Abstract
Introduction
Calcitonin-gene related peptide (CGRP) is a vasoactive neuropeptide involved in the pathophysiology ofmigraine. CGRP has been targeted for both preventive and acute treatment of migraine.
Objective
Provide a summary of the most clinically relevant literature surrounding CGRP modulating therapies.
Methods
This update on CGRP modulating therapies includes articles selected as most clinically relevant by theauthors.
Conclusion
CGRP modulating therapies are an exciting new addition to migraine treatment given their safety andtolerability. Additionally, compared to traditional migraine preventive medication these treatments are migrainespecific.Further real-world and clinical data is ongoing to better understand these treatments that continue to gainfavor in the management of migraine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Role of CGRP in Migraines: Brief Overview of Pathophysiology
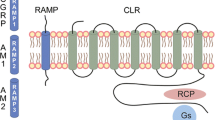
Over the past several decades, there have been many different theories regarding the pathophysiology of migraine, but both clinical and preclinical studies have outlined the role of calcitonin-gene related peptide (CGRP) in migraine. CGRP is a neuropeptide with two different forms, the alpha isoform and the beta isoform. The alpha subunit is highly expressed in both the peripheral and central nervous system, whereas the beta subunit is mainly found in the enteric nervous system [1].
As it is currently understood, CGRP is released from the trigeminal ganglion and/or nerve fibers running along the meningeal and cerebral blood vessels through the process of calcium-dependent exocytosis [2, 3]. CGRP receptors are expressed in multiple anatomical sites associated with migraine pathophysiology including the trigeminal ganglion, trigeminal nucleus caudalis, thalamus, and cerebral and meningeal vasculature. CGRP receptor binding leads to sensitization of nociceptors trigeminal neurons which is thought to relay migraine pain signal through the brainstem into the brain, resulting in pain that is experienced in a migraine attack [4]. CGRP release results in meningeal vasodilation and neurogenic inflammation, which is thought to be due to mast cell activation and degranulation [1, 3].
Several studies have demonstrated the role of CGRP in migraine. Early studies revealed elevated serum levels of CGRP in the jugular vein during a migraine attack – specifically, a higher level in those with chronic migraine versus episodic migraine [5, 6]. Another study showed that the intravenous infusion of CGRP resulted in a delayed migraine attack in patients with history of migraine, but no headache or other somatic pain response in otherwise healthy patients [7]. Studies evaluating CGRP levels in patients with chronic migraine who were treated with onabotulinumtoxinA found that one month after treatment, those who responded to onabotulinumtoxinA treatment had a reduction in CGRP serum levels compared to those who were not responsive [8, 9]. Taken together, these studies suggest that CGRP plays a strong role in the manifestation of migraine through activation of downstream signaling cascades.
CGRP Monoclonal Antibodies
There are currently four CGRP monoclonal antibodies approved for preventive treatment of migraine, eptinezumab, erenumab, fremanezumab, and galcanezumab. They vary by binding site, antibody, and mode of administration. Please refer to Table 1 for further details of the four antibodies. Since their approval, our understanding of CGRP monoclonal antibodies has continued to expand with ongoing research.
Erenumab
Erenumab was the first FDA approved monoclonal antibody (mAb) against a G-protein-coupled receptor that directly blocks access of ligands to the receptor with high affinity and selectivity, which inhibits its downstream signaling cascade [10].
Currently, CGRP monoclonal antibodies are not the first-line migraine preventive treatment, but this has been called into question given their tolerability and efficacy. One study compared the efficacy and tolerability of erenumab versus topiramate for preventive treatment of migraine [11]. The primary endpoint was medication discontinuation due to an adverse event and the secondary endpoint was patients that achieved ≥ 50% reduction from baseline in monthly migraine days within the three months. A statistically significant number of participants discontinued topiramate (38.9%) compared to erenumab (10.6%) due to adverse events. The main reasons that led to discontinuation of topiramate were paresthesia, disturbance in attention, and negative effects on mood. The erenumab group had a statistically significant reduction in monthly migraine days compared to the topiramate group [11]. This is an important study as current consensus requires patients to trial two oral generic preventive drugs (topiramate, divalproex sodium/valproate sodium, beta-blockers, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitor, or other level A and B) for at least six weeks (discontinuation secondary to inability to tolerate or adverse events) prior to CGRP therapy consideration [12]. Further data is needed to guide decisions regarding first-line preventive treatment.
