Abstract
Currently, the measurement of areal bone mineral density (aBMD) is used at NASA to evaluate the effects of spaceflight on the skeletal health of astronauts. Notably, there are precipitous declines in aBMD with losses >10 % detected in the hip and spine in some astronauts following a typical 6-month mission in space. How those percentage changes in aBMD relate to fracture risk in the younger-aged astronaut is unknown. Given the unique set of risk factors that could be contributing to this bone loss (eg, adaptation to weightlessness, suboptimal diet, reduced physical activity, perturbed mineral metabolism), one might not expect skeletal changes due to spaceflight to be similar to skeletal changes due to aging. Consequently, dual-energy X-ray absorptiometry (DXA) measurement of aBMD may be too limiting to understand fracture probability in the astronaut during a long-duration mission and the risk for premature osteoporosis after return to Earth. Following a brief review of the current knowledge-base, this paper will discuss some innovative research projects being pursued at NASA to help understand skeletal health in astronauts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In its vision for space exploration after 2020, NASA intends to take humans beyond low Earth orbit to explore and inhabit celestial bodies - whether it will be 6-month habitation on the lunar surface, a 6-month transit to a near-Earth asteroid or a 6-month transit and subsequent return from an 18-month stay on Mars. Therefore, in the NASA world of integrated systems, the role of the biomedical scientists and medical officers is to mitigate the risks to the “human system”—the astronaut—to ensure a readiness for, and a successful completion of, these spaceflights referred to as exploration class missions.
To address the bone health of astronauts throughout their active career and lifetime, NASA has developed a research and bone health monitoring program that collects biomedical data used to define occupational risks in the astronauts during and due to prolonged exposure to spaceflight.
However, defining risks to the skeletal health in the astronaut can be challenging. Bone is a complex tissue with considerable heterogeneity. It is difficult to detect skeletal trends in a cohort with a low number of subjects, ie, long-duration astronauts that typically fly 180-day missions aboard the International Space Station (ISS). Currently, there are less than 50 long-duration astronauts and the total number is not expected to increase appreciably in the next 10 years. In addition, the slow acquisition of data, the multiple and novel factors of space travel, and the limited reference data on skeletal health of younger persons (<45 years) further complicate the ability to substantiate an osteoporosis risk, and to define it well enough to develop a therapeutic course of action before the ISS is no longer available (in 2020, possibly 2028) and prior to exploration spaceflights.
Ideally, biomedical data from astronauts are surrogate measures for physiological outcomes of interest to undergird any clinical decision-making. Therefore, the measurement of areal bone mineral density (aBMD) as a surrogate measure for bone strength and fracture risk in ISS astronauts was a medical requirement by the time of the first expedition in 2000. The aBMD measurement as a sole surrogate, however, may not be sufficient for fracture prediction, especially in the complicated patient whose bone loss is not related to aging. This disconnect may be even more evident in the long-duration astronaut where some characterization of skeletal biology suggests that changes to bones are unlike terrestrial age-related bone loss and thus require an expanded and more detailed evaluation beyond dual-energy X-ray absorptiometry (DXA) aBMD.
Regardless of the constraints that might impede the collection of astronaut data, understanding changes in bone mass and bone quality while in the space environment is the first step to defining an osteoporosis risk, and establishing whether an intervention is required to mitigate that risk. In the absence of fracture data, however, there may not be enough data to conclude that an intervention during spaceflight, vs after return to Earth, is required to mitigate a risk to long term health. Moreover, there is an overarching concern that an Earth-based intervention could do more harm than good when provided to a typically young and healthy cohort and in a remote and extreme environment.
This review will describe the biomedical data acquired from long-duration astronauts to outline what is known about skeletal biology of humans in space and will describe some research directions that are being pursued to increase the understanding of human skeletal biology in order to plan for longer duration spaceflights.
