Abstract
The 2012 renewed Chapel Hill Consensus Conference (CHCC) officially named three clinicopathological entities, i.e. granulomatosis with polyangiitis (GPA), eosinophilic granulomatosis with polyangiitis (EGPA), and microscopic polyangiitis (MPA), as major variants of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV). Recent genetic and cohort studies revealed the need for further differentiation between the entities, for example regarding differences in outcome. As well as ANCA reactivity, upper and lower airway disease were found to be differentiating factors for AAV variants, improving prognostic ability regarding relapse prediction and associated clinical features. Extravascular granulomatosis, or “granuloma”, which describes both clinically relevant granulomatous manifestations and histopathologically documented granulomatous inflammation, is characteristic of localized and systemic GPA, but not MPA. This review summarizes new knowledge regarding granuloma in the head and neck region of AAV, its histomorphological equivalents in the upper and lower respiratory tract, and evidence for a granulomatous phenotype of a persistent localized GPA variant. This comprises the development of disease activity and damage scores for extravascular lesions in the ear, nose, and throat (ENT) regions, and imaging techniques. In addition, findings linking extravascular manifestations to granulomatous inflammation are described. We hypothesize that, as for ANCA, necrotizing granulomatous inflammation and its clinical manifestations are discriminators, assisting subclassification of AAV and/or GPA subphenotypes which will be useful both for designing clinical trials and for treating patients successfully.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 2012 revised Chapel Hill Consensus Conference (CHCC) defined microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA, formerly WG), eosinophilic granulomatosis with polyangiitis (EGPA, formerly CSS), and single-organ manifestations (e.g. renal-limited forms) as the major clinicopathological variants of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV) [1]. They belong to the category of vasculitides predominantly affecting small vessels, have a pauci-immune vasculitis, and are probably pathogenetically associated with either proteinase 3-ANCA (PR3-ANCA) or myeloperoxidase-ANCA (MPO-ANCA) [2]. Because the diseases are rare and there is some overlap between them, most clinical trials have recruited a mixture of AAV patients, or at least of both GPA and MPA patients [3]. AAV patients commonly have signs or symptoms of systemic vasculitis in the lungs and kidneys, and have a poor prognosis if left untreated [4]. In recent years management has improved, and even life and/or organ-threatening AAV is today a treatable condition [5, 6]. European Vasculitis Society (EUVAS) randomized controlled trials (RCT) defining all AAV patients as belonging to a single category reported reduced survival, mainly caused by adverse events and/or chronically relapsing disease progression [7–9]. However, small and large cohorts comprising only GPA patients had better outcomes, with fewer deaths and reduced relapse [10, 11].
The need to recognize different clinical phenotypes observed within AAV [12] and to distinguish between different forms of one entity, e.g. GPA, has been discussed before [13]. Recent genome-wide association (GWAS) data, cohort studies and further improvement of diagnostic tools have provided support for the hypothesis that MPA, GPA, and otherwise defined entities should be studied separately [14]. New diagnostic or classification criteria that enable more accurate discrimination between different entities (or courses) have been acknowledged, suggesting that documentation of both ANCA specificity and granulomatous manifestations improves prognostic ability regarding clinical outcomes [15, 16].
Extravascular granulomatosis featuring necrotizing granulomatous inflammation, or “granuloma”, which describes both clinically relevant granulomatous manifestation and a histopathological characteristic, is symptomatic of both localized and systemic GPA. Granulomatous inflammation of the ENT region is associated with tissue and bone destruction [17] and, similarly to PR3-ANCA, upper and/or lower respiratory tract involvement seems to be associated with frequent relapse [18]. Interestingly, GPA relapses, especially those involving the upper airways, were reduced after treatment with co-trimoxazole [19, 20]; this topic has recently been reviewed [6]. The main way in which MPA differs from GPA is that extravascular granulomatosis is, by definition, absent, but vasculitis symptoms and renal disease are more prominent. The most important predictors of renal outcome are creatinine level at diagnosis, sclerotic lesions, and the number of normal glomeruli in the kidney biopsy [21]. EGPA is the least common of the major AAV entities. It seems to progress in stages similar to those of GPA, but extravascular features differ histomorphologically and clinically. Hypereosinophilia and asthma are central clinical manifestations, the latter usually preceding vasculitis by some years and being associated with symptoms of allergic rhinitis [22, 23]. Necrotizing granulomatous inflammation of the ENT tract with cartilage or bone destruction is not observed. Extravascular (eosinophil-rich) interstitial inflammation with involvement of the myocardium is associated with bad prognosis [23, 24]. In contrast with MPA, the time of testing is critical to identifying ANCA-positive EGPA patients [25]. As well as the idiopathic phenotypes listed above, ANCA-associated vasculitis can have other etiologies; for example, it may be drug or infection-induced (reviewed in Ref. [26]).
