Abstract
Purpose of Review
Deep brain stimulation (DBS) is an established treatment in several movement disorders, including Parkinson’s disease, dystonia, tremor, and Tourette syndrome. In this review, we will review and discuss the most recent findings including but not limited to clinical evidence.
Recent Findings
New DBS technologies include novel hardware design (electrodes, cables, implanted pulse generators) enabling new stimulation patterns and adaptive DBS which delivers potential stimulation tailored to moment-to-moment changes in the patient’s condition.
Better understanding of movement disorders pathophysiology and functional anatomy has been pivotal for studying the effects of DBS on the mesencephalic locomotor region, the nucleus basalis of Meynert, the substantia nigra, and the spinal cord.
Eventually, neurosurgical practice has improved with more accurate target visualization or combined targeting. A rising research domain emphasizes bridging neuromodulation and neuroprotection.
Summary
Recent advances in DBS therapy bring more possibilities to effectively treat people with movement disorders. Future research would focus on improving adaptive DBS, leading more clinical trials on novel targets, and exploring neuromodulation effects on neuroprotection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: Current State of the Art in Movement Disorders
Deep brain stimulation (DBS) is currently an established treatment option in several movement disorders, including Parkinson’s disease (PD), dystonia, tremor, and Tourette syndrome [1, 2].
However, despite several advances in technology within the last decade, there are still some limitations with DBS surgery. For example, several symptoms are still not responsive to DBS whatsoever the disease or the target, and there is a critical need for improving the accuracy of the simulation pattern, finding more effective targets to tackle intractable symptoms, increasing targeting accuracy during implantation, and updating the hardware itself.
Parkinson’s Disease
Recent work has confirmed the effects of DBS not only in motor but also in non-motor signs. Subthalamic nucleus (STN) DBS in PD has been reported as effective and safe more than 15 years after the surgery, and importantly, postoperative dementia risk is not increased in the long term [3, 4••]. Moreover, an anterior location of STN electrodes has been suggested to give a better non-motor improvement such as sleep and urinary incontinence [5] and restless leg syndrome [6].
Both STN-DBS and globus pallidus internus (GPi) DBS have been considered effective to treat motor symptoms in PD [7]. A recent meta-analysis yielded no overall difference between targets in tremor reduction at various time points up to 12 months after surgery [8]. Similarly, another study compared the effects of STN-DBS and GPi-DBS on PD rest tremor and action tremor. Both targets improved action and rest tremor at 6 and 12 months, with STN-DBS being more effective on action tremor at 6 months but not at the 12 months follow-up [9]. A recent review states that thalamic ventralis intermedius nucleus (Vim) DBS better addresses action tremor in PD [10••].
Over the last several years, following the EARLYSTIM study, results proving that DBS can be used when motor complications occur early [11], the timing of the DBS surgery for PD has changed. Newer findings from this cohort strengthen earlier surgery as an option, since it is associated with better behavioral outcomes (hyperdopaminergic symptoms and neuropsychiatric fluctuations) in STN-DBS patients compared to patients with only medical treatment [12]. Moreover, noticeable improvements are reported in freezing of gait at 2-year follow-up [13]. Furthermore, the early surgical group demonstrated better social, occupational, and psychosocial function [14].
Dystonia
Although there is evidence that isolated dystonia is improved by both GPi [15] and STN DBS [1], there is still lack of strong evidence for focal dystonia [2]. The issue is even more important with combined dystonia (i.e., dystonia occurring with other neurological symptoms like chorea, spasticity, ataxia) that is much less addressed by DBS in the literature [1]. However, there is still an overall GPi-DBS nonresponder rate in dystonia as high as 25% [2].
One solution to tackle this issue may be to select other targets. A prospective study focused on Vim-DBS in dystonic tremor, and essential tremor (ET) found that dystonic tremor and ET were significantly improved during the first 5 years, but not afterwards [16]. Improvement of activities of daily living was nonsignificant after 2 to 3 years postimplantation, possibly due to a lack of control on dystonia itself. Nevertheless, this work highlights the possibility to stimulate the Vim if tremor is disabling in dystonic patients. Another interesting target is the cerebellum [17]. Stimulation of the dentate nucleus has been reported to be effective in a few cases of secondary dystonia [18,19,20].