It is currently recommended that women stop CGRP monoclonal antibodies five months before trying to conceive [13]. One case report has been published of a woman who chose to continue erenumab monthly injections throughout the entirety of her pregnancy [14]. There were no complications during the pregnancy. She had a vaginal delivery, and the infant was healthy without congenital malformations. Reportedly, the child continued to meet all their developmental milestones at the 6-month pediatrician visit. Further data is needed to better understand the safety of CGRP targeted treatment in pregnancy [14].
Many times, in practice, hypertension is both an underlying medical condition and side effect that must be acknowledged to adequately titrate medications for individual patients. It has been shown that hypertension has been noted more commonly in people with migraine versus migraine-free individuals [15]. The physiological mechanism of CGRP has strong vasodilatory effects, which may help patients combat hypertension [16]. One study looked at various phase 2 and 3 studies to see the effect of elevated blood pressure while on erenumab [16]. The data showed elevated blood pressures usually occurred with the first 7 days (28/61, 46%), although it was seen throughout erenumab use as well. In addition, it was found that 27 out of the 61 cases of documented elevated blood pressure needed pharmacological intervention or emergency room visit/hospitalization [17]. Thus, from the data, the FDA had labeled hypertension as a side effect of erenumab. One crucial limitation in this study was that the study population had limited individuals with a history of hypertension or high blood pressure. In a post-hoc analysis of clinical trial and post-marketing data of erenumab treated individuals, it was shown that patients with a history of hypertension or risk of hypertension were more prone to have elevated blood pressures as an adverse effect, although again, the study population did not take into account a larger sample size of patients with these co-morbidities [18]. Future studies still need to be conducted to assess the risk of hypertension while being on erenumab or other CGRP therapies.
Galcanezumab
Galcanezumab is a humanized monoclonal antibody that binds CGRP and prevents its biological activity without blocking the CGRP receptor [19].
Galcanezumab has shown efficacy for prevention of migraine and cluster headache, which makes it unique. Galcanezumab 300 mg was effective in treating episodic cluster headache 1–3 weeks after the first injection. The primary outcome for this study was mean change in weekly frequency of cluster headaches attacks, and the secondary end point assessed at least 50% decrease in weekly frequency of cluster headache attacks. Results were significant for mean reduction in weekly attacks in galcanezumab treated patients (8.7) compared to placebo (5.2). There was also a reduction of at least 50% in weekly frequency of cluster headaches in galcanezumab treated patients (71%) compared to placebo (53%) [19].
While galcanezumab is effective for the treatment of episodic cluster headache, data has not shown significant results for the treatment of chronic cluster headache. A phase 3 randomized, placebo-control trial evaluated weekly frequency of headache reduction in patients with chronic cluster headache. Galcanezumab, 300 mg was not statistically significant in reducing weekly headache frequency [20].
Eptinezumab
Many patients in clinical practice not only present with chronic migraine, but medication overuse headache. In the clinical trial for eptinezumab for treatment of chronic migraine, 40.2% of participants had medication overuse headache. Acute medication use was decreased across 24 weeks of treatment with eptinezumab in individuals diagnosed with medication overuse headache and chronic migraine [21].
