Pang et al. [67] used a semi-automated segmentation technique that was validated against BLOKS scoring of BML, and Ratzlaff et al. also used a semi-automated method that was validated against the WORMS scoring of BML [68]. Although both methods were validated against established semiquantitative scoring systems, it is yet to be determined whether these novel techniques will have an important function in OA clinical trials in the future.
MRI of Muscles
MRI enables excellent delineation of muscles, and thus can be used to measure cross-sectional areas or lengths of lower limb muscles. Sattler et al. revealed that knees with frequent pain have lower anatomical cross-sectional areas and experience less force from the quadriceps (but not from other thigh muscles) compared with contralateral knees without knee pain at the same radiographic stage [69]. Frequent pain did not seem to affect the correlation between anatomical cross-sectional area and strength of OA knees. These findings suggested that quadriceps-strengthening exercise might be useful for treating symptomatic knee OA. Building on this, Ruhdorfer et al. revealed there were no significant side differences of quadriceps (or other thigh muscle) anatomical cross-sectional area between knees with medial joint space narrowing (JSN) on radiography and knees without JSN, or between specific medial JSN knee strata and contralateral knees without JSN, for either men or women [70]. Two-year longitudinal changes in thigh muscle anatomical cross-sectional areas were small and did not differ significantly between medial JSN knees and knees without JSN. These longitudinal findings indicated that a more advanced radiographic stage of knee OA is not necessarily associated with a longitudinal decline in muscle function.
Conclusions
MRI has become a powerful research tool for the OA research community, and an increasing amount of MRI-derived data is being published in the literature. MRI overcomes the inherent limitations of radiography and enables evaluation of a variety of tissues and pathological features associated with pain. Semiquantitative and quantitative approaches are available for morphological analysis, and newer compositional MRI techniques have promise for imaging of pre-morphological changes within tissue including cartilage and meniscus. Compared with NEMRI, CEMRI enables more accurate assessment of synovitis in knee OA, although the former is still a valid alternative when CEMRI is not available. Provided that investigators use the appropriate MRI procedures to achieve the objectives of their study, MRI will help to identify the pathological basis of OA and, hopefully, enable the development of a long-desired DMOAD.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Guermazi A, Roemer FW, Felson DT. Inadequacy of radiographs: one cause for failure of clinical trials to identify a disease modifying drug for osteoarthritis. Arthritis Rheum. 2013. doi:10.1002/art.38086.
Guermazi A, Niu J, Hayashi D, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study). BMJ. 2012;345:e5339.
Eckstein F, Wirth W, Nevitt MC. Recent advances in osteoarthritis imaging—the Osteoarthritis Initiative. Nat Rev Rheumatol. 2012;8:622–30.
Guermazi A, Roemer FW, Haugen IK, et al. MRI-based semiquantitative scoring of joint pathology in osteoarthritis. Nat Rev Rheumatol. 2013;9:236–51.
Crema MD, Roemer FW, Guermazi A. Magnetic resonance imaging in knee osteoarthritis research: semiquantitative and compositional assessment. Magn Reson Imaging Clin N Am. 2011;19:295–321.
Roemer FW, Kwoh CK, Hannon MJ, et al. Semiquantitative assessment of focal cartilage damage at 3T MRI: a comparative study of dual echo at steady state (DESS) and intermediate-weighted (IW) fat suppressed fast spin echo sequences. Eur J Radiol. 2011;80:e126–31.
Hayashi D, Guermazi A, Kwoh CK, et al. Semiquantitative assessment of subchondral bone marrow edema-like lesions and subchondral cysts of the knee at 3T MRI: a comparison between intermediate-weighted fat-suppressed spin echo and dual echo steady state sequences. BMC Musculoskelet Disord. 2011;12:198.
• Roemer FW, Nevitt MC, Felson DT, et al. Predictive validity of within-grade scoring of longitudinal changes of MRI-based cartilage morphology and bone marrow lesion assessment in the tibio-femoral joint—the MOST study. Osteoarthritis Cartilage. 2012;20:1391–8. Describes the validity of within-grade scoring, which is currently standard practice in semiquantitative scoring of knee osteoarthritis features in research studies.
Hunter DJ, Guermazi A, Lo GH, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI osteoarthritis knee score). Osteoarthritis Cartilage. 2011;19:990–1002.
Laberge MA, Baum T, Virayavanich W, et al. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle aged subjects—data from the Osteoarthritis Initiative. Skeletal Radiol. 2012;41:633–41.