Herein, we summarize evidence that ANCA specificity can be used to distinguish between AAV varieties, and argue that extravascular manifestations, especially granuloma, can be used for the same purpose. The strongest argument for granuloma’s differentiating function comes from the description of a localized GPA variant that did not develop into full-blown disease, which was reported by two independent studies [27•, 28•]. By analogy with the use of ANCA to discriminate between AAV phenotypes, we discuss the potential use of granuloma for future subgroup differentiation.
ANCA Specificity is a Discriminating Factor in AAV
Genetic Studies: Single Nucleotide Polymorphisms and Alleles are Associated with MPO-ANCA or PR3-ANCA
Initial data generated from candidate genes revealed heterogenic backgrounds for GPA, EGPA, and MPA. In an extended association screening of apoptosis-linked genes, a haplotype surrounding the RXRB gene (encoding the retinoid X receptor β) on chromosome 6q21.3 was highly significantly associated with GPA [29]. This haplotype includes the HLA-DPB1 gene, which had previously been associated with chronic beryllium disease, another granulomatous disease. This association was subsequently confirmed for an independent sample, with impressive effect size (odds ratio 3.38). The association with this allele (HLA-DPB1*0401) was only observed for ANCA-positive GPA [30]. Kelley and co-workers [31] observed IgA and IgG ANCA engagement of Fc receptor genetic variants, which might also affect the outcome of GPA. A strong association of EGPA with IL-10 promoter polymorphisms was detected [32]. In the search for genetic links in AAV, a major collaborative GWAS study organized by the EUVAS group combined single nucleotide polymorphisms (SNP) screening with candidate gene screening. The results confirmed previous associations between GPA and the HLA-DP region, SERPINA1, and PR3; risk of MPA is associated with the HLA-DQ region. Unexpectedly, the strongest associations with risk genes were observed for ANCA subtypes, i.e. PR3-ANCA or MPO-ANCA [33••], revealing the relevance of autoimmune mechanisms and the pathogenic function of ANCA. However, a warning was given not to convert the data immediately into “direct clinical implications in terms of disease classification or diagnosis”, because of inherent limitations in the first GWAS study [34] and, for example, a lack of functional consequences of the PR3 promoter SNP (rs62132295) had already been described some time previously [35]. A second GWAS study, organized by the VCRC group in the US, re-confirmed the strong association between GPA and the HLA-DP allele [36]. Interestingly, polymorphisms in the toll-like receptor 9 gene also differed, being either PR3-ANCA+ and MPO-ANCA+ AAV or GPA and MPA, respectively [37]. Transcription profiles of different cellular subsets identified for vasculitis and for individual entities, including GPA [38, 39], could help to identify (bio)markers and assist separation of subphenotypes. One could also envisage future genetic studies analyzing extreme subphenotypes, on the basis of cohort studies resembling GPA studies already performed [27•, 28•] (Fig. 1), to determine whether specific risk genes or molecular profiles can be identified.
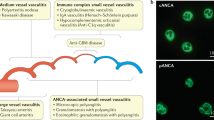
Definition of GPA according to the EULAR/EUVAS scheme [3], revealing that improved understanding of disease variations has led to higher resolution with regard to identification of subphenotypes and transitions. Left side: computed tomographic scan of the head with a destroyed orbital wall (white arrow) (from Ref. [73]; reprinted with permission from the Massachusetts Medical Society; copyright 2005)
Cohort Studies: ANCA Specificity is Useful for Prognostic Subclassification
Confirming and extending previous results [40–42], a recent study has found ANCA to be superior to CHCC and European Medicines Agency classifications for predicting relapse. PR3-ANCA+ patients were almost twice as likely to relapse as those with MPO-ANCA. Moreover, it was observed that PR3-ANCA was prevalent in patients with granulomatous inflammation and that almost all patients with destructive lesions of the upper airways were PR3-ANCA+ [43•]. Two cluster analyses of GPA and MPA patients yielded five classes associated with different outcomes. The cluster model which included ANCA specificity was regarded as more accurate and simple to use [44]. With regard to ANCA negativity, there are probably true and false ANCA-negative variants in AAV. A recent study provided an intriguing explanation of why MPO-ANCA positivity is likely to be missed [45].
Altogether, and despite limitations, genetic and cohort studies strongly support the use of ANCA specificity for subclassification of AAV. Thus, because of the evidence summarized above indicating the usefulness of ANCA subspecificities in classification and differences in outcome [14], future clinical study cohorts incorporating GPA, MPA and EGPA should add the prefix PR3-ANCA, MPO-ANCA, or ANCA-negative [1]. This also applies to variants of GPA and EGPA without clinically overt signs of vasculitis; in most cases, these are retrospectively recognized as localized variants and usually develop into systemic vasculitis within a variable time frame [3, 46]. The 2012 CHCC consensus is to “add the prefix MPO-ANCA, PR3-ANCA or ANCA-negative”.