Tremor
The Vim nucleus has long been established as the main DBS target to treat tremor, specifically ET, being safe and efficacious when compared to historical thalamotomy [10••]. A prospective, controlled multicenter study including 122 patients showed that both unilateral and bilateral Vim-DBS are effective. Tremor was improved by 2.49 + / − 0.96 points on the Essential Tremor Rating Scale 1 year after implantation [21]. A recent review yielded that 1-year tremor reductions ranged from 53 to 63% with unilateral Vim-DBS. Overall, bilateral Vim-DBS treated both upper extremities with increased likelihood of addressing head tremor and thus demonstrated more improvement in tremor reduction (66–78%). Several studies show ongoing beneficial effects up to 5 years and beyond [22, 23].
Nevertheless, some adverse effects (gait ataxia, dysarthria) may either be caused by the progression of the underlying disease or on target stimulation itself [24]. Indeed, Vim-DBS could induce antidromic cerebellar stimulation and inappropriate plastic remodeling causing early ataxia and gait impairment. Long-term habituation may explain a later loss of therapeutic effect, but this must be weighed against the possibility of disease progression [25].
Tourette Syndrome
Tourette syndrome is a complex neuropsychiatric disease that commonly manifests with obsessive–compulsive disorder (OCD), vocal and/or motor tics, depression, and disruptive behavior disorders. Disease heterogeneity can be the reason why clinical results from DBS are still debated. A recent prospective multicenter study focused on 185 patients with medically refractory Tourette syndrome who underwent DBS, either targeting the centromedian thalamic region, the anterior GPi, the posterior GPi, or the anterior limb of the internal capsule [26]. Motor and vocal tic severity globally improved at 1 year after DBS, but there was a significant adverse event rate (35.4%). The most common stimulation-induced adverse effects were dysarthria (6.3%) and paresthesia (8.2%). However, due to small sample size in some target groups, comparing tic improvement between targets is difficult [26]. A retrospective multicenter review on 123 patients showed that DBS significantly improved tics and OCD regardless of the target (GPi, centromedian thalamus, nucleus accumbens) site [27•].
Another work reported long-term tic improvement 48 months after anterior GPi-DBS in 19 patients. This study also revealed that 75% patients were good responders, and that none of those having self-injury at baseline showed such behavior after DBS [28]. These promising results need to be confirmed with larger trials.
New DBS Technologies
Even though DBS device technology seems to be evolving at slow pace, there are now several advances that could improve both the way in which therapy is delivered and the patient-physician relationship. New implementations of DBS technology involve electrode design (allowing the delivery of different spatial stimulation patterns), pulse generator hardware design (allowing an increase in the stimulation parameter choice and add features aimed to provide a wider knowledge on patient’s state like neural activity sensing, accelerometers), and pulse generator communication capabilities (introducing new ways of DBS programming and interaction) [29].
New Concepts in Stimulation Patterns
Stimulation patterns represent the way electrical pulses are delivered to the brain tissue. They can be modulated spatially, thus shaping the electrical field in the tissue, or temporarily, using different stimulus waveforms or pulse trains.
The classical stimulation pattern in DBS is represented by charge-balanced biphasic square waves delivered at a fixed frequency by cylindrical contacts in a monopolar or bipolar fashion. In PD, 60-µs pulse width and 130-Hz frequency with variable amplitude are usually considered effective, while longer pulse widths (> 120 µs) are mainly used in dystonia. However, recent evidence has shown that shorter pulsed (< 60 µs) stimulation can reduce stimulation-induced adverse events, like ataxia with Vim-DBS [30, 31].
Spatial modulation relies on the availability of asymmetric current sources, either steering the stimulation in a specific direction (directional leads) or providing independent current sources with different stimulation parameters. Directional leads are characterized by axially segmented electrodes that can be activated in order to focus the stimulation on a specific direction in a plane orthogonal to the electrode axis. This widens the therapeutic window, thus improving DBS effectiveness [32] while further reducing antiparkinsonian medication as compared to classical omnidirectional DBS [33]. The availability of DBS devices able to deliver independent stimulation at each contact (multiple independent constant current controlled, MICC) provides further means for directing the electric field in a non-isotropic way [34•]. Having independent sources also supports the effective use of directional leads providing to each contact the full spectrum of parameters.
As an alternative to conventional DBS delivery, a stimulation algorithm where weak high-frequency pulse trains are delivered to different electrode contacts at different times in order to contract pathological synchronization by resetting the phase of the targeted neurons (coordinated reset stimulation) has been proposed [35].