Crema MD, Felson DT, Roemer FW, et al. Prevalent cartilage damage and cartilage loss over time are associated with incident bone marrow lesions in the tibiofemoral compartments: the MOST study. Osteoarthritis Cartilage. 2013;21:306–13.
Roemer FW, Felson DT, Wang K, et al. Co-localisation of non-cartilaginous articular pathology increases risk of cartilage loss in the tibiofemoral joint – the MOST study. Ann Rheum Dis. 2013;72:942–8.
Carnes J, Stannus O, Cicuttini F, et al. Knee cartilage defects in a sample of older adults: natural history, clinical significance and factors influencing change over 2.9 years. Osteoarthritis Cartilage. 2012;20:1541–7.
Virayavanich W, Alizai H, Baum T, et al. Association of frequent knee bending activity with focal knee lesions detected with 3T MRI—data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2013. doi:10.1002/acr.22017.
Bennell KL, Bowles KA, Wang Y, et al. Higher dynamic medial knee load predicts greater cartilage loss over 12 months in medial knee osteoarthritis. Ann Rheum Dis. 2011;70:1770–4.
Eckstein F, Cotofana S, Wirth W, et al. Greater rates of cartilage loss in painful knees than in pain-free knees after adjustment for radiographic disease stage: data from the Osteoarthritis Initiative. Arthritis Rheum. 2011;63:2257–67.
Eckstein F, Nevitt M, Gimona A, et al. Rates of change and sensitivity to change in cartilage morphology in healthy knees and in knees with mild, moderate, and end-stage radiographic osteoarthritis: results from 831 participants from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2011;63:311–9.
Eckstein F, Kwoh CK, Boudreau RM, et al. Quantitative MRI measures of cartilage predict knee replacement: a case–control study from the Osteoarthritis Initiative. Ann Rheum Dis. 2013;72(5):707–14.
Schneider E, Nevitt M, McCulloch C, et al. Equivalence and precision of knee cartilage morphometry between different segmentation teams, cartilage regions, and MR acquisitions. Osteoarthritis Cartilage. 2012;20:869–79.
Eckstein F, McCulloch CE, Lynch JA, et al. How do short-term rates of femorotibial cartilage change compare to long-term changes? four year follow-up data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2012;20:1250–7.
McAlindon T, LaValley M, Schneider E, et al. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: a randomized controlled trial. JAMA. 2013;309:155–62.
Cao Y, Stannus OP, Aitken D, et al. Cross-sectional and longitudinal associations between systemic, subchondral bone mineral density and knee cartilage thickness in older adults with or without radiographic osteoarthritis. Ann Rheum Dis. 2013. doi:10.1136/annrheumdis-2013-203691.
Widmyer MR, Utturkar GM, Leddy HA, et al. High body mass index is associated with increased diurnal strains in the articular cartilage of the knee. Arthritis Rheum. 2013. doi:10.1002/art.38062.
McAlindon TE, Nuite M, Krishnan N, et al. Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: a pilot randomized controlled trial. Osteoarthritis Cartilage. 2011;19:399–405.
Souza RB, Stehling C, Wyman BT, et al. The effects of acute loading on T1rho and T2 relaxation times of tibiofemoral articular cartilage. Osteoarthritis Cartilage. 2010;18:1557–63.
Hovis KK, Stehling C, Souza RB, et al. Physical activity is associated with magnetic resonance imaging-based knee cartilage T2 measurements in asymptomatic subjects with and those without osteoarthritis risk factors. Arthritis Rheum. 2011;63:2248–56.
Lin W, Alizai H, Joseph GB, et al. Physical activity in relation to knee cartilage T2 progression measured with 3 T MRI over a period of 4 years: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2013. doi:10.1016/j.joca.2013.06.022.
Anandacoomarasamy A, Leibman S, Smith G, et al. Weight loss in obese people has structure-modifying effects on medial but not on lateral knee articular cartilage. Ann Rheum Dis. 2012;71:26–32.
Raya JG, Horng A, Dietrich O, et al. Articular cartilage: in vivo diffusion-tensor imaging. Radiology. 2012;262:550–9.
Madelin G, Babb JS, Xia D, et al. Reproducibility and repeatability of quantitative sodium magnetic resonance imaging in vivo in articular cartilage at 3 T and 7 T. Magn Reson Med. 2012;68:841–9.
Newbould RD, Miller SR, Toms LD, et al. T2* measurement of the knee articular cartilage in osteoarthritis at 3T. J Magn Reson Imaging. 2012;35:1422–9.
Englund M, Roemer FW, Hayashi D, et al. Meniscus pathology, osteoarthritis and the treatment controversy. Nat Rev Rheumatol. 2012;8:412–9.