Granuloma as Another Differentiating Factor in AAV
Extravascular granulomatosis is a major factor differentiating MPA from GPA [1], and in EGPA extravascular lesions are usually not associated with necrotizing granulomatous inflammation [22]. We summarize findings on clinical manifestations in the ENT region that are likely to be induced by or associated with granulomatous inflammation. We assume that, for example, destructive or expanding tumor-like granulomatous manifestations not only follow different pathogenic pathways from vasculitis manifestations but also enable differentiation of additional disease subphenotypes, at least for GPA.
The Neglected Granulomatous Manifestation and its Clinical Phenotypes
Similarly to PR3-ANCA, involvement and/or disease of the ENT region has been recognized as a predictor of relapse in AAV [38, 39]. Despite this, granulomatous manifestations in the head and neck region are sometimes neglected, because they have been regarded as mild and can be occult, at least in the beginning. However, nasal blockage, nasal bridge collapse, chronic sinusitis, hearing loss and subglottic stenosis are severe manifestations suggestive of GPA and commonly contributing to the vasculitis damage index (VDI) score. A survey of 701 North-American GPA patients revealed that, after fatigue and joint pain, symptoms in the ENT region were the predominant presenting features at diagnosis [47]. Further, a small study of GPA (24 patients) reported sinusitis and subglottic stenosis as the most commonly observed head and neck manifestations at diagnosis [48]. Regarding disease-related damage the upper airways were prominent—secondary to kidney involvement in a Turkish cohort of 50 GPA patients [49]. A cross-sectional study of 283 AAV patients comparing VDI and combined damage assessment (CDA) scores reported high incidence of ENT-related organ damage [50]. This is complemented by a study using EUVAS RCT data from 183 patients and revealing an association between ENT disease at baseline and subsequent ENT damage [51]. This must be taken into account, especially in fully-developed disease with life-threatening systemic vasculitis when granulomatous manifestations may seem of minor importance. When remission is induced, “grumbling” disease should be re-evaluated by means of an interdisciplinary approach, e.g. by ENT specialists, and using new imaging techniques to assist in finding “occult” granulomas. Comprehensive overviews of airway manifestations in adults and children have been published [52, 53], revealing the destructive potential of GPA-related granulomatous inflammation in the head and neck region.
Subglottic stenosis of the trachea is the most severe manifestation in the ENT region, because it can turn into a life-threatening condition and requires increased dilatations, open airway reconstruction, and other local management procedures [54, 55]. In our experience, activity-adapted immunosuppression combined with moderate intervention may be a promising strategy. Orbital masses, also called “granuloma(tous) masses”, are a rare and treatment-resistant granulomatous manifestation, which was observed in 5 % of a GPA cohort of 1,142 patients [56•]. In this study nearly half the patients were refractory to highly potent immunosuppression, approximately three-quarters had visual impairment, and approximately every fifth patient suffered from blindness. Similar observations were documented for a French AAV cohort of 1,286 patients, among whom GPA was the most frequent diagnosis; 6.6 % of these patients had orbital inflammatory tumors [57]. Promising new therapy, for example rituximab, does not always lead to remission or response, especially for refractory GPA and if granulomatous manifestations, e.g. retro-orbital granulomas, are present [58–60]. Localized GPA, especially with involvement of the upper respiratory tract, has been successfully treated with co-trimoxazole, which is recommended (either alone or with glucocorticoids) for induction of remission and for maintenance therapy [6].
The need for improved diagnosis and therapy for granulomatous manifestations is revealed by an EUVAS-based analysis of long-term patient survival [8]. New disease activity scores for granuloma-induced ENT signs have recently been developed, with better sensitivity and specificity than the scores currently used in outcome studies. The proposed scoring system is designed to complement the Birmingham vasculitis activity score (BVAS) but has yet to be adapted for reliability and intraobserver variation [61, 62]. There is also a recognized lack of a best practice biomarker and/or global imaging procedure for quantifying the volume of the granuloma mass and its distribution and progression, including the degree of scarring. However, magnetic resonance imaging can detect early subglottic stenosis, differentiate between active and inactive subglottic inflammation, recognize early proptosis, and monitor the response of treatment-resistant retro-orbital granulomatous masses [51, 63, 64•]. In future, when studying treatment-resistant or refractory GPA caused by subglottic or orbital involvement, imaging procedures should supplement AAV scoring systems for disease activity and for damage.