Adaptive DBS (aDBS)
The possibility to deliver a stimulation tailored to moment-to-moment changes in the patient’s condition seems to be the most attractive feature of next-generation DBS technologies. Until now, available pulse generators were only capable of providing a fixed stimulation, which was, however, sufficient to provide satisfactory results.
The first question concerning aDBS is what variable should be used as a biomarker for a patient’s clinical state. Design considerations include the feasibility of the recording, the battery drain induced by biomarker sensing, the need for additional implants, and the stability and reliability of the biomarker [36]. At present, in PD, the most promising approach is based on the recording of local neural activity (local field potentials, LFPs) using implanted DBS lead. This poses, however, the technological challenge of signal-to-noise ratio when LFPs have to be recorded during DBS ON [37].
In PD, where stimulation is continuously provided to patients, sensing during stimulation is essential, and, after several years of research, there are now two commercially available devices approved for LFP sensing during stimulation, the Medtronic Percept [38] and the Newronika AlphaDBS [39]. Both devices are capable of aDBS which is currently under investigation.
The current state of the art of LFP-based aDBS for PD experiments is built on amplitude modulations (AM) of STN-LFP beta band (13–35 Hz) as a feedback control biomarker. Beta band showed consistent correlation with levodopa dynamics [40, 41], and motor status, mainly akinesia and rigidity [42,43,44]. Beta activity is also stable over time after DBS implantation, as shown in chronic recordings [45]. Therefore, the first experiments on aDBS in humans relied on beta detection and subsequent either “on demand” [46] or “linear adaptive” [44] change of DBS amplitude.
Despite being simple, LFP beta activity amplitude alone is not able to capture the complex dynamics of PD neurophysiology. Beta burst dynamics and beta phase, instead of beta-band amplitude, could be used as aDBS biomarker that anticipates beta AM and therefore could trigger stimulation that anticipates instead of following the beta AM [47••]. In addition, other LFP frequency bands, or even electrocorticogram bands [48], could be targeted to better represent other symptoms such as dyskinesia [48] or tremor [49].
The long-term integration of LFP recordings and wearable technologies in ecologic environments could help validating biomarkers and further improving aDBS [50•].
Telemedicine
DBS pulse generators are usually controlled by short-range connectivity and dedicated devices (e.g., for in-clinic programming). However, increased capabilities of smart applications open the way for telemetry and remote access. The first example is the Abbott NeuroSphere Virtual Clinic [51]. This technology is based on an in-app video chat and integrated remote programming via secured connection, allowing the physician to assess the patient’s current condition and to change stimulation settings if needed. Although it may not replace in person consultation, this technology may prove useful to remote patients and in cases in which health systems and transportation are globally disrupted. Recent work showed high patient and physician satisfaction when using the NeuroSphere Virtual Clinic in chronic pain patients [52].
Also, in the near future, the new implantable aDBS (or simply sensing) systems will have the potential to record brain signals in humans 24/7, thus demanding effective solutions to manage, store, analyze such data, and integrate them with other information streams including but not limited to wearables and direct patient input. This would help in exploiting the full potential of aDBS, including the application of more sophisticated machine learning or deep learning algorithms for decoding brain states [53].
New DBS Targets
Parkinson’s Disease
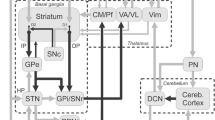
Although dopaminergic medications and both STN- and GPi-DBS significantly ameliorate cardinal motor symptoms in PD, other symptoms such as gait and balance are less predictable and not well sustained in the long term. Additionally, cognitive symptoms are usually not affected by or can even worsen after stimulation. In this context, researchers have explored alternative brain and spinal targets to tackle these symptoms of poor response to the conventional DBS targets [54].
Mesencephalic Locomotor Region
DBS of the mesencephalic locomotor region (MLR), composed of the pedunculopontine (PPN) and cuneiform (CuN) nuclei, has been proposed to treat dopa-resistant gait and balance disorders in PD. Indeed, this region is composed of a collection of cholinergic, glutamatergic, and GABAergic neurons with an impressive array of reciprocal connections with basal ganglia, motor cortex, and spinal cord motor neurons [55].
