Abstract
Chronic migraine is a debilitating disorder that affects 2 % of the global population and imparts a significant societal and economic impact. The cornerstones of chronic migraine management include making an accurate diagnosis, patient education, treatment of comorbid conditions, and selection of an appropriate, evidence-based acute and preventive treatment regimen. Although it is common to treat chronic migraine with preventive medications effective for episodic migraine, a number of treatment options exist with specific evidence for effectiveness in chronic migraine. Currently, onabotulinumtoxinA injections are the only FDA-approved preventive treatment for chronic migraine. A number of non-medication treatment options including occipital nerve and supraorbital nerve stimulation have shown promise as effective prevention for patients either unable to tolerate or unable to obtain relief from oral medications, but more research is necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic migraine is a debilitating neurologic disorder with a significant and negative impact on the lives of patients and their families. It also imparts a substantial societal and economic burden. Chronic migraine affects approximately 2 % of the general population, and the International Burden of Migraine Study has demonstrated that patients with chronic migraine have a lower health-related quality of life and a higher likelihood of experiencing an inability to work, are less likely to be able to attend social functions or perform household chores, and experience a markedly higher degree of disability than patients with episodic migraine [1, 2, 3•]. Eighty percent of patients with chronic migraine are misdiagnosed or underdiagnosed and an even greater number are undertreated [1]. To decrease the burden of chronic migraine, we must address not only gaps in identification and diagnosis, but also the application of evidence-based medicine for the treatment of chronic migraine [4••, 5]. This narrative review provides an updated review of evidence-based options for the preventive treatment of chronic migraine.
Identification and Diagnosis of Chronic Migraine
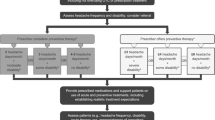
The first step in optimizing the treatment of chronic migraine starts with an accurate and timely diagnosis. The diagnostic criteria for chronic migraine, as defined by the International Classification of Headache Disorders, 3rd edition, is 15 or more headache days per month for at least 3 months in which 8 or more headache days per month either meet criteria for migraine with or without aura or respond to migraine-specific treatment [6]. As patients with chronic migraine frequently under-report or fail to recall their “mild” headache days, the diagnosis of chronic migraine can often be missed or delayed. Asking a patient, “Do you feel like you have a headache of some type on 15 or more days per month?” [7] and “How many days each month on average are you completely free from any type of headache?” [8•] can be helpful to establish an accurate monthly headache frequency. ID-CM is a case-finding tool based on headache frequency and four symptoms (moderate/severe pain intensity, photophobia, phonophobia, and migraine-related nausea) that have a positive predictive value of 96 % and can be a simple, accurate tool for the diagnosis of chronic migraine by general neurologists and non-neurologists [9••].
Evidence of Preventive Options in Chronic Migraine
Once chronic migraine is accurately diagnosed, an effective treatment plan should address both the preventive and the acute or as needed treatment of exacerbations [10]. For the purpose of this review, we will focus solely on evidence-based preventive options for chronic migraine for which the goal is to reduce the number of migraine attacks and overall number of headache days which, in turn, will reduce the need for acute treatment and will also help to prevent medication overuse. In this review, specific preventive options for the treatment of chronic migraine are listed alongside their level of evidence based on the number and the types of trials performed (randomized placebo-controlled versus open label).