The Granulomatous Phenotype of Persistent Localized GPA
Localized GPA usually is regarded as transition stage, developing over time into the generalized and sometimes immediately dangerous stages of early systemic, generalized, severe and refractory disease (Fig. 1) which include organ and/or life-threatening complications, for example alveolar hemorrhage and/or nephritis [3, 6]. However, a rare variant of persistent localized GPA was described by two newer and comparable studies [27•, 28•]. This persistent localized GPA variant is characterized by granulomatous inflammation confined to the ENT region, i.e. without identifiable evidence of systemic vasculitis. In the first study, long-term persistent localized GPA was observed in ~5 % of a German monocentric cohort (1,024 GPA patients). Nearly half the patients with localized GPA remained ANCA-negative and long-term survival was excellent, but frequent relapse and damage in the ENT region were observed which tended to be refractory to standard treatment. Nasal cartilage erosion and lysis, septum perforation, destruction of the inner nose (e.g. loss of conchae), and retro-orbital granulomatous masses were observed. Major clinical consequences were severe pain and organ damage with functional loss, i.e. blindness or airway obstruction. Many parts of the ENT tract can be affected by obstruction, e.g. stenosis of the subglottic region and trachea, the Eustachian tube, or the lacrimal duct. In the second study, the French Vasculitis Study Group observed that 3.2 % of 494 GPA patients had disease localized to one organ. Clinical manifestations were of the granulomatous type, for example orbital granulomas, ENT disease, or isolated lung nodules. These patients remained in the localized stage throughout follow-up. Patients with long-term localized GPA have—according to the 2012 CHCC definition—“no identifiable evidence of systemic vasculitis but when they exhibit clinical and pathological changes identical to those seen in GPA respiratory tract involvement and, especially if they are ANCA-positive, they should be included in the GPA category” [1]. In EGPA extravascular manifestations can occur, but compared with GPA they are less frequent, rarely granulomatous and/or destructive, and affect different organs [22–24]. Definition of a persistent “localized EGPA variant” seems to be difficult, and is the subject of current research [46].
Association of Necrotizing Granulomatous Inflammation with Extravascular Manifestations
Granulomatous and nongranulomatous extravascular inflammation is common in GPA and has a variety of morphologies. During the early phase, neutrophilic microabscesses with scattered multi-nucleated giant cells are prevalent. In later phases, degenerating neutrophils and necrotic debris form a central, irregularly outlined necrotic zone (geographic necrosis), which is surrounded by poorly defined granulomatous inflammation with palisades of elongated macrophages and scattered multi-nucleated giant cells. The mixed inflammatory infiltrate includes monohistiocytic epitheloid cells and giant cells and a large and heterogeneous population of other cells, including clusters of T and B lymphocytes with partial follicular organization, activated and follicular dendritic cells, plasma cells, PR3-expressing neutrophils, scattered eosinophils, mast cells, debris-laden macrophages, and high endothelial venules suggestive of ectopic lymphoid-like tissue neoformation. All characteristic symptoms of GPA can be found in nasal biopsies of active disease [65]. We believe that elucidating molecular and cellular patterns within the granulomatous inflammation, although time-consuming, is worthwhile because it may result in better understanding of the pathogenesis and possibly also in identification of prognostic markers. Interestingly, B cells derived from granulomatous inflammation of an ANCA-negative patient with localized GPA produced autoantibodies directed against a transmembrane protein, but not PR3 [66, 67]. Whether this is caused by a lack of PR3-ANCA+ B lymphocytes in the subphenotype of ANCA-negative localized GPA or by difficulty in finding local PR3-ANCA-producing cells remains to be investigated. If pathogenic ANCA are evolving from dysregulated natural homeostatic autoimmunity and exogenous factors have an effect [2], there must still be some local site(s) where the dysregulation is initiated and either occurs or does not. Somatic mutations and clonal expansion of immunoglobulin genes derived from granulomatous inflammation (in the ENT region) indicate local ongoing immune responses in GPA [66, 68, 69], which in most, but not all, cases may lead to or favor the formation of pathogenic PR3-ANCA [70]. Of note, on the transcriptional level the primary (nasal) tissue in GPA had a different profile from that of other inflammatory disorders, with involvement of the nasal mucosa [71]. Increased heterogeneity was observed in the GPA transcription patterns, which might also be indicative of subphenotypes. It was recently revealed that fibroblasts lining bone or cartilage, sometimes in a pannus-like pattern (Fig. 2), contribute to destruction of bone or cartilage, for example via expression of matrix metalloproteases [72•]. These results support the theory that granulomatous inflammation actively participates in the development of clinically relevant extravascular manifestations, including midline destruction or ulcerative lesions described above. Because not all GPA patients seem to have lesions resulting in destruction of the nose or eye, these features might indicate specific subphenotypes of GPA.