The Human Research Program at NASA
For the 40+ years of manned spaceflight, the increased risks for fracture and for early onset osteoporosis due to spaceflight are probably the most recognized risks to astronaut health both within NASA and in the public sector. Because of the paradigm shift in the osteoporosis field for requiring more than aBMD for assessing bone strength and fracture risk, however, these skeletal risks in the astronaut population are often misunderstood and remain poorly defined. In the general community, osteoporosis is commonly associated with geriatric ages. The average age of the long-duration astronaut, however, is 46.8 ± 4.3 years (range 36.8–55.3); it could take 10–15 years after return from a mission for the incidence of low trauma or fragility fractures due to spaceflight. The earlier fracture probability is presumed to be the result of irreversible or persistent declines in the skeleton with spaceflight combined with the expected declines with aging. A fragility fracture at this age (~60s) would be considered premature and avoidable. Bone loss with spaceflight is a biomedical change recognized by astronauts, but they have yet to see a clinical effect of this putative occupational health risk. Thus, defining how bone loss in astronauts could be a risk to health or performance is critical not only to prophylactic health management of astronauts but also for planning exploration class missions. To this aim, whether astronauts are predisposed to fragility or atraumatic fractures is dependent upon (1) the biomedical measures used to describe spaceflight-induced changes to bone, (2) the technology to conduct those measures, (3) the translation of those measures to fracture outcome, and (4) the application of those measures for the surveillance of the astronaut’s bone health.
Biomedical Data of the Long-Duration Astronaut
During the 1990s, it was believed at NASA that those critical bone measures could be acquired by DXA. Moreover, the current NASA medical standards for bone health are based upon aBMD T-scores of the hip and lumbar spine where a T-score above −1 is required to qualify as an applicant for astronaut candidacy and the efficacy of an in-flight intervention is determined by its ability to preserve aBMD T-scores above −2.0 at the end of the mission (“a permissible outcome”).
Skeletal evaluations of astronauts have been conducted since early in the manned space program. With the demonstrated capability of DXA technology and biochemical assays for bone turnover [1–3] to detect profound changes in skeletal biology in Russian cosmonauts and American astronauts, it became medically required at NASA to systematically assess skeletal health in ISS astronauts by conducting DXA scans and assays of biomarkers for bone metabolism before and after spaceflight. DXA scans can also be performed on a triennial basis after the astronaut retires.
Areal Bone Mineral Density
A seminal report of BMD data, obtained from DXA scans of cosmonauts serving on the Russian Mir space craft, first reported the accelerated decline in bone mass by calculating an average loss of aBMD as 1 %–1.5 % per month determined from preflight and postflight DXA scans [1]. Recognizably, this rate of BMD losses (1 %–1.5 % aBMD /month) exceeds the aBMD declines (0.5 %–1 % aBMD annually) observed with primary osteoporosis in older individuals. In addition, the report highlighted the site-specificity of aBMD declines substantiating that losses predominate in skeletal regions that are typically weight-bearing on Earth. These declines were later corroborated in a retrospective analysis of BMD recovery after long-duration spaceflight [4], which asserted a trend in aBMD to be restored to preflight levels in astronauts after spaceflight, albeit over a prolonged period and with high variability. Currently, skeletal integrity is assessed by the T-score at the end of mission referencing astronauts to a sex-specific (ie, Official Position of International Society for Clinical Densitometry [ISCD]) database. To date, no long-duration astronaut has returned from spaceflight with a T- or Z-scores less than −2.0, even after losing as much as 10 %–15 % BMD in hip or spine. Notably, DXA aBMD would not provide enough information to predict fracture in this astronaut cohort, which already has a very low incidence of fractures and a very small total number of subjects.
This variability in aBMD response, during and after spaceflight, is being further investigated through epidemiological analyses using an algorithm developed from the aBMD data of the Rochester Bone Health Study [5]. The Mayo cohort-derived algorithm generates a predicted value of aBMD loss that is compared with an observed value of aBMD loss obtained from the repository of astronaut medical data. Using age, pre-flight aBMD and follow-up time, the post-flight aBMD for each US astronaut is predicted; the predicted and observed aBMD, and the rates of aBMD change, immediately and 3 years after spaceflight, have been determined in 24 male astronauts. The mean aBMD for various sites (hip, lumbar spine, ultra-distal radius, mid-shaft radius) was either stable or improved by 3 years relative to the mean immediate postflight aBMD. However, a deficit for the total hip was evident at 3 years after return [5] indicating that the male (which was the only sex evaluated) space station astronauts continue to have aBMD lower than what would be expected in a population not exposed to flight—raising a potential concern regarding future hip fracture risk.