Topiramate has three randomized placebo-controlled trials demonstrating efficacy in the prevention of chronic migraine [11–13]. Silberstein et al. conducted the largest of these studies with an intent-to-treat population of 306 subjects (topiramate, n = 153; placebo, n = 153) and with the primary efficacy endpoint being the change from baseline in the mean number of migraine/migrainous headache days per month. At a mean dose of 86 mg/day for 90 days, topiramate resulted in a statistically significant mean reduction in the number of migraine/migrainous headache days (topiramate −6.4 ± 5.8 vs. placebo −4.7 ± 6.1, p = 0.01) [12]. Although this study excluded patients that were overusing acute medications, the two other topiramate studies for chronic migraine included patients with medication overuse [11, 13]. Diener et al. conducted a smaller study which included only 59 patients in the intent-to-treat population, 32 of which received topiramate and 27 of which received placebo. The target dose of topiramate was 100 mg per day for 90–100 days. The primary efficacy endpoint was the change in the number of migraine days from the 28th day baseline phase to the last 28 days of the double-blind phase in the intent-to-treat population. Topiramate significantly reduced the number of migraine days per month (topiramate −3.5 ± 6.3 vs. placebo −0.2 ± 4.7, p < 0.05) [11]. In addition, this study demonstrated an improvement in migraine disability in the topiramate group based on MIDAS scores [11]. The third study, by Silvestrini and colleagues, was the smallest of the 3 studies with only 28 patients that were randomized in a 1:1 ratio to receive topiramate or placebo. During the 8-week maintenance phase, the treatment group received a low dose of topiramate at 50 mg daily. During the last 4 weeks of the maintenance phase, patients receiving topiramate experienced a significantly lower 28-day headache frequency compared to those treated with placebo (topiramate 8.1 ± 8.1 vs. placebo 20.6 ± 3.4, p < 0.0007) [13]. The most common adverse events reported in all 3 studies were paresthesias, anorexia and weight loss, fatigue, difficulty with concentration, nausea, and dyspepsia. These three randomized placebo-controlled studies provide a high level of evidence that topiramate is effective for the prevention of chronic migraine with or without medication overuse [11–13].
OnabotulinumtoxinA injections for the preventive treatment of chronic migraine have been studied in 2 large multicenter randomized placebo-controlled studies with identical study designs, Phase 3 Research Evaluating Migraine Prophylaxis Therapy 1 and 2 (PREEMPT 1 and 2) trials [14, 15, 16••, 17]. In the pooled analysis, a total of 1384 subjects were randomized to OnabotulinumtoxinA (n = 688) or placebo (n = 696). Of note, over 60 % of all patients enrolled were overusing acute headache pain medications in both studies. Subjects received injections of onabotulinumtoxinA every 12 weeks in a 31 fixed-site, fixed-dose protocol of 155 units versus placebo for 2 injection cycles. The primary endpoint for the pooled analysis was mean change from baseline in frequency of headache days at 24 weeks. At 24 weeks, the pooled analysis demonstrated that onabotulinumtoxinA injections, 155 U every 12 weeks, had a greater reduction in the number of headache days compared to placebo (−8.4 vs. −6.6, p < 0.001) [16••]. The most common adverse events with onabotulinumtoxinA injections included neck pain, injection site pain, ptosis, muscular weakness, and headache. Overall, these 2 randomized, placebo-controlled studies provided a high level of evidence that onabotulinumtoxinA 155 U delivered in the PREEMPT protocol is effective for the prevention of chronic migraine with or without medication overuse [14, 15, 16••, 17]. At this time, onabotulinumtoxinA administered per the PREEMPT protocol is the only FDA-approved treatment for prevention of chronic migraine.