GPA in sinonasal mucosa (H&E, ×100): granulomatous inflammation comprising the characteristic ill-defined Wegener’s granuloma with epitheloid cells and several giant cells (arrowheads) on the left side, inflammatory destruction of adjacent bone (B) by pannus-like tissue (asterisks) on the right side, and typical dense lymphoplasmocytic inflammatory background with beginning of the formation of follicular structures (dotted circles) below (from Ref. [70], by permission of Oxford University Press)
Conclusions
Whereas systemic vasculitis is common to all AAV, extravascular manifestations seem to differ between the main subtypes of AAV. On the basis of the association with relapse, identification of a persistent localized GPA variant, and indications of a mechanistic link between granulomatous inflammation and ENT destruction, we propose that granuloma might be another differentiating factor in AAV, or at least in GPA. Confirmation of this theory will require identification of appropriate surrogate markers for the granuloma, similar to the ANCA, which, for example, are suggestive of a destructive disease course, specific extravascular manifestations, or treatment resistance.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11.
Jennette JC, Falk RJ. Pathogenesis of ANCA-associated vasculitis: observations, theories and speculations. La Presse Med Q Med Rev. 2013;42:493–8.
Hellmich B, Flossmann O, Gross WL, Bacon P, Cohen-Tervaert JW, Guillevin L, et al. EULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: focus on anti-neutrophil cytoplasm antibody-associated vasculitis. Ann Rheum Dis. 2007;66:605–17.
Berden A, Göçeroglu A, Jayne D, Luqmani R, Rasmussen N, Bruijn JA, et al. Diagnosis and management of ANCA associated vasculitis. Br Med J. 2012;16:344.
Geetha D, Seo P. Advances in therapy for ANCA-associated vasculitis. Curr Rheumatol Rep. 2012;14:509–15.
Schönermarck U, Gross WL, de Groot K. Treatment of ANCA-associated vasculitis. Nat Rev Nephrol. In press.
Little MA, Nightingale P, Verburgh CA, Hauser T, De Groot K, Savage C, et al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis. 2010;69:1036–43.
Flossmann O, Berden A, de Groot K, Hagen C, Harper L, Heijl C, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis. 2011;70:488–94.
Luqmani R. Maintenance of clinical remission in ANCA-associated vasculitis. Nat Rev Rheumatol. 2013;9:127–32.
Eriksson P, Jacobsson L, Lindell A, Nilsson JA, Skogh T. Improved outcome in Wegener’s granulomatosis and microscopic polyangiitis? A retrospective analysis of 95 cases in 2 cohorts. J Intern Med. 2009;265:496–506.
Holle JU, Gross WL, Latza U, Nölle B, Ambrosch P, Heller M, et al. Improved outcome in 445 patients with Wegener’s granulomatosis in a German vasculitis center over four decades. Arthritis Rheum. 2011;63:257–66.
Hoffman GS, Langford CA. Are there different forms of life in the antineutrophil cytoplasmic antibody universe? Ann Intern Med. 2005;143:683–5.
Bacon PA. The spectrum of Wegener’s granulomatosis and disease relapse. N Engl J Med. 2005;352:330–2.
Watts RA, Scott DG. ANCA vasculitis: to lump or split? Why we should study MPA and GPA separately. Rheumatology. 2012;51:2115–7.
Millet A, Pederzoli-Ribeil M, Guillevin L, Witko-Sarsat V, Mouthon L. Antineutrophil cytoplasmic antibody-associated vasculitides: is it time to split up the group? Ann Rheum Dis. 2013;72:1273–9.
Fervenza FC, Specks U. Vasculitis: refining phenotypes in ANCA-associated vasculitis. Nat Rev Nephrol. 2013;9:6–8.
Gadola S, Gross WL. Vasculitis in 2011: the renaissance of granulomatous inflammation in AAV. Nat Rev Rheumatol. 2012;8:74–6.
Holle JU. Seropositive and negative ANCA-associated vasculitis, anti-MPO and PR3-vasculitis: different outcomes? La Presse Med Q Med Rev. 2013;42:616–9.
Stegeman CA, Tervaert JW, de Jong PE, Kallenberg CG. Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. N Engl J Med. 1996;335:16–20.
Reinhold-Keller E, De Groot K, Rudert H, Nölle B, Heller M, Gross WL. Response to trimethoprim/sulfamethoxazole in Wegener’s granulomatosis depends on the phase of the disease. Q J Med. 1996;89:15–23.
Sinico RA, Di Toma L, Radice A. Renal involvement in anti-neutrophil cytoplasmic autoantibody associated vasculitis. Autoimmun Rev. 2013;12:477–82.