Quantitative Computed Tomography (QCT)
Research technologies for bone imaging have been used in flight studies of ISS astronauts to expand the assessment of bone changes in space to sub-regions of the hip and spine. QCT, for example, determined that there were sub-regional changes in whole hip bone (ie, the cortical and cancellous bone of the proximal femur) [6] and the tibia [7] and that these sub-regional changes (ie, relative to preflight) occurred at different rates [6]. This expanded capability suggests that 3-d imaging of bone provided a fuller, more sensitive description of spaceflight effects on whole bone, especially for clinically-relevant sites of hip and spine. Though there is only a modest improvement in fracture prediction with QCT parameters of the hip for non-astronauts [8], QCT’s ability to detect effects of spaceflight on bone architecture and changes to bone compartments (cortical and cancellous) increases the understanding of skeletal biology in space beyond that provided by DXA. QCT scanning of the lumbar spine, however, did not provide additional information for the vertebrae above and beyond DXA measurement of aBMD, which was attributed to the thin cortex (~0.25 mm) and the trabecular-rich nature of vertebral bodies.
Importantly, the delineation of sub-regions of bone by QCT is important when assessing different interventions that may have separate effects on distinct compartments of whole bone. This concept has been demonstrated in the PATH study [9] with parathyroid hormone and alendronate. In addition, there is a recent report of a flight study suggesting that the combination of sufficient nutrition and resistive exercise (Advanced Resistive Exercise Device [ARED]) is capable of mitigating bone loss in ISS astronauts [10•]. Because there is a higher excretion of bone turnover markers (N-teleopeptide, C-telopeptide among others) and calcium throughout the spaceflight [10•], one cannot exclude the possibility that the reduced decline in aBMD actually represents an increase in cortical bone mass by stimulated periosteal apposition concurrent with loss of bone mass in the cancellous bone.
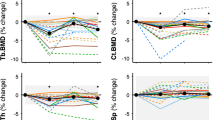
The Fig. 1 illustrates this effect in a larger total of crewmembers divided between those who flew with no exercise hardware (Mir), those who flew before ARED (using an interim resistive exercise device “iRED” with only ~300 pound force [lbf]) and after ARED hardware (with 600 lbf) became available on-board the ISS.
Declines in DXA aBMD in Long-duration Astronauts on Mir and ISS Spaceflights. Percentage change of preflight areal BMD per month was calculated by subtracting the first postflight DXA BMD measurement from the preflight measurement and normalizing by the mission duration (typically 4–6 months). ARED is an acronym for Advanced Resistive Exercise Device [ARED], which is exercise hardware capable of providing up to 600 pounds of force (lbf). Data are plotted for groups of crewmembers who served on the Mir (n = 28 cosmonauts and 7 U.S. astronauts), on the International Space Station [ISS] pre-ARED (n = 24 U.S. astronauts exercising on an interim Resistive Exercise Device [iRED]), and on ISS after access to ARED hardware was available (n = 11 U.S. astronauts). BMD changes are reported for lumbar spine, femoral neck, trochanter, total hip, and wrist. (Wrist is 1/3 radius + ulna, and is for ISS crewmembers only.) All group mean aBMD changes from preflight to postflight were significant (P < 0.05), except at the wrist, as determined by 1-tailed, unpaired t-tests using absolute data. Likewise, except for the effect on the wrist, the comparison of exercise effects between ARED and iRED suggests an improved “step function” with ARED exercise hardware - attenuating the expected declines in aBMD with spaceflight. The impact on actual bone strength and on fracture risk remains unknown