Eptinezumab is the only anti–calcitonin gene-related peptide monoclonal antibody preventive treatment that has been evaluated when initiated during a migraine attack [21]. A phase 3 trial was conducted to evaluate the efficacy of eptinezumab during a migraine attack (258). The study treated participants with eptinezumab 100 mg or placebo during a moderate to severe attack within 6 h of attack. The primary endpoints studied were time to headache pain freedom and time to absence of most bothersome symptoms. The study showed statistically significant faster headache pain freedom in both 2 h (23.5% in eptinezumab vs. 12.0% in placebo) and 4 h (46.6% in eptinezumab and 26.4%) after infusion. It also showed a greater absence of bothersome symptoms (eptinezumab 55.5% vs. placebo 35.8%). The most common side effect was hypersensitivity reactions, however there were no adverse events [22]. While it may not be feasible to always administer eptinezumab during a migraine attack, this study indicates that treatment can work during an attack and further analysis indicated a delay until the next migraine attack. When discussing preventive treatment options with patients, this information can be beneficial to some when deciding between options.
Wearing-off
In clinical practice, when CGRP mAbs are used for migraine prevention, a portion of patients report wearing-off as the next dose of medication is due. The phenomenon of wearing-off has been evaluated for all CGRP mAbs, but with different definitions of wearing-off. For the evaluation of galcanezumab, wearing-off was defined as an increase in greater than two weekly headache days in the last week of treatment cycle in comparison to the second week of treatment, within the last two months of treatment. In clinical trials of patients treated with galcanezumab for both episodic and chronic migraine, while the data showed a numerical trend of increase in wearing off after two months of treatment for both episodic (0–1.4% placebo and 0–4.0% galcanezumab) and chronic migraine (0–2.3% placebo and 5.2–8.2% galcanezumab), these findings were not statistically significant [23].
Erenumab wearing-off was documented in 34.8% of participants in a self-reported 6-month real-world observational study of individuals with episodic and chronic migraine. The majority of wearing-off, 80% occurred 1 week before the next injection. There was variability in the frequency of the wearing-off as only 32% of participants reported it occurred with each injection. The wearing-off occurred at different time intervals with 20%, 8% and 20% reported during months 1–2, months 3–4, and months 5–6, respectively. While another 12% of patients did not have a pattern to their wearing-off [24].
A 64-week erenumab efficacy and safety study assessed wearing-off as lack of sustained efficacy over the treatment course. The study showed no wearing-off effect over the 64 weeks of erenumab treatment [25].
Fremanezumab wearing-off effect was assessed for both monthly and quarterly dosing intervals in patients with episodic or chronic migraine. Wearing-off as defined as worsening of symptoms before the next dose and was assessed by comparing mean weekly migraine days. There was no change in mean weekly migraine days between weeks 1–2 and weeks 3–4 or weeks 1–3 and week 4 at months 3, 6, 9, and 15. Additionally, when assessing first (months 1–3) and second quarter treatment (months 4–6) there was no change in mean weekly migraine days between weeks 1–2 and weeks 11–12. There was no evidence of wearing-off for monthly or quarterly fremanezumab toward the end of the dosing interval [26].
Switching between CGRP Therapies
In our practice, if an individual has side effects or an inadequate response to one CGRP monoclonal antibody, they may be switched to another monoclonal antibody. Until recently, the efficacy of switching within the class of medication was not known, but recent data suggests it may be effective [26]. One study evaluated treatment response when individuals with lack of response to erenumab were switched to galcanezumab or fremanzumab. A lack of response was defined as no significant improvement after 3 months of erenumab treatment. One-third of patients met the primary endpoint of ≥ 30% responder rate three months after the switch. Additionally, 12% of patients switched to an alternative CGRP monoclonal antibody achieved a ≥ 50% response rate. This data suggests that if an individual does not respond to the first CGRP monoclonal antibody, there may be benefit in trying another [27].
Oral CGRP Receptor Antagonist (Gepant)
Small molecule calcitonin gene-related peptide receptor antagonist, are now available for both the acute and preventive treatment of migraine. Ubrogepant was the first oral CGRP receptor antagonist approved for the acute treatment of migraine. Soon after rimegepant was approved for both acute and preventive treatment of migraine and atogepant was approved for preventive treatment of migraine. Most recently, zavegepant was approved for acute treatment of migraine.