Cordier JF, Cottin V, Guillevin L, Bel E, Bottero P, Dalhoff K, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss). La Presse Med Q Med Rev. 2013;42:507–10.
Moosig F, Bremer JP, Hellmich B, Holle JU, Holl-Ulrich K, Laudien M, et al. A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg-Strauss, EGPA): monocentric experiences in 150 patients. Ann Rheum Dis. 2013;72:1011–7.
Moosig F, Richardt G, Gross WL. A fatal attraction: eosinophils and the heart. Rheumatology. 2013;52:587–9.
Bremer JP, Csernok E, Holle J, Gross WL, Moosig F. Getting rid of MPO-ANCA: a matter of disease subtype. Rheumatology. 2013;52:752–4.
Csernok E, Lamprecht P, Gross WL. Clinical and immunological features of drug-induced and infection-induced proteinase 3-antineutrophil cytoplasmic antibodies and myeloperoxidase-antineutrophil cytoplasmic antibodies and vasculitis. Curr Opin Rheumatol. 2010;22:43–8.
• Holle JU, Gross WL, Holl-Ulrich K, Ambrosch P, Noelle B, Both M, et al. Prospective long-term follow-up of patients with localised Wegener’s granulomatosis: does it occur as persistent disease stage? Ann Rheum Dis. 2010;69:1934–9. This is the first study to identify a subset of GPA patients with a persistent variant of localized granulomatous disease.
• Pagnoux C, Stubbe M, Lifermann F, Decaux O, Pavic M, Bérezné A, et al. French Vasculitis Study Group. Wegener’s granulomatosis strictly and persistently localized to one organ is rare: assessment of 16 patients from the French vasculitis study group database. J Rheumatol. 2011;38:475–8. This is the second study to identify a subgroup of GPA patients with localized granulomatous disease only.
Jagiello P, Gencik M, Arning L, Wieczorek S, Kunstmann E, Csernok E, et al. New genomic region for Wegener’s granulomatosis as revealed by an extended association screen with 202 apoptosis-related genes. Hum Genet. 2004;114:468–77.
Heckmann M, Holle JU, Arning L, Knaup S, Hellmich B, Nothnagel M, et al. The Wegener’s granulomatosis quantitative trait locus on chromosome 6p21.3 as characterised by tagSNP genotyping. Ann Rheum Dis. 2008;67:972–9.
Kelley JM, Monach PA, Ji C, Zhou Y, Wu J, Tanaka S, et al. IgA and IgG antineutrophil cytoplasmic antibody engagement of Fc receptor genetic variants influences granulomatosis with polyangiitis. Proc Natl Acad Sci. 2011;108:20736–41.
Wieczorek S, Holle JU, Epplen JT. Recent progress in the genetics of Wegener’s granulomatosis and Churg-Strauss syndrome. Curr Opin Rheumatol. 2010;22:8–14.
•• Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367:214–23. The first GWAS study on ANCA-associated vasculitides provides convincing evidence that risk genes linked to autoantibodies differentiate AAV phenotypes.
Cid M. The search for genetic links in ANCA-associated vasculitis and its variants. N Engl J Med. 2012;367:271–3.
Pieters K, Pettersson A, Gullberg U, Hellmark T. The −564 A/G polymorphism in the promoter region of the proteinase 3 gene associated with Wegener’s granulomatosis does not increase the promoter activity. Clin Exp Immunol. 2004;138:266–70.
Xie G, Roshandel D, Sherva R, Monach PA, Lu Y, Kung T, et al. Granulomatosis with polyangiitis (Wegener’s) is associated with HLA-DPB1*04 and SEMA6A gene variants: evidence from a genome-wide analysis. Arthritis Rheum. 2013;65:2457–68.
Husmann CA, Holle JU, Moosig F, Mueller S, Wilde B, Cohen Tervaert JW, et al. Genetics of toll like receptor 9 in ANCA associated vasculitides. Ann Rheum Dis. 2013. doi:10.1136/annrheumdis-2012-202803.
Lyons PA, McKinney EF, Rayner TF, Hatton A, Woffendin HB, Koukoulaki M, et al. Novel transcription signatures identified by transcriptional analysis of separated leukocyte subsets in systemic lupus erythematosus and vasculitis. Ann Rheum Dis. 2010;69:1208–13.
Cheadle C, Berger AE, Andrade F, James R, Johnson K, Watkins T, et al. Transcription of proteinase 3 and related myelopoiesis genes in peripheral blood mononuclear cells of patients with active Wegener’s granulomatosis. Arthritis Rheum. 2010;62:1744–54.
Hogan SL, Falk RJ, Chin H, Cai J, Jennette CE, Jennette JC, et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med. 2005;143:621–31.