Moreover, the QCT hip scans of astronauts captured changes in hip bone structure, both with spaceflight and with re-ambulation on Earth [6, 11]. Data of integral bone (cortical plus cancellous bone compartments) and of cancellous compartments alone suggested that endocortical resorption reduced cortical bone volume during spaceflight and that re-ambulation in Earth’s 1 G environment stimulated periosteal expansion at the femoral neck [11]. This anatomical response is not completely unexpected as it is similarly observed in QCT evaluations of aging populations [12] in whom cross-sections of the femoral neck increase with age (by periosteal apposition) in the context of age-related bone loss and thinning of the cortex . However, integral BMD (g/cm3) and calculations of compressive and bending strength were still decreased relative to baseline measures when hip scans were performed 1 year after return to Earth [11]. This increase in radial bone growth in ISS crewmembers raises the possibility that the geometrical changes in the femoral neck, as it adapts to spaceflight and readapts to Earth, will repeat itself on a second mission possibly reducing bone strength and impacting fracture risk. Likewise, it is unclear how these changes in the ISS astronaut, who is at the average age of 46, will combine with the expected change observed after age 70 years [12]. As a follow-up, QCT hip scans performed in 8 of these same astronauts, at time periods 2–4 years after return to Earth, revealed that cancellous BMD displayed a second phase of decline, and recovery to preflight measures was not observed in all subjects [13•].
Finite Element Models of QCT Data
With this additional information on bone architecture, QCT scanning has facilitated the development of Finite Element Models (FEM) with which to estimate failure loads at the hip [14]. This computational tool was applied to serial QCT scans performed in astronauts before and after ISS missions to evaluate the impact of spaceflight on the biomechanics of the hip [15]. Hip bone strength for 2 loading orientations (1-legged stance and a postero-lateral fall) were estimated from FEM of 11 sets of QCT scans with sufficient quality (scan quality became an issue with an unanticipated replacement in QCT instruments at a local Houston hospital) [15]. As reported, the percent changes in hip strength over a mission were highly variable ranging between −4 % and −30 % for stance loading and +3 % and −24 % for fall loading in crewmembers (cosmonauts and astronauts) [15]. More importantly, the changes in hip strength during spaceflight as detected by changes in aBMD, as a surrogate measure of hip strength, did not correlate with the changes in FE estimates of hip strength (R 2 = 0.05 fall and R 2 = 0.23 stance) [15]. When one compares group mean values, the FEM estimates a 2-fold decline in strength (kN) for every 1-fold decline in the surrogate measure of aBMD by DXA (in g/cm2). Thus, QCT captures more changes to whole hip than can be measured by DXA [15], which supports the conclusion that DXA aBMD may not be sufficient to assess the effect of spaceflight on hip bone strength.
Bone Turnover Biomarkers
In addition, there is a medical requirement to assess biochemical markers of bone degradation and of bone formation in long-duration astronauts before and after ~4-month to 6-month spaceflight missions [16]. As reported, N-teloepeptide is increased in urine specimens obtained early in flight (relative to preflight measurements), remains high in urine samples collected through the mission, but returns to preflight levels in urine collected on landing and up to 4 months postflight [16]. In contrast, bone specific alkaline phosphatase [BAP] and osteocalcin show no change from preflight measures in any of the sera obtained throughout the flight.
Furthermore, increased levels of sclerostin, an inhibitor of bone formation produced by SOST gene in osteocytes in response to skeletal unloading, were reported in stroke patients [17], with spinal cord injury [18], and in preclinical studies [19]. These reports undergirded the measurement of sclerostin in frozen sera of male human subjects in a bed rest analog for spaceflight [20]. Results indicate that both 28 and 60 days of bed rest led to increases in circulating sclerostin relative to pre-bed rest [20]. Parathyroid hormone was reduced while bone resorption biomarkers (eg, N-teleopeptide, deoxypyridinoline, pyridinoline) were increased in these subjects [20].