Both ubrogepant 50 mg and 100 mg compared to placebo were statistically significant in meeting the co-primary endpoints of pain freedom at 2 h and absence of both bothersome symptoms at 2 h [28]. In a 52-week extension open-label trial, as needed use of ubrogepant 50 mg or 100 mg for a migraine attack was found to be safe. The most common treatment related adverse events were nausea (1.5–1.7%), dizziness (0.5–1.5%), and somnolence (1.2–1.5%) [29]. Co-adminstration of ubrogepant and sumatriptan is well tolerated and shows no clinically meaningful alteration in pharmacokinetics for either drug [30].
Rimegepant was initially approved the acute treatment of migraine attacks and is the first orally disintegrating gepant. In the phase 3 trial, rimegepant 75 mg was superior to placebo for the co-primary endpoints, pain freedom and freedom from most bothersome symptoms 2 h after dosing. The most common adverse event was nausea [31]. Acute treatment with rimegepant is effective in individuals who have failed two or more triptans [32]. Individuals with > 6 monthly migraine days using PRN rimegepant for acute treatment of migraine attacks showed reduction in migraine frequency over 52-weeks [33]. There was no associated increased tablet utilization when patients were allowed to use rimegepant 75 mg PRN up to once daily for 52 weeks. These individuals also showed improved headache related quality of life [34].
Rimegepant was also shown to be effective when taken every other day for preventive treatment of episodic migraine. The phase 2/3 study met the primary end point of change in monthly migraine days with a mean change of -4.3 days in the rimegepant group compared to -3.5 days with placebo [35]. Rimegepant every other day was found to be similar to erenumab and galcanezumab in mean difference in monthly migraine days and migraine specific quality of life in a matching-adjusted indirect comparison study [36].
Atogepant is used for preventive treatment of episodic and chronic migraine. In the phase 3 trial, atogepant 10 mg, 30 mg and 60 mg all met the primary end point of change from baseline in number of monthly migraine days across 12 weeks [37]. Atogepant dosing can be adjusted if a patient is taking a concomitant strong CYP3A4 inhibitors and OATP inhibitors (38). The most common adverse events seen in clinical trials were constipation and nausea [37]. In our clinical practice, as we start atogepant, we encourage our patients to increase fiber intake.
Atogepant may be an option for individuals who have failed CGRP-targeted monoclonal antibodies and/or onabotulinumtoxinA due to its unique mechanism of action. Atogepant partially inhibits both thinly myelinated Aδ-fibers and unmyelinated C-fibers in a rat CSD model. This suggests a different mechanism of action than fremanezumab, which inhibits only Aδ-fibers, and onabotulinumtoxinA, which inhibits C-fibers. This data suggests that atogepant may be beneficial in individuals who have not responded to CGRP-targeted monoclonal antibodies or onabotulinumtoxinA given the different mechanism of action [38].
In clinical practice, atogepant’s short half-life, 11 h, compared to CGRP mAbs, 27–31 days, is beneficial for certain patient populations. For example, women of childbearing age who are considering pregnancy in the next year, atogepant can be stopped when a woman decides she wants to try to conceive. Typically we recommend stopping atogepant 4 weeks before trying to conceive. CGRP monoclonal antibodies require a 5-month washout period prior to attempting to conceive.
Given that gepants are approved for both preventive and acute treatment of migraine, clinical questions have arisen surrounding combining therapies. Two studies have evaluated the efficacy and tolerability of combining two gepants. When evaluating the drug-drug interactions of oral atogepant and ubrogepant, the combination was found to be safe, well tolerated and without parmacokinetic changes [39]. The TANDEM trial evaluated the combined use of atogepant 60 mg daily and ubrogepant 100 mg as needed for migraine attacks. Again this combination was found to be safe and well tolerated [40].