Sodium valproate was studied in one prospective, double-blind, randomized, placebo-controlled trial for chronic daily headache [18]. In a total of 70 patients, 29 had a diagnosis of chronic migraine and 41 had a diagnosis of chronic tension-type headache. Seventeen of the chronic migraine patients in this study received 500 mg of sodium valproate twice daily for 3 months versus 12 chronic migraine patients who received placebo for the same duration. End-points included reduction in number of days per month with pain as well as decrease in subjective pain scores as rated using a visual analog scale. At the end of the first month and at 3 months, the sodium valproate treatment group had significantly lower maximum pain visual analog scale scores and reduced pain frequency as defined by the number of days with pain in 1 month. Pain frequency in chronic migraine patients treated with sodium valproate were 22.05 ± 6.6 before treatment, 7.0 ± 4.8 after 1 month of treatment, and 5.2 ± 5.0 after 3 months of treatment compared to the placebo group whose pain frequencies were 22.3 ± 6.9 before placebo, 22.4 ± 6.4 after 1 month of placebo, and 22.3 ± 6.4 after 3 months of treatment (p < 0.001 at 1 month and p < 0.001 at 3 months) [18]. In this small study, the incidence of adverse events was low in the group taking sodium valproate 500 mg twice daily and included somnolence, tremor, and hair loss. Although hematologic and chemistry panels that were performed at 4 and 12 weeks were within normal limits, periodic monitoring of complete blood count and liver function tests is recommended while on sodium valproate, especially during the first 6 months of treatment.
Tizanidine is an alpha-2 adrenergic agonist that modulates and decreases the release of norepinephrine in the brainstem, may have anti-nociceptive effects that are independent of the endogenous opioid system, and is also an effective centrally acting muscle relaxant. Tizanidine has been studied in one prospective, double-blind, placebo-controlled, multi-center outcomes study [19]. Ninety-two patients (tizanidine n = 45, placebo n = 47) completed 8 weeks of the study and 85 patients (tizanidine n = 44, placebo n = 41) completed the total 12 weeks of the study. All subjects reported greater than 15 headache days per month for at least 3 months and greater than 75 % met the diagnostic criteria for chronic migraine. The primary endpoint was the overall improvement in the headache index which was calculated as the product of the number of headache days, average intensity, and duration of each headache divided by 28 days during each 4 week interval (baseline, treatment week 1 through 4, week 5 through 8, and week 9 through 12). Tizanidine was slowly titrated over 4 weeks to 24 mg or max dose tolerated with a mean of 18 mg per day in three divided intervals. Overall, tizanidine was shown to be superior to placebo in reducing the overall headache index (p = 0.0025) [19]. In addition, there was a reduction in the mean headache days per week (p = 0.0193), severe headache days per week (p = 0.0211), average headache intensity (p = 0.0108), peak headache intensity (p = 0.0020), and mean headache duration (p = 0.0127) [19]. Somnolence was a common adverse event and was reported by almost 50 % of the patients on tizanidine. Other adverse events include dizziness, dry mouth, asthenia, and elevation of liver enzymes. It is reported that about 5 % of patients could develop an elevation of liver enzymes and is recommended to perform liver function tests at baseline and 1, 3, and 6 months after initiating treatment.
Gabapentin was studied in one double-blind, placebo-controlled, crossover trial for the treatment of chronic daily headache in which 22 of 95 total subjects had chronic migraine and 58 had a combination of migraine and tension-type headache [20]. Gabapentin was titrated over 2 weeks to a final dose of 2400 mg daily. The primary endpoint was the difference in the percentage of headache days while on gabapentin compared to placebo. Primary efficacy results revealed a mean difference in the number of headache days between gabapentin and placebo of 9.1 % ± 20.9, indicating that patients experienced a significantly higher number of headache-free days while on gabapentin compared to placebo (p < 0.001) [20]. Adverse events on gabapentin were common and reported by 39 % of patients on gabapentin compared to 14 % on placebo and included somnolence, ataxia, and nausea [20].