Several clinical trials have been performed targeting the MLR area DBS in PD, showing that unilateral or bilateral stimulation could improve gait freezing in both the off- and on-medication states early after surgery. However, the degree of improvement has been highly variable, and benefits often were not maintained [56, 57]. MLR area DBS may also have the potential to reduce falls, though the impact on postural instability is unclear [56]. A recent study showed that MLR stimulation improved intermittent switching of postural sway, feedback gains in the proportional-integral-derivative model, and clinical balance impairment [58]. Unfortunately, it is unclear whether such partial benefits on gait and balance are clinically meaningful as assessment of quality of life is seldom reported [55].
Substantia Nigra Pars Reticulata
The substantia nigra pars reticulata (SNr) is a primary output nucleus of the basal ganglia together with the GPi. The SNr sends GABAergic projections to the pedunculopontine nucleus. In PD, the SNr is abnormally overactivated, which leads to inhibition of the locomotor region, contributing to the axial problems typical of PD progression [54].
Dual stimulation of the SNr (using ventral DBS contacts) and the STN (using dorsal DBS contacts) using the same electrode has been applied in PD to restore locomotor function and was superior in controlling freezing of gait compared to STN stimulation alone [59]. Another recent study demonstrates that high-frequency stimulation of the SNr but not of the STN ameliorates the anticipatory postural adjustments in PD [60].
A crossover, randomized trial investigated the effects of simultaneous stimulation in both the STN and SNr at different frequencies in PD (126 Hz in STN and 63 Hz in SNr) with the SNr stimulation alone. For most patients, the combined stimulation achieved the best freezing and balance control [61]. Freezing of gait has also been reported to be improved by combined STN and SNr stimulation [62].
Although promising, there are still uncertainties regarding the best stimulation parameters and the hotspot of stimulation within SNr to improve locomotion. Few studies suggest that stimulation in the lateral SNr is less effective for treating gait disturbances in PD than stimulation in the medial SNr region [63], while stimulation of the medial portion of the SNr has been shown to induce depression and hypomania [55].
Spinal Cord
Spinal cord stimulation (SCS) is a well-established therapy for the treatment of chronic neuropathic pain due to its good efficacy profile and safety. In the last few years, SCS has been suggested to improve axial symptoms in PD patients, especially gait changes and posture abnormalities [64]. The potential therapeutic application of SCS received considerable interest after a study in rodent, and monkey models of parkinsonism demonstrated that SCS could improve locomotion [65].
An open-label study including 15 PD patients with low back and/or lower limb pain and thoracic SCS reported a significant improvement in pain intensity, postural stability, and gait speed over 12 months of follow-up [66••]. Another open-label study reported improvements in several gait parameters after thoracic SCS for 6 months in five PD patients [67]. More recently, an open-label study with 6 pain-free PD patients failed to show any benefit 12 months after thoracic SCS [68].
Despite the overall good outcomes of SCS in treating gait problems in most studies (Table 1), there are still challenges ahead because a relatively small number of PD patients have been evaluated so far with variable study populations. Additionally, the stimulation produces tangible sensations which might be responsible for a placebo effect in a subset of patients. Moreover, many papers included patients with lower limb and back pain, which is a confounding bias because pain improvement after SCS can affect gait performance [55].
A study population with better-defined inclusion criteria, multicenter trials, and long-term follow-up are the next steps to establish SCS as a potentially neuromodulatory tool for gait in PD. Double-blind approaches designed with an amplitude subthreshold for paresthesia, very high frequencies (below the sensory threshold) [65], or new paradigms such as burst stimulation might certainly guide future trials to avoid placebo effects [74].
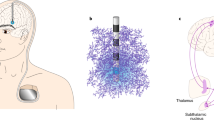
Nucleus Basalis of Meynert
The nucleus basalis of Meynert (NBM) is largely involved in cognitive and behavioral functions, including arousal, attention, perception, and memory [76]. Cognitive impairment and dementia are an important source of disability and reduction in quality of life for both patients and caregivers in PD and dementia with Lewy bodies (DLB) [77].
To date, six primary clinical studies, including three case reports and three randomized crossover studies, involving patients with PD with dementia and DLB have been performed [78]. Although DBS seems to be safe, no significant improvement of cognitive scores between sham vs. NBM DBS has been observed.
Although the primary outcomes were not achieved among the trials, decreased neuropsychiatric inventory scores, which were primarily driven by a reduction of visual hallucination and apathy, were noticed in some patients [79, 80]. Additionally, improvement of functional connectivity in the frontoparietal and default mode network in DLB subjects was also observed [79]. Besides, increased metabolic activity at the superior lingual gyrus following NBM DBS was shown, while cognitive function continued unchanged [81]. Moreover, combined GPi and NBM-DBS in PD resulted in reduced right frontal and parietal metabolism without improving cognition [82]. More preclinical evidence is needed to optimize NBM DBS clinically such as patient selection, the hotspot of stimulation, and DBS parameters.