It is also feasible to consider in clinical practice that individuals taking CGRP monoclonal antibodies for prevention may also use oral small molecule CGRP receptor antagonists as an acute treatment. While many individuals are prescribed both classes of medications, the safety and efficacy of their combined usage needs further evaluation. A phase 1b drug-drug interaction trial evaluated pharmacokinetic properties and safety of ubrogepant when co-administered with erenumab or galcanezumab. The plasma concentration of ubrogepant before and after a single dose of erenumab and galcanezumab remained unchanged. No new adverse events emerged with the combined treatment [40]. Further research is needed to assess the safety and long-term consequence of dual CGRP suppression.
The newest CGRP modulating therapy is intranasal zavegepant, a third generation CGRP receptor antagonist. A phase two dose-ranging randomized controlled trial showed that in 1673 patients randomized to various doses (5 mg, 10 mg, 20 mg) versus placebo, zavegepant 10 mg and 20 mg demonstrated a statistically significant improvement in the primary end points of pain freedom and most bothersome symptom freedom as compared to placebo [41]. The safety profile of zavegepant was also favorable, with its phase one study demonstrating no serious adverse events and the phase two study demonstrating the most common side effects to be dysgeusia and nasal discomfort [41, 42]. The greatest benefit of zavegepant is its intranasal formulation, allowing migraine patients who experience severe nausea and/or vomiting an alternative to the current existing gepants, all in oral formulations.
Conclusion
CGRP modulating therapies are effective as both preventive and acute treatment of episodic and chronic migraine. The benefit of these treatments is their efficacy and tolerability. They have provided treatment options for individuals that cannot tolerate our standard migraine medications due to side effects or contraindication to therapy. Our knowledge of these therapies continues to expand and new agents are continually being developed. More research is needed to better understand the effect of combining these medications and the long-term consequences of CGRP blockade.
Data Availability
No datasets were generated or analysed during the current study.
References
Iyengar S, Johnson KW, Ossipov MH, Aurora SK. CGRP and the Trigeminal System in Migraine. Headache. 2019;59(5):659–81.
Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol [Internet]. 2018 Jun 1 [cited 2022 Jun 3];14(6):338–50. https://pubmed.ncbi.nlm.nih.gov/29691490/.
Wattiez AS, Sowers LP, Russo AF. Calcitonin gene-related peptide (CGRP): role in migraine pathophysiology and therapeutic targeting. https://doi.org/101080/1472822220201724285 [Internet]. 2020 Feb 1 [cited 2022 Jun 3];24(2):91–100. https://www.tandfonline.com/doi/abs/https://doi.org/10.1080/14728222.2020.1724285.
Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev Mol Med [Internet]. 2011 [cited 2022 Jun 3];13:e36.
Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol [Internet]. 1988 Feb 1 [cited 2022 Jun 3];23(2):193–6. https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1002/ana.410230214.
PJ G. L E, R E. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol [Internet]. 1990 [cited 2021 Jul 15];28(2):183–7. https://pubmed.ncbi.nlm.nih.gov/1699472/.
Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen. & J. CGRP may play a causative role in migraine.
Cernuda-Morollón E, Martínez-Camblor P, Ramón C, Larrosa D, Serrano-Pertierra E, Pascual J. Research Submissions CGRP and VIP levels as predictors of efficacy of Onabotulinumtoxin Type A in Chronic Migraine. 2014.
Cernuda-Morollón E, Ramón C, Martínez-Camblor P, Serrano-Pertierra E, Larrosa D, Pascual J. OnabotulinumtoxinA decreases interictal CGRP plasma levels in patients with chronic migraine. Pain [Internet]. 2015 May 1 [cited 2022 Apr 20];156(5):820–4. https://pubmed.ncbi.nlm.nih.gov/25735000/.
Garces F, Mohr C, Zhang L, Huang CS, Chen Q, King C et al. Molecular Insight into Recognition of the CGRPR Complex by Migraine Prevention Therapy Aimovig (Erenumab). Cell Rep [Internet]. 2020 Feb 11 [cited 2022 Jun 8];30(6):1714–1723.e6. https://pubmed.ncbi.nlm.nih.gov/32049005/.