Amitriptyline is a tricyclic antidepressant and has a high level of evidence that it is effective for the preventive treatment of episodic migraine. In an unblinded, non-placebo controlled trial of 72 patients with chronic migraine subjects were randomized 1:1 to receive either amitriptyline or onabotulinumtoxinA injections (250 units) [21]. A reduction of at least 50 % in the number of pain episodes, intensity of pain, number of drug doses for pain, and subjective reports of improvement by patient or examiner were the main endpoints. Amitriptyline at doses of 25–50 mg daily was found to have similar benefits to onabotulinumtoxinA injections (250 U) delivered into 15 pre-established points around the head. Patients (67.8 %) in the onabotulinumtoxinA group had a reduction of at least 50 % in the number of days of pain compared to 72 % in the amitriptyline group (p = 0.78). Statistically similar improvements of at least 50 % in the intensity of pain were also noted for both groups (50 % onabotulinumtoxinA versus 55.6 % amitriptyline, p = 0.79), as well as a greater than 50 % reduction in the number of pain drug doses (77 % onabotulinumtoxinA versus 71 % amitriptyline, p = 0.76) [21]. The occurrence of adverse events, however, was higher in the amitriptyline group (somnolence, weight gain, dry mouth, and constipation) compared to the onabotulinumtoxinA injection group.
Several medications have been studied in open-label trials. Although open-label trials are a lower quality of evidence to make clinical decisions, they do lay the foundation for future double-blind, randomized, placebo-controlled trials to better define efficacy of treatment options. Medications that have been studied in open-label trials for chronic migraine prevention include memantine up to 10–20 mg daily [22], zonisamide up to 400 mg daily [23], atenolol 50 mg daily [24], and pregabalin 150 mg twice daily [25]. Despite the lack of randomized controlled trials supporting their efficacy, medications that are used for the prevention of episodic migraine are frequently used for the off-label treatment of chronic migraine based on the assumption that these conditions share a similar pathophysiology. Although it can be assumed that these medications may be effective, future randomized control trials are needed to provide an evidence-base for their routine use.
Two open-label trials have been performed to investigate the efficacy of nerve blocks in the prevention of chronic migraine. In one study, 150 chronic migraine patients received occipital nerve block with a local anesthetic and steroid. A total of 52 % of treated patients experienced a 50 % or greater reduction in headache days the month following the procedure compared to their pretreatment baseline [26]. A second study investigated nerve blocks administered via a fixed-site, fixed-dose design using 0.25 % bupivacaine (1 cc at greater and lesser occipital nerves, 0.5 cc at auriculotemporal and zygomaticotemporal, and 0.2 cc at supraorbital and supratrochlear areas bilaterally) to 218 patients with chronic migraine at 3 months intervals in a prospective open-label fashion. After 12 months, 53.2 % of patient had greater than 50 % reduction in the mean frequency of headache days [27]. A recent randomized, multicenter, double-blind, placebo-controlled study on greater occipital nerve blockade for the treatment of chronic migraine using saline versus bupivacaine demonstrated a greater reduction in the number of headache days in the bupivacaine group versus placebo (p = 0.004) [28]. Sphenopalatine ganglion blockade in the treatment of chronic migraine has been evaluated in a double-blind, placebo-controlled, randomized study of 41 subjects (bupivacaine, n = 26 versus saline, n = 12) that demonstrated that 0.5 % bupivacaine delivered via a noninvasive device twice a week for 6 weeks was superior to saline with a statistically significant difference in the numerical pain score at 15 min, 30 min, and 24 h after the procedure [29]. Endpoints for longer term efficacy, however, such as the number of headache days 1 month post-treatment, did not meet statistical significance in this small study [30]. Larger randomized, controlled trials of nerve blocks are necessary to further define the efficacy of these treatments.
A number of open-label studies have suggested that occipital nerve stimulation (ONS) may be a promising treatment option for refractory chronic migraine patients who have not adequately responded to or are unable to tolerate oral or injectable preventative options; however, only three randomized, sham-controlled have been conducted. In general, these studies identified ONS as a promising potential treatment option but failed to provide definitive evidence of efficacy as one study was an underpowered feasibility study and the other two studies failed to meet their primary endpoints (despite meeting a number of secondary endpoints) [31–33]. Combined supraorbital nerve stimulation (SONS) and ONS has also been investigated in two open-label studies both of which have provided encouraging preliminary results [34, 35]. Drawbacks to ONS and SONS include the fact that it is an invasive surgical procedure with a number of potential adverse events which include implant site infection, nontarget area sensory syndrome, implant site pain, and lead migration and fractures. A more thorough discussion about the existing data on ONS and combined ONS-SONS as chronic migraine prophylaxis is beyond the scope of this review.