Collectively, all of these biochemical studies suggest that an uncoupling of cell signaling occurs during spaceflight, resulting in an unbalanced, net loss in bone mass from the entire skeleton [16]. Sclerostin may play a role in lack of a formation response to the stimulated resorption during spaceflight; measurements in astronauts are in progress. An imbalance at the level of the bone remodeling unit could contribute to decreased bone quality and strength of the whole bone but, to-date, there are no biopsy data from astronauts to substantiate this impact at the tissue level. However, a rebound in bone formation biomarkers at 30 days postflight suggests that balanced bone remodeling can be restored; the long-term impact on bone quality in the astronaut, and its contribution to skeletal integrity, remain unknown.
To sum, spaceflight data-to-date suggest that the adaptation of astronaut bones to the mechanical unloading of spaceflight is complex. Briefly, X-ray based imaging technologies (DXA and QCT) characterize a precipitous loss in bone mass, a site-specific effect on normally weight-bearing skeletal regions, and sub-regional changes in bone morphology. A FEM of QCT data indicates significant declines in estimated hip strength for 2 loading scenarios. DXA does not appear to detect all of these changes. Bone turnover markers reflect an uncoupling of bone resorption (increased) from bone formation (stable or decreased). Collectively, the imaging data and bone turnover data suggest structural adaptations to the hip occur during prolonged spaceflight [11]. Uncertainty still remains because biochemical assays and bone imaging data are indirect measures of skeletal integrity. How flight surgeons and program directors can use biomedical data from astronauts to mitigate a potential osteoporosis risk in the astronaut and to prioritize research for risk mitigation is uncertain.
Directed Research Tasks in NASA Bone Discipline
The astronaut dataset represents a varied and limited assortment of biomedical measurements acquired from research flight studies and/or medical assessment tests. Medical assessment tests are compulsory tests while research studies generate data— some of which are very relevant to skeletal biology (eg, gonadal hormones)—only from astronaut volunteers. Regardless, the data strongly suggest that the skeletal changes due to spaceflight are not like the terrestrial bone loss and the skeletal deconditioning that are observed with aging. This raises the requirement to expand the current clinically-accepted method and index for bone health (ie, DXA aBMD) to be more relevant to the astronaut following exposure to spaceflight.
To this aim, the author, as the Bone Discipline Lead at JSC, used the insight of osteoporosis experts to maximize the characterization of spaceflight effects in the astronaut [21••]. This panel of experts reviewed the biomedical data (both research and medical) from 35 long-duration astronauts accumulated by the time of this NASA Bone Summit (2010) [21••]. On the whole, the panel agreed that an intervention was required to mitigate the risk for early onset osteoporosis, but that there was insufficient information for it to recommend an intervention (the type and timing) for risk mitigation in astronauts serving on the planned exploration class missions. Hence, this clinical advisory panel recommended bone measurements to monitor as part of a risk surveillance program.
Consequently, there are 2 merit-reviewed, Directed Research projects in the Human Research Program (HRP) that are based upon the concerns raised by the Bone Summit panel [21••]. The first study will test the feasibility of QCT for risk surveillance in ISS astronauts. QCT hip scans will be performed in astronauts before and after spaceflight, at 1 year, and possibly at 2 years after return (1) to describe the effects of in-flight interventions to mitigate volumetric BMD [vBMD] loss in sub-regions of hip, and (2) to monitor the recovery of vBMD in the hip trabecular compartment. A previous NASA flight study documented an absence of recovery in ISS astronauts. These data suggest irreversible changes to trabecular BMD, and possibly to bone microarchitecture, that may require therapy.
In addition, QCT will generate data to describe the skeletal effects of spaceflight on parameters of bone structure that could improve the forecasting of fractures during a mission [22]. Hip QCT data will describe the hypothesized effect of biochemical interventions from the effect of mechanical countermeasures on trabecular and cortical regions of bone, respectively. In other words, QCT will demonstrate its ability to differentiate the modeling effect of resistive exercise from the remodeling effect of bisphosphonates in subjects who display increased hip aBMD by DXA. Moreover, the QCT data will be used to generate an FEM to estimate changes in hip strength with spaceflight and to describe recovery of hip strength while back on Earth.