Reuter U, Ehrlich M, Gendolla A, Heinze A, Klatt J, Wen S et al. Erenumab versus topiramate for the prevention of migraine-a randomised, double-blind, active-controlled phase 4 trial. https://clinicaltrials.gov/ct2/show/NCT03828539.
The American Headache Society Position Statement On Integrating New Migraine Treatments Into Clinical Practice. Headache [Internet]. 2019 Jan 1 [cited 2022 Jun 8];59(1):1–18. https://pubmed.ncbi.nlm.nih.gov/30536394/.
Noseda R, Bedussi F, Gobbi C, Ceschi A, Zecca C. Safety profile of monoclonal antibodies targeting the calcitonin gene-related peptide system in pregnancy: updated analysis in VigiBase®. Cephalalgia. 2023;43(4):3331024231158083.
Vig SJ, Garza J, Tao Y. The use of erenumab for migraine prophylaxis during pregnancy: A case report and narrative review. Headache [Internet]. 2022 [cited 2022 Jun 8]; https://pubmed.ncbi.nlm.nih.gov/35467013/.
Bigal ME, Kurth T, Santanello N, Buse D, Golden W, Robbins M et al. Migraine and cardiovascular disease. Neurology [Internet]. 2010 Feb 23 [cited 2022 Jun 8];74(8):628–35. https://n.neurology.org/content/74/8/628.
Favoni V, Giani L, Al-Hassany L, Asioli GM, Butera C, De Boer I et al. CGRP and migraine from a cardiovascular point of view: What do we expect from blocking CGRP? J Headache Pain [Internet]. 2019 Mar 12 [cited 2022 Jun 8];20(1):1–7. https://thejournalofheadacheandpain.biomedcentral.com/articles/https://doi.org/10.1186/s10194-019-0979-y.
Saely S, Croteau D, Jawidzik L, Brinker A, Kortepeter C, Hypertension. A new safety risk for patients treated with erenumab. Headache J Head Face Pain [Internet]. 2021 Jan 1 [cited 2022 Jun 8];61(1):202–8. https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1111/head.14051.
Dodick DW, Tepper SJ, Ailani J, Pannacciulli N, Navetta MS, Loop B et al. Risk of hypertension in erenumab-treated patients with migraine: Analyses of clinical trial and postmarketing data. Headache [Internet]. 2021 Oct 1 [cited 2022 Jun 8];61(9):1411–20. https://pubmed.ncbi.nlm.nih.gov/34591982/.
Goadsby PJ, Dodick DW, Leone M, Bardos JN, Oakes TM, Millen BA et al. Trial of Galcanezumab in Prevention of Episodic Cluster Headache. N Engl J Med [Internet]. 2019 Jul 11 [cited 2022 Jun 8];381(2):132–41. https://pubmed.ncbi.nlm.nih.gov/31291515/.
Dodick DW, Goadsby PJ, Lucas C, Jensen R, Bardos JN, Martinez JM et al. Phase 3 randomized, placebo-controlled study of galcanezumab in patients with chronic cluster headache: Results from 3-month double-blind treatment. Cephalalgia [Internet]. 2020 Aug 1 [cited 2022 Jun 8];40(9):935–48. https://pubmed.ncbi.nlm.nih.gov/32050782/.
Marmura MJ, Diener HC, Cowan RP, Tepper SJ, Diamond ML, Starling AJ et al. Preventive migraine treatment with eptinezumab reduced acute headache medication and headache frequency to below diagnostic thresholds in patients with chronic migraine and medication-overuse headache. Headache [Internet]. 2021 Oct 1 [cited 2022 Jun 8];61(9):1421–31. https://pubmed.ncbi.nlm.nih.gov/34551130/.