Pitfalls in the Management of Chronic Migraine
Treatment of chronic migraine must be multidisciplinary, and it should start with patient education. Medication overuse, lack of medication adherence, and the presence of undiagnosed or undertreated comorbidities are the main pitfalls in the management of chronic migraine. Based on the existing evidence, medication overuse headache can occur with the use of opiates and butalbital-containing medications 8 or more days per month, triptans 10 or more days per month, simple analgesics 15 or more days per month, or any combination of as needed medications 15 or more days per month for 3 months [36]. Overused medications should be judiciously tapered and discontinued and can be accomplished simultaneously with the initiation of preventive treatment options. Caffeine should also be strictly limited or discontinued in chronic migraine as caffeine withdrawal is a common trigger for chronic headache [37]. Similar to many other chronic diseases, a lack of medication compliance and adherence is a common pitfall in the management of chronic migraine as less than 25 % of patients with chronic migraine adhere to oral migraine preventive regimen 1 year after treatment [38•]. This may be a due to a lack of proper patient education versus poor medication tolerability neither of which is mutually exclusive. Patients should to be educated that preventive medications frequently take 6–8 weeks before improvement is noted [39]. To help improve the tolerability of medications, it is generally recommended to start low and go slow when titrating medications. Table 1 lists the recommended titration schedules for the medications outlined in this review. Additionally, patients should be educated that some side effects will attenuate with continued use. Many medical and psychiatric diagnoses are comorbid with chronic migraine including obesity, obstructive sleep apnea, insomnia, depression, and anxiety and should be addressed and treated for optimal benefit to the patient [40, 41]. Risk factor modification and maintaining a scheduled, balanced lifestyle by losing weight, exercising, avoiding alcohol, developing coping mechanisms for handling stressors, and maintaining a steady, sufficient sleep schedule can help to reduce overall migraine disability [42–44]. With proper patient education, strict limits on the use of as needed medications, patience with preventive options, risk factor modification, and treatment of medical and psychiatric comorbidities, chronic migraine patients can live with lower levels of disability and pain.
Conclusion
Although chronic migraine is a debilitating medical condition that affects millions of individuals worldwide, a thoughtful and methodical approach can be rewarding for both the patient and the provider. Frequently, the largest barrier to effective treatment stems from the lack of a clear diagnosis. This suggests the importance of a familiarity with the diagnostic criteria for chronic migraine and a need to solicit accurate information from the patient including the number of headache days per month and an account of the presence of migraine associated features. Once the diagnosis of chronic migraine is made, evidence-based treatment options should be incorporated into the treatment plan. As with many chronic health conditions, education is of paramount importance for patients with chronic migraine and should include counseling about the important diagnostic features, discussion of potential medication side effects, and establishment of reasonable expectations for treatment. Recognition and treatment of commonly occurring comorbid medical and psychiatric conditions including obstructive sleep apnea, obesity, depression, anxiety, and medication overuse can frequently lead to better outcomes than treatment of the headache disorder alone. Rather than relying on subjective reports of treatment success or failure, quantitative response to treatment should be monitored by recording features such as change in average or maximal pain severity, number of headache days, or frequency of acute medication use. Further research on chronic migraine is certainly warranted to better understand its pathophysiology and to find new and innovative treatments which are both safe and effective.
References
Paper of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bigal ME, Serrano D, Reed M, et al. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71(8):559–66.
Natoli JL, Manack A, Dean B, et al. Global prevalence of chronic migraine: a systematic review. Cephalalgia. 2010;30(5):599–609.
Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia. 2011;31(3):301–15. This study demonstrated that patients with chronic migraine suffer from a lower healthcare-related quality of life and a higher degree of disability and burden compared to episodic migraine.