Pagnoux C, Hogan SL, Chin H, Jennette JC, Falk RJ, Guillevin L, et al. Predictors of treatment resistance and relapse in anti-neutrophil cytoplasmic antibody-associated small vessel vasculitis: comparison of two independent cohorts. Arthritis Rheum. 2008;58:2908–18.
Walsh M, Flossmann O, Berden A, Westman K, Höglund P, Stegeman C, et al. Risk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2012;64:542–8.
• Lionaki S, Blyth ER, Hogan SL, Hu Y, Senior BA, Jennette CE, et al. Classification of antineutrophil cytoplasmic autoantibody vasculitides. Arthritis Rheum. 2012;64:3452–62. This study reveals that ANCA specificity is superior to clinical classifications (CHCC, EMA) regarding prediction of disease outcome, for example relapse.
Mahr A, Katsahian S, Varet H, Guillevin L, Hagen EC, Höglund P, et al. Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: a cluster analysis. Ann Rheum Dis. 2013;72:1003–10.
Roth AJ, Ooi JD, Hess JJ, van Timmeren MM, Berg EA, Poulton CE, et al. Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest. 2013;123:1773–83.
Pagnoux C, Guillevin L. Churg-Strauss syndrome: evidence for disease subtypes? Curr Opin Rheumatol. 2010;22:21–8.
Abdou NI, Kullman GJ, Hoffman GS, Sharp GC, Specks U, McDonald T, et al. Wegener’s granulomatosis: survey of 701 patients in North America. Changes in outcome in the 1990s. J Rheumatol. 2002;29:309–16.
Taylor SC, Clayburgh DR, Rosenbaum JT, Schindler JS. Progression and management of Wegener’s granulomatosis in the head and neck. Laryngoscope. 2012;122:1695–700.
Kamali S, Erer B, Artim-Esen B, Gul A, Ocal L, Konice M, et al. Predictors of damage and survival in patients with Wegener’s granulomatosis: analysis of 50 patients. J Rheumatol. 2010;37:374–8.
Suppiah R, Flossman O, Mukhtyar C, Alberici F, Baslund B, Brown D, et al. Measurement of damage in systemic vasculitis: a comparison of the Vasculitis damage index with the combined damage assessment index. Ann Rheum Dis. 2011;70:80–5.
Martinez Del Pero M, Walsh M, Luqmani R, Flossmann O, Mukhtyar C, Jani P, et al. Long-term damage to the ENT system in Wegener’s granulomatosis. Eur Arch Otorhinolaryngol. 2011;268:733–9.
Pagnoux C, Wolter NE. Vasculitis of the upper airways. Swiss Med Wkly. 2012;142:w13541.
Fowler NM, Beach JM, Krakovitz P, Spalding SJ. Airway manifestations in childhood granulomatosis with polyangiitis (Wegener’s). Arthritis Care Res. 2012;64:434–40.
Wester JL, Clayburgh DR, Stott WJ, Schindler JS, Andersen PE, Gross ND. Airway reconstruction in Wegener’s granulomatosis-associated laryngotracheal stenosis. Laryngoscope. 2011;121:2566–71.
Arebro J, Henriksson G, Macchiarini P, Juto JE. New treatment of subglottic stenosis due to Wegener’s granulomatosis. Acta Otolaryngol. 2012;132:995–1001.
• Holle JU, Voigt C, Both M, Holl-Ulrich K, Nölle B, Laudien M, et al. Orbital masses in granulomatosis with polyangiitis are associated with a refractory course and a high burden of local damage. Rheumatology. 2013;52:875–82. This study characterizes orbital masses as a rare but resistant granulomatous manifestation in a large cohort of GPA patients.
Rothschild PR, Pagnoux C, Seror R, Brézin AP, Delair E, Guillevin L. Ophthalmologic manifestations of systemic necrotizing vasculitides at diagnosis: a retrospective study of 1286 patients and review of the literature. Semin Arthritis Rheum. 2013;42:507–14.
Martinez Del Pero M, Chaudhry A, Jones RB, Sivasothy P, Jani P, Jayne D. B-cell depletion with rituximab for refractory head and neck Wegener’s granulomatosis: a cohort study. Clin Otolaryngol. 2009;34:328–35.
Roubaud-Baudron C, Pagnoux C, Méaux-Ruault N, Grasland A, Zoulim A, LE Guen J, et al. Rituximab maintenance therapy for granulomatosis with polyangiitis and microscopic polyangiitis. J Rheumatol. 2012;39:125–30.
Holle JU, Dubrau C, Herlyn K, Heller M, Ambrosch P, Noelle B, et al. Rituximab for refractory granulomatosis with polyangiitis (Wegener’s granulomatosis: comparison of efficacy in granulomatous versus vasculitic manifestations. Ann Rheum Dis. 2012;71:327–33.