Englund M, Felson DT, Guermazi A, et al. Risk factors for medial meniscal pathology on knee MRI in older US adults: a multicentre prospective cohort study. Ann Rheum Dis. 2011;70:1733–9.
Kim HA, Kim I, Song YW, et al. The association between meniscal and cruciate ligament damage and knee pain in community residents. Osteoarthritis Cartilage. 2011;19:1422–8.
Stehling C, Souza RB, Hellio Le Graverand MP, et al. Loading of the knee during 3.0T MRI is associated with significantly increased medial meniscus extrusion in mild and moderate osteoarthritis. Eur J Radiol. 2012;81:1839–45.
Wang Y, Dempsey AR, Lloyd DG, et al. Patellofemoral and tibiofemoral articular cartilage and subchondral bone health following arthroscopic partial medial meniscectomy. Knee Surg Sports Traumatol Arthrosc. 2012;20:970–8.
Crema MD, Roemer FW, Felson DT, et al. Factors associated with meniscal extrusion in knees with or at risk for osteoarthritis: the Multicenter Osteoarthritis Study. Radiology. 2012;264:494–503.
Raynauld JP, Martel-Pelletier J, Haraoui B, et al. Risk factors predictive of joint replacement in a 2-year multicentre clinical trial in knee osteoarthritis using MRI: results from over 6 years of observation. Ann Rheum Dis. 2011;70:1382–8.
Badlani JT, Borrero C, Golla S, et al. The effects of meniscus injury on the development of knee osteoarthritis: data from the Osteoarthritis Initiative. Am J Sports Med. 2013;41:1238–44.
Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368:1675–84.
• Guermazi A, Hayashi D, Jarraya M, et al. Medial posterior meniscal root tears are associated with development or worsening of medial tibiofemoral cartilage damage: The Multicenter Osteoarthritis Study. Radiology. 2013. Describes meniscal root tear as an important feature of knee osteoarthritis.
Wenger A, Englund M, Wirth W, et al. Relationship of 3D meniscal morphology and position with knee pain in subjects with knee osteoarthritis: a pilot study. Eur Radiol. 2012;22:211–20.
Wenger A, Wirth W, Hudelmaier M, et al. Meniscus body position, size, and shape in persons with and persons without radiographic knee osteoarthritis: quantitative analyses of knee magnetic resonance images from the Osteoarthritis Initiative. Arthritis Rheum. 2013;65:1804–11.
Li W, Edelman RR, Prasad PV. Delayed contrast enhanced MRI of meniscus with ionic and non-ionic agents. J Magn Reson Imaging. 2011;33:731–5.
Wang L, Chang G, Xu J, et al. T1rho MRI of menisci and cartilage in patients with osteoarthritis at 3T. Eur J Radiol. 2012;81:2329–36.
Williams A, Qian Y, Golla S, et al. UTE-T2* mapping detects sub-clinical meniscus injury after anterior cruciate ligament tear. Osteoarthritis Cartilage. 2012;20:486–94.
Baker K, Grainger A, Niu J, et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann Rheum Dis. 2010;69:1779–83.
• Guermazi A, Roemer FW, Hayashi D, et al. Assessment of synovitis with contrast-enhanced MRI using a whole-joint semiquantitative scoring system in people with, or at high risk of, knee osteoarthritis: the MOST study. Ann Rheum Dis. 2011;70:805–11. Describes comprehensive semiquantitative scoring of synovitis in knee osteoarthritis using contrast-enhanced MRI.
Zhang Y, Nevitt M, Niu J, et al. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum. 2011;63:691–9.
Crema MD, Felson DT, Roemer FW, et al. Peripatellar synovitis: comparison between non-contrast-enhanced and contrast-enhanced MRI and association with pain. The MOST study. Osteoarthritis Cartilage. 2013;21:413–8.
Knoop J, Dekker J, Klein JP, et al. Biomechanical factors and physical examination findings in osteoarthritis of the knee: associations with tissue abnormalities assessed by conventional radiography and high resolution 3.0 Tesla magnetic resonance imaging. Arthritis Res Ther. 2012;14:R212.
•• Loeuille D, Sauliere N, Champigneulle J, et al. Comparing non-enhanced and enhanced sequences in the assessment of effusion and synovitis in knee OA: associations with clinical, macroscopic and microscopic features. Osteoarthritis Cartilage. 2011;19:1433–9. Important study revealing scientific evidence that contrast-enhanced MRI enables more accurate assessment of synovitis in knee osteoarthritis than non-enhanced MRI.