Diener HC, Holle D, Dodick D. Treatment of chronic migraine. Curr Pain Headache Rep. 2011;15(1):64–9. This is an excellent review on the multidisciplinary approach to the treatment of chronic migraine.
Katsarava Z, Buse DC, Manack AN, et al. Defining the differences between episodic migraine and chronic migraine. Curr Pain Headache Rep. 2012;16(1):86–92.
The International Classification of Headache Disorders, 3rd edition (beta version). 2013; 33 (9): 629-808
Lipton RB. Chronic migraine, classification, differential diagnosis, and epidemiology. Headache. 2011;51 Suppl 2:77–83.
Starling AJ, Dodick DW. Best practices for patients with chronic migraine: burden, diagnosis, and management in primary care. Mayo Clin Proc. 2015;90(3):408–14. This review defines the role of a primary care physician (PCP) in the treatment of chronic migraine.The PCP plays a critical role in the identification of chronic migraine patients, timely referral to a headache specialist, and treatment of comorbidities and risk factors associated with chronic migraine.
Lipton RB, Serrano D, Buse DC, et al. Improving the detection of chronic migraine: development and validation of identify chronic migraine (ID-CM). Cephalalgia: an international journal of headache 2015. This study validated an easy-to-use case finding tool for the identification of patients with chronic migraine. It can be used by general neurologists and non-neurologists
Dodick DW, Silberstein SD. Migraine prevention. Pract Neurol. 2007;7(6):383–93.
Diener HC, Bussone G, Van Oene JC, et al. Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia. 2007;27(7):814–23.
Silberstein SD, Lipton RB, Dodick DW, et al. Efficacy and safety of topiramate for the treatment of chronic migraine: a randomized, double-blind, placebo-controlled trial. Headache. 2007;47(2):170–80.
Silvestrini M, Bartolini M, Coccia M, et al. Topiramate in the treatment of chronic migraine. Cephalalgia. 2003;23(8):820–4.
Aurora SK, Dodick DW, Turkel CC, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30(7):793–803.
Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30(7):804–14.
Dodick DW, Turkel CC, DeGryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50(6):921–36. This pooled analysis demonstrated that onabotulinumtoxinA injections per the PREEMPT protocol are effective for the prevention of chronic migraine with or without medication overuse.
Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51(9):1358–73.
Yurekli VA, Akhan G, Kutluhan S, et al. The effect of sodium valproate on chronic daily headache and its subgroups. J Headache Pain. 2008;9(1):37–41.
Saper JR, Lake 3rd AE, Cantrell DT, et al. Chronic daily headache prophylaxis with tizanidine: a double-blind, placebo-controlled, multicenter outcome study. Headache. 2002;42(6):470–82.
Spira PJ, Beran RG. Gabapentin in the prophylaxis of chronic daily headache: a randomized, placebo-controlled study. Neurology. 2003;61(12):1753–9.
Magalhaes E, Menezes C, Cardeal M, et al. Botulinum toxin type A versus amitriptyline for the treatment of chronic daily migraine. Clin Neurol Neurosurg. 2010;112(6):463–6.
Bigal M, Rapoport A, Sheftell F, et al. Memantine in the preventive treatment of refractory migraine. Headache. 2008;48(9):1337–42.
Pascual-Gomez J, Alana-Garcia M, Oterino A, et al. Preventive treatment of chronic migraine with zonisamide: a study in patients who are refractory or intolerant to topiramate. Rev Neurol. 2008;47(9):449–51.
Edvardsson B. Atenolol in the prophylaxis of chronic migraine: a 3-month open-label study. Springerplus. 2013;2:479.
Calandre EP, Garcia-Leiva JM, Rico-Villademoros F, et al. Pregabalin in the treatment of chronic migraine: an open-label study. Clin Neuropharmacol. 2010;33(1):35–9.