Winner PK, Mcallister P, Chakhava G, Ailani J, Ettrup A, Mette ; et al. Effects of Intravenous Eptinezumab vs Placebo on Headache Pain and Most Bothersome Symptom When Initiated During a Migraine Attack A Randomized Clinical Trial. 2021; https://jamanetwork.com/.
Ailani J, Kuruppu DK, Rettiganti M, Oakes T, Schroeder K, Wietecha L et al. Does wearing off of efficacy occur in galcanezumab-treated patients at the end of the monthly treatment cycle? Post hoc analyses of four phase III randomized trials. Headache [Internet]. 2022 Feb 1 [cited 2022 Jun 8];62(2):198–207. https://pubmed.ncbi.nlm.nih.gov/35076090/.
Robblee J, Devick KL, Mendez N, Potter J, Slonaker J, Starling AJ. Real-World Patient Experience With Erenumab for the Preventive Treatment of Migraine. Headache J Head Face Pain [Internet]. 2020 Oct 1 [cited 2022 Jul 10];60(9):2014–25. https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1111/head.13951.
Goadsby PJ, Reuter U, Lanteri-Minet M, Paiva G, Lima S, Hours-Zesiger P et al. Long-term Efficacy and Safety of Erenumab. Neurology [Internet]. 2021 Jun 1 [cited 2022 Jul 12];96(22):e2724–35. https://n.neurology.org/content/96/22/e2724.
Blumenfeld AM, Stevanovic DM, Ortega M, Cohen JM, Seminerio MJ, Yang R et al. No Wearing-Off Effect Seen in Quarterly or Monthly Dosing of Fremanezumab: Subanalysis of a Randomized Long-Term Study. Headache J Head Face Pain [Internet]. 2020 Nov 1 [cited 2022 Jul 10];60(10):2431–43. https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1111/head.13994.
Overeem LH, Peikert A, Hofacker MD, Kamm K, Ruscheweyh R, Gendolla A et al. Effect of antibody switch in non-responders to a CGRP receptor antibody treatment in migraine: A multi-center retrospective cohort study. Cephalalgia [Internet]. 2022 Apr 1 [cited 2022 Jun 8];42(4–5):291.
Dodick DW, Lipton RB, Ailani J, Lu K, Finnegan M, Trugman JM et al. Ubrogepant for the Treatment of Migraine. N Engl J Med [Internet]. 2019 Dec 5 [cited 2021 Nov 8];381(23):2230–41. https://www.nejm.org/doi/full/https://doi.org/10.1056/NEJMoa1813049.
RB JA. L, S H, K K, K L, M B, Long-Term Safety Evaluation of Ubrogepant for the Acute Treatment of Migraine: Phase 3, Randomized, 52-Week Extension Trial. Headache [Internet]. 2020 Jan 1 [cited 2021 Jul 15];60(1):141–52. https://pubmed.ncbi.nlm.nih.gov/31913519/.
Jakate A, Boinpally R, Butler M, Lu K, McGeeney D, Periclou A. Evaluation of the Pharmacokinetic Interaction of Ubrogepant Coadministered with Sumatriptan and of the safety of Ubrogepant with triptans. Headache. 2020;60(7):1340–50.
PJ RC, DA G, CM S, EG CMF. S, Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet (London, England) [Internet]. 2019 Aug 31 [cited 2021 Jul 15];394(10200):737–45. https://pubmed.ncbi.nlm.nih.gov/31311674/.
Jensen C, Lipton R, Blumenfeld A, Croop R, Thiry A, L’Italien G et al. Rimegepant for the Acute treatment of Migraine in patients with a history of Triptan Treatment failure: pooled results from 3 phase 3 clinical trials (4914). Neurology. 2021;96(15 Supplement).
L’italien G, Popoff E, Johnston K, Mcgrath D, Conway CM, Powell L et al. Rimegepant 75 mg for acute treatment of migraine is associated with significant reduction in monthly migraine days: Results from a long-term, open-label study. https://us.sagepub.com/en-us/nam/open-access-at-sage.