The second project follows up on a recommendation to explore the FEM data from population studies (eg, Age, Gene/Environment Susceptibility [AGES] Reykjavik Study, Rochester Bone Health Study, MrOs) to determine if it were possible to derive standards for bone health in astronauts that supplement DXA aBMD measurements. Consequently, the author assembled a task group composed of the principal investigators (or a designee) of the mentioned population cohorts as well as experts in FEM, some of whom generated FEM for these same studies [23–25]. This task group suggested that bone health standards for astronauts could be derived from data obtained from populations combined to cover the age range of the astronauts and with a single method of FEM applied across the hip QCT scans. Following the generation of this FEM hip strength dataset, various statistical methods would be applied (eg, receiver operating characteristics and area under the curve) to identify a nonpermissible outcome for bone health following prolonged spaceflight with the aim of reducing the risk of hip fracture as the astronaut ages. This analysis will integrate the declines in FEM hip strength observed with aging (cross-sectional comparisons) and the declines observed with spaceflight (serial measurements in astronauts) [15]. FEM data from the first study will be used to inform the declines with spaceflight further.
The result of this innovative study is not expected to be perfect nor intended to predict fracture. However, unlike aBMD, which may underestimate the effect of spaceflight and poorly estimate the efficacy of interventions, QCT and FEM data have already demonstrated an ability to capture additional effects of spaceflight on hip bone. These research and clinical advisory panels will periodically reconvene to review the expanded bone data (and any risk factor data) from ISS astronauts accumulated to date, which will include data from QCT, FEM, and from other innovative research technologies like Trabecular Bone Score “TBS” [26, 27].
Even with these important contributions to skeletal risk surveillance, large uncertainties in the fundamental nature of bone loss in space persist. If the aim of HRP is to generate enough information to mitigate all risks to human health and performance for upcoming exploration class missions, then the knowledge gaps of the mechanisms of bone loss also need to be addressed. This understanding will most likely need to come from animal research.
Conclusions
The constraints of acquiring biomedical data from astronauts associated with spaceflight may preclude using conventional approaches for evaluating spaceflight as a risk factor for osteoporosis, ie, by prospective studies with fracture outcome. Moreover, DXA measurement of aBMD is not sufficient for capturing the effects of spaceflight on skeletal integrity and there may be a risk of underestimating the efficacy of in-flight interventions for bone loss if aBMD and T-scores are the sole standards for bone health. NASA may likely need to use innovative and novel assessments of skeletal integrity in astronauts in order to formulate risk mitigation strategies and/or clinical practice guidelines prior to engaging in longer-duration spaceflights. Overall, understanding fundamental bone biology in space will help ensure that human research in space-induced physiological deconditioning is appropriately prioritized by the Human Research program both within the bone discipline and across disciplines of human physiology.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
LeBlanc A, Schneider V, Shackelford L, et al. Bone mineral and lean tissue loss after long-duration spaceflight. J Musculoskelet Neuronal Interact. 2000;1(2):157–60.
Smith SM, Nillen JL, Leblanc A, et al. Collagen cross-link excretion during space flight and bed rest. J Clin Endocrinol Metab. 1998;83(10):3584–91.
Smith SM, Wastney ME, Morukov BV, et al. Calcium metabolism before, during, and after a 3-mo spaceflight: kinetic and biochemical changes. Am J Physiol. 1999;277(1 Pt 2):R1–R10.
Sibonga JD, Evans HJ, Sung HG, et al. Recovery of spaceflight-induced bone loss: bone mineral density after long-duration missions as fitted with an exponential function. Bone. 2007;41(6):973–8.
Amin S, Achenbach S, Atkinson EJ, et al. Bone density following three years of recovery from long-duration space flight. [abstract 2172] Presented at the18th Humans in Space Symposium of the International Academy of Astronautics. Houston, TX, April 11–15, 2011.
Lang T, LeBlanc A, Evans H, et al. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19(6):1006–12.
Vico L, Collet P, Guignandon A, et al. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet. 2000;355(9215):1607–11.
Black DM, Bouxsein ML, Marshall LM, et al. Proximal femoral structure and the prediction of hip fracture in men: a large prospective study using QCT. J Bone Miner Res. 2008;23(8):1326–33.