Krasnokutsky S, Belitskaya-Lévy I, Bencardino J, et al. Quantitative magnetic resonance imaging evidence of synovial proliferation is associated with radiographic severity of knee osteoarthritis. Arthritis Rheum. 2011;63:2983–91.
Roemer FW, Felson DT, Yang T, et al. The association between meniscal damage of the posterior horns and localized posterior synovitis detected on T1-weighted contrast-enhanced MRI—the MOST study. Semin Arthritis Rheum. 2013;42:573–81.
Baker KR, Matthan NR, Lichtenstein AH, et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthritis Cartilage. 2012;20:382–7.
Peterfy CG, Guermazi A, Zaim S, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90.
Kornaat PR, Ceulemans RY, Kroon HM, et al. MRI assessment of knee osteoarthritis: knee osteoarthritis scoring system (KOSS)—inter-observer and intra-observer reproducibility of a compartment-based scoring system. Skeletal Radiol. 2005;34:95–102.
Hunter DJ, Lo GH, Gale D, et al. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds osteoarthritis knee score). Ann Rheum Dis. 2008;67:206–11.
Xu L, Hayashi D, Roemer FW, et al. Magnetic resonance imaging of subchondral bone marrow lesions in association with osteoarthritis. Semin Arthritis Rheum. 2012;42:105–18.
Kwoh CK. Clinical relevance of bone marrow lesions in OA. Nat Rev Rheumatol. 2013;9:7–8.
Kazakia GJ, Kuo D, Schooler J, et al. Bone and cartilage demonstrate changes localized to bone marrow edema-like lesions within osteoarthritic knees. Osteoarthritis Cartilage. 2013;21:94–101.
Ip S, Sayre EC, Guermazi A, et al. Frequency of bone marrow lesions and association with pain severity: results from a population-based symptomatic knee cohort. J Rheumatol. 2011;38:1079–85.
Hayashi D, Englund M, Roemer FW, et al. Knee malalignment is associated with an increased risk for incident and enlarging bone marrow lesions in the more loaded compartments: the MOST study. Osteoarthritis Cartilage. 2012;20:1227–33.
Stein V, Li L, Lo G, et al. Pattern of joint damage in persons with knee osteoarthritis and concomitant ACL tears. Rheumatol Int. 2012;32:1197–208.
Dore D, de Hoog J, Giles G, et al. A longitudinal study of the association between dietary factors, serum lipids, and bone marrow lesions of the knee. Arthritis Res Ther. 2012;14:R13.
Laslett LL, Doré DA, Quinn SJ, et al. Zoledronic acid reduces knee pain and bone marrow lesions over 1 year: a randomised controlled trial. Ann Rheum Dis. 2012;71:1322–8.
Pang J, Driban JB, Destenaves G, et al. Quantification of bone marrow lesion volume and volume change using semi-automated segmentation: data from the Osteoarthritis Initiative. BMC Musculoskelet Disord. 2013;14:3.
Ratzlaff C, Guermazi A, Collins J, et al. A rapid, novel method of volumetric assessment of MRI-detected subchondral bone marrow lesions in knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:806–14.
Sattler M, Dannhauer T, Hudelmaier M, et al. Side differences of thigh muscle cross-sectional areas and maximal isometric muscle force in bilateral knees with the same radiographic disease stage, but unilateral frequent pain—data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2012;20:532–40.
Ruhdorfer A, Dannhauer T, Wirth W, et al. Thigh muscle cross-sectional areas and strength in advanced versus early painful osteoarthritis: an exploratory between-knee, within-person comparison in Osteoarthritis Initiative participants. Arthritis Care Res (Hoboken). 2013;65:1034–42.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Ali Guermazi has served as a consultant for Sanofi Aventis, Merck Serono, and TissueGene and has held stock/stock options in Boston Imaging Core Lab, LLC. C. Kent Kwoh has served on an advisory board for Pfizer, has received grant support from Abbvie, has received payment for development of educational presentations (including service on speakers bureaus) from Dinora and Creative Educational Concepts, and has served on a data safety and monitoring board for Novartis. Daichi Hayashi declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with animal subjects performed by any of the authors. With regard to the authors’ research cited in this paper, all procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Imaging
Rights and permissions
About this article
Cite this article
Hayashi, D., Guermazi, A. & Kwoh, C.K. Clinical and Translational Potential of MRI Evaluation in Knee Osteoarthritis. Curr Rheumatol Rep 16, 391 (2014). https://doi.org/10.1007/s11926-013-0391-6
Published:
DOI: https://doi.org/10.1007/s11926-013-0391-6