Garske U, Haack A, Beltrán O, Flores-Suárez LF, Bremer JP, Lamprecht P, et al. Intra- and Inter-rater reliability of endonasal activity estimation in granulomatosis with polyangiitis (Wegener’s). Clin Exp Rheumatol. 2012;30:S22–8.
Martinez Del Pero M, Rasmussen N, Chaudhry A, Jani P, Jayne D. Structured clinical assessment of the ear, nose and throat in patients with granulomatosis with polyangiitis (Wegener’s). Eur Arch Otorhinolaryngol. 2013;270:345–54.
Muhle C, Reinhold-Keller E, Richter C, Duncker G, Beigel A, Brinkmann G, et al. MRI of the nasal cavity, the paranasal sinuses and orbits in Wegener’s granulomatosis. Eur Radiol. 1997;7:566–70.
• Klink T, Holle J, Laudien M, Henes FO, Moosig F, Platzek C, et al. Magnetic resonance imaging in patients with granulomatosis with polyangiitis (Wegener’s) and subglottic stenosis. MAGMA. 2013;26:281–90. The study describes the value of magnetic resonance imaging for detection and grading of subglottic stenosis in GPA patients.
Lamprecht P, Holl-Ulrich K, Gross WL. Granulomatosis with polyangiitis – Pathogenesis. In: Ball GV, Fessler SL, Bridges BJ, editors. Oxford Textbook on Rheumatology: Vasculitis, 3rd ed. Oxford: Oxford University Press. 2014. In press.
Voswinkel J, Assmann G, Held G, Pitann S, Gross WL, Holl-Ulrich K, et al. Single cell analysis of B lymphocytes from Wegener’s granulomatosis: B cell receptors display affinity maturation within the granulomatous lesions. Clin Exp Immunol. 2008;154:339–45.
Thurner L, Müller A, Cérutti M, Martin T, Pasquali JL, Gross WL, et al. Wegener’s granuloma harbors B lymphocytes with specificities against a proinflammatory transmembrane protein and a tetraspanin. J Autoimmun. 2011;36:87–90.
Voswinkel J, Mueller A, Kraemer JA, Lamprecht P, Herlyn K, Holl-Ulrich K, et al. B lymphocyte maturation in Wegener’s granulomatosis: a comparative analysis of VH genes from endonasal lesions. Ann Rheum Dis. 2006;65:859–64.
Zhao Y, Odell E, Choong LM, Barone F, Fields P, Wilkins B, et al. Granulomatosis with polyangiitis involves sustained mucosal inflammation that is rich in B-cell survival factor and autoantigen. Rheumatology. 2012;51:1580–6.
Mueller A, Holl-Ulrich K, Lamprecht P, Gross WL. Germinal centre-like structures in Wegener’s granuloma: the morphological basis for autoimmunity? Rheumatology. 2008;47:1111–3.
Laudien M, Häsler R, Wohlers J, Böck J, Lipinski S, Bremer L, et al. Molecular signatures of a disturbed nasal barrier function in the primary tissue of Wegener’s granulomatosis. Mucosal Immunol. 2011;4:564–73.
• Kesel N, Köhler D, Herich L, Laudien M, Holl-Ulrich K, Jüngel A, et al. Cartilage destruction in granulomatosis with polyangiitis (Wegener’s granulomatosis) is mediated by human fibroblasts after transplantation into immunodeficient mice. Am J Pathol. 2012;180:2144–55. This study identifies fibroblasts in nasal mucosa as cellular mediators of cartilage and/or bone destruction and thus suggests a direct link between granulomatous inflammation and destruction in GPA.
Aries PM, Both M. Destructive eye lesions in Wegener’s granulomatosis. N Engl J Med. 2005;352:392.
Acknowledgments
The work of the authors is supported by the German Research Society (DFG), Clinical Research Unit (CRU) KFO170. The authors would like to thank Claudia Möck and Stefanie Dörner for helping with preparation of the manuscript. This review is dedicated to Professor emeritus Wolfgang Müller-Ruchholtz on the occasion of his 85th birthday to recognize his valuable contributions to and thoughtful advice on immunological research.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Antje Mueller, Konstanze Holl-Ulrich, and Wolfgang L. Gross declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of Topical Collection on Vasculitis
Rights and permissions
About this article
Cite this article
Mueller, A., Holl-Ulrich, K. & Gross, W.L. Granuloma in ANCA-Associated Vasculitides: Another Reason to Distinguish Between Syndromes?. Curr Rheumatol Rep 15, 376 (2013). https://doi.org/10.1007/s11926-013-0376-5
Published:
DOI: https://doi.org/10.1007/s11926-013-0376-5