Johnston K, Harris L, Powell L, Popoff E, Coric V, L’Italien G et al. Monthly migraine days, tablet utilization, and quality of life associated with Rimegepant – post hoc results from an open label safety study (BHV3000–201). J Headache Pain [Internet]. 2022 Dec 1 [cited 2022 May 5];23(1):1–8. https://thejournalofheadacheandpain.biomedcentral.com/articles/https://doi.org/10.1186/s10194-021-01378-5.
Croop R, Lipton RB, Kudrow D, Stock DA, Kamen L, Conway CM et al. Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet [Internet]. 2021 Jan 2 [cited 2021 Nov 8];397(10268):51–60. http://www.thelancet.com/article/S0140673620325447/fulltext.
Popoff E, Johnston K, Croop R, Thiry A, Harris L, Powell L, et al. Matching-adjusted indirect comparisons of oral rimegepant versus placebo, erenumab, and galcanezumab examining monthly migraine days and health-related quality of life in the treatment of migraine. Headache. 2021;61(6):906–15.
Ailani J, Lipton RB, Goadsby PJ, Guo H, Miceli R, Severt L et al. Atogepant for the Preventive Treatment of Migraine. https://doi.org/101056/NEJMoa2035908 [Internet]. 2021 Aug 18 [cited 2021 Oct 11];385(8):695–706. https://www.nejm.org/doi/full/https://doi.org/10.1056/NEJMoa2035908.
HIGHLIGHTS OF PRESCRIBING INFORMATION. [cited 2021 Nov 15]; Available from: http://www.fda.gov/medwatch.
Strassman AM, Melo-Carrillo A, Houle TT, Adams A, Brin MF, Burstein R. Atogepant – an orally-administered CGRP antagonist – attenuates activation of meningeal nociceptors by CSD: https://doi.org/101177/03331024221083544 [Internet]. 2022 Mar 25 [cited 2022 May 6];033310242210835.
Jakate A, Blumenfeld AM, Boinpally R, Butler M, Borbridge L, Contreras-De Lama J, et al. Pharmacokinetics and safety of ubrogepant when coadministered with calcitonin gene-related peptide-targeted monoclonal antibody migraine preventives in participants with migraine: A randomized phase 1b drug-drug interaction study. Headache [Internet]. 2021 Apr 1 [cited 2022 Jun 3];61(4):642–52. Available from: https://pubmed.ncbi.nlm.nih.gov/33818780/.
Croop R, Madonia J, Conway C, Thiry A, Forshaw M, Murphy A, et al. Intranasal Zavegepant is Effective and Well Tolerated for the Acute Treatment of Migraine: A Phase 2/3 Dose-Ranging Clinical trial (4976). Neurology. 2021;96(15 Supplement).
Bertz R, Donohue M, Madonia J, Bhardwaj R, Stringfellow J, Anderson M, et al. Safety, Tolerability, and Pharmacokinetics of Single and Multiple Ascending Doses of Intranasal Zavegepant in Healthy Adults (S31.004). Neurology. 2022;98(18 Supplement).
Author information
Authors and Affiliations
Contributions
K.B, T.S. and S.V wrote the main manuscript. J.A. created the outline for the article and was the main reviewer of the article.
Corresponding author
Ethics declarations
Competing Interests
Jessica Ailani, MD, reports personal fees from AbbVie, Aeon, Amgen, Axsome Dr. Reddy, Eli Lilly, eneura, Pfizer, GlaxoSmithKline, Gore, Impel, Parema, Linpharma, Lundbeck Merz, Neurolief, Scilex, Satsuma, Theranica, clinical trial support from AbbVie, Impel, Lundbeck, Parema . She has provided editorial services to Current Pain and Headache Reports, Medscape, and SELF magazine.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bedrin, K., Shah, T., Vaidya, S. et al. CGRP Modulating Therapies: An Update. Curr Neurol Neurosci Rep 24, 453–459 (2024). https://doi.org/10.1007/s11910-024-01363-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-024-01363-w