Weibelt S, Andress-Rothrock D, King W, et al. Suboccipital nerve blocks for suppression of chronic migraine: safety, efficacy, and predictors of outcome. Headache. 2010;50(6):1041–4.
Kaniecki RG. Management of chronic migraine with quarterly pericranial nerve blocks: a prospective 12-month trial. Headache 2012; 52:908: P85.
Inan LE, Inan N, Karadas O, et al. Greater occipital nerve blockade for the treatment of chronic migraine: a randomized, multicenter, double-blind, and placebo-controlled study. Acta Neurol Scand 2015.
Cady R, Saper J, Dexter K, et al. A double-blind, placebo-controlled study of repetitive transnasal sphenopalatine ganglion blockade with tx360((R)) as acute treatment for chronic migraine. Headache. 2015;55(1):101–16.
Cady RK, Saper J, Dexter K, et al. Long-term efficacy of a double-blind, placebo-controlled, randomized study for repetitive sphenopalatine blockade with bupivacaine vs. saline with the Tx360 device for treatment of chronic migraine. Headache. 2015;55(4):529–42.
Saper JR, Dodick DW, Silberstein SD, et al. Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia. 2011;31(3):271–85.
Silberstein SD, Dodick DW, Saper J, et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia. 2012;32(16):1165–79.
Lipton RB, Goadsby PJ, Cady RK, et al. PRISM study: occipital nerve stimulation for treatment-refractory migraine [abstract P047]. Cephalalgia. 2009;29:30.
Hann S, Sharan A. Dual occipital and supraorbital nerve stimulation for chronic migraine: a single-center experience, review of literature, and surgical considerations. Neurosurg Focus. 2013;35(3):E9.
Reed KL, Black SB, Banta 2nd CJ, et al. Combined occipital and supraorbital neurostimulation for the treatment of chronic migraine headaches: initial experience. Cephalalgia. 2010;30(3):260–71.
Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71(22):1821–8.
Griffiths RR, Evans SM, Heishman SJ, et al. Low-dose caffeine physical dependence in humans. J Pharmacol Exp Ther. 1990;255(3):1123–32.
Hepp Z, Dodick DW, Varon SF, et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35(6):478–88. This is the first study evaluating oral medication adherence in chronic migraine patients. It demonstrated the adherence is poor at 6 months and even lower at 12 months.
Dodick D. Finding a fit: strategies for chronic migraine prophylaxis. Johns Hopkins Adv Stud Med 2006; 6(4D)
Chen YC, Tang CH, Ng K, et al. Comorbidity profiles of chronic migraine sufferers in a national database in Taiwan. J Headache Pain. 2012;13(4):311–9.
Teixeira AL, Costa EA, Da Silva Jr AA, et al. Psychiatric comorbidities of chronic migraine in community and tertiary care clinic samples. J Headache Pain. 2012;13(7):551–5.
Verrotti A, Agostinelli S, D'Egidio C, et al. Impact of a weight loss program on migraine in obese adolescents. Eur J Neurol. 2013;20(2):394–7.
Pryse-Phillips WE, Dodick DW, Edmeads JG, et al. Guidelines for the nonpharmacologic management of migraine in clinical practice. Can Headache Soc Cmaj. 1998;159(1):47–54.
Varkey E, Cider A, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31(14):1428–38.
Compliance with Ethics Guidelines
Conflict of Interest
Amaal J. Starling declares no potential conflicts of interest. Bert B. Vargas is a section editor for Current Pain and Headache Reports.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Episodic Migraine
Rights and permissions
About this article
Cite this article
Starling, A.J., Vargas, B.B. A Narrative Review of Evidence-Based Preventive Options for Chronic Migraine. Curr Pain Headache Rep 19, 49 (2015). https://doi.org/10.1007/s11916-015-0521-0
Published:
DOI: https://doi.org/10.1007/s11916-015-0521-0