Keaveny TM, Hoffmann PF, Singh M, et al. Femoral bone strength and its relation to cortical and trabecular changes after treatment with PTH, Alendronate, and their combination as assessed by finite element analysis of quantitative CT scans. J Bone Miner Res. 2008;23(12):1974–82.
• Smith SM, Heer MA, Shackelford L, et al. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: evidence from biochemistry and densitometry. J Bone Miner Res. 2012;27(9):1–11. This paper reports that modifying risk factors for bone loss, ie, suboptimal nutrition and resistive exercise during spaceflight, improves the DXA aBMD outcome in ISS astronauts after ~ 6months in space.
Lang TF, LeBlanc AD, Evans HJ, et al. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. J Bone Miner Res. 2006;21(8):1224–30.
Riggs BL, Melton III LJ, Robb RA, et al. Population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23(2):205–14.
• Carpenter RD, LeBlanc AD, Evans H, et al. Long-term changes in the density and structure of the human hip and spine after long-duration spaceflight. Acta Astronautica. 2010;67:71–81. QCT scans of hip detect the failure of the hip trabecular volumetric BMD to be restored to preflight levels in ISS astronauts by 2-years after return to Earth.
Keyak JH, Kaneko TS, Tehranzadeh J, et al. Predicting proximal femoral strength using structural engineering models. Clin Orthop Relat Res. 2005;437:219–28.
Keyak JH, Koyama AK, LeBlanc A, et al. Reduction in proximal femoral strength due to long-duration spaceflight. Bone. 2009;44(3):449–53.
Smith SM, Wastney ME, O’Brien KO, et al. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the Mir space station. J Bone Miner Res. 2005;20(2):208–18.
Gaudio A, Pennisi P, Bratengeier C, et al. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab. 2010;95:2248–53.
Morse LR, Sudhakar S, Danilack V, et al. Association between sclerostin and bone density in chronic spinal cord injury. J Bone Miner Res. 2012;27:352–9.
Robling AG, Niziolet PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–75.
Spatz JM, Fields EE, Yu EW, et al. Serum sclerostin increases in healthy adult men during bed rest. J Clin Endocrinol Metab. 2012;97(9):E1736–40.
•• Orwoll ES, Adler RA, Amin S, et al. Invited review: skeletal health in long-duration astronauts: nature, assessment and management recommendations from the NASA Bone Summit. 2013. In press. This review paper outlines the recommendations of osteoporosis experts, charged with recommending a surveillance program for bone health in long-duration astronauts, after reviewing the bone-relevant research and medical data from 35 astronauts.
Nelson ES, Lewandowski B, Licata A, et al. Development and validation of a predictive bone fracture risk model for astronauts. Ann Biomed Eng. 2009;37(11):2337–59.
Orwoll ES, Marshall LM, Nielson CM, et al. Osteoporotic fractures in Med Study Group. Finite element analysis of the proximal femur and hip fracture risk in older men. J Bone Miner Res. 2009;24(3):475–83.
Keaveny TM, Kopperdahl DL, Melton III LJ, et al. Age-dependence of femoral strength in white women and men. J Bone Miner Res. 2010;25(5):994–1001.
Keyak JH, Sigurdsson S, Karlsdottir G, et al. Male–female differences in prediction of hip fracture during finite element analysis. Bone. 2011;48(6):1239–45.
Pothaud L, Carceller P, Hans D. Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone. 2008;42(4):775–87.
Rabier B, Heraud A, Grand-Lenois C, et al. A multicenter, retrospective case–control study assessing the role of trabecular bone scores (TBS) in menopausal Caucasian women with low areal bone mineral density (aBMD): analyzing the odds of vertebral fracture. Bone. 2010;46(10):176–81.
Disclosure
JD Sibonga declares that she has no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sibonga, J.D. Spaceflight-induced Bone Loss: Is there an Osteoporosis Risk?. Curr Osteoporos Rep 11, 92–98 (2013). https://doi.org/10.1007/s11914-013-0136-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-013-0136-5