Ataxia
Cerebellar ataxia is a disabling neurological symptom with hereditary and acquired etiologies. Overall, management is undertaken via rehabilitation since no medical treatment has yet been shown to be effective [83]. Recently, noninvasive stimulation has been shown to be effective in alleviating symptoms in post-lesion or degenerative ataxia [84, 85]. Invasive cerebellar stimulation in animal models suggests the possibility of modulating aberrant dentato-thalamo-cortical loops known to be dysfunctional in refractory ataxic patients due to different etiologies [86]. In case reports, dentate nucleus DBS was effective for treating ataxia in SCA type 3, cerebellar stroke, and dystonia [18, 19, 87].
A recent randomized double-blind crossover pilot trial enrolled five patients with spinocerebellar ataxia type 3 or post-lesion ataxia [88]. Active or sham phases were randomly performed 3 months apart. The effects on the primary outcome (Scale for the Assessment and Rating of Ataxia) were numerically better, but not statistically significant, after active versus sham stimulation. Regarding the secondary measures, dentate nucleus DBS caused a significant improvement in cerebellar tremor and the global impression of change after comparing active to sham stimulations.
Tremor
For medication-refractory cases, DBS is an established, effective, and safe treatment for ET [89]. The Vim is the traditional DBS target in ET, but the posterior subthalamic area (PSA) has been suggested as an alternative target [90].
A randomized, double-blind crossover study showed that PSA-DBS significantly reduced tremor severity and improved quality of life in ET patients [91]. There were no relevant differences in quality and frequency of stimulation side effects between VIM and PSA, with a tendency toward greater tremor improvement with PSA stimulation. Clinical benefit was achieved at lower stimulation amplitudes in the PSA, and the majority of patients remained with PSA-DBS after 1 year of follow-up. More recently, using probabilistic fiber tracking, a clinical trial in ET showed that PSA contacts were closer to the dentato-rubro-thalamic tract (DRTT) and led to a greater improvement in tremor scores than VIM contacts [92]. Proximity to the DRTT was also related to lower amplitudes of stimulation and higher DBS efficiency. It seems that the DRTT is potentially a common tremor-reducing structure since it also has been shown to be involved in Parkinson’s tremor, multiple sclerosis, and dystonic head tremor [93]. Besides, a new randomized double-blind controlled crossover trial compared PSA and Vim-DBS on action tremor in each patient. The four-plot electrodes were implanted so that they reached both targets, and PSA was superior to Vim-DBS at 6 months [94].
Neurosurgeon’s Practice
DBS requires high targeting accuracy to achieve the best result; therefore, surgical practice is critically impacted by technological breakthroughs in imaging and connectivity that help targeting not only nuclei but also tracts. Besides, recent preclinical discoveries hint DBS’ ability to play a role in neuroprotection by combining electrical stimulation and its biological effects in the nervous tissue.
Improving Visualization
Good DBS outcomes rely on accurate nucleus targeting. Robot-assisted surgery may allow for another option for lead placement compared with conventional frame-based approaches. The mean duration of DBS surgery is significantly reduced with robot-assisted DBS [95]. A meta-analysis compiling 2409 DBS trajectories confirmed the superiority of robot-assisted surgery as the pooled mean target error was decreased by 0.788 mm [96••].
Another improvement in implantation precision may come from better target visualization. For instance, GPi imaging may benefit from the use of 7-Tesla MRI which helps visualize the motor part of the GPi (posterolateral region) with cortex-striatum-GPi tractography or “reverse” tractography originating from the thalamus backwards to the GPi [97]. Such use of each patient’s connectivity pattern may improve DBS by finding functional hotspots that will eventually give the best outcome and may also assist with programming after surgery by functional interrogation of circuits. Indeed, an exploratory GPi imaging study in PD using directional leads recorded a strong 5–35 Hz activity pattern in the posterolateral region of the GPi. This activity correlated with the best motor results and the plots in contact with this region [98].
Quite similarly, STN imaging may benefit from 7Tesla MRI, by allowing accurate patient-specific nucleus parcellation, thanks to STN-cortex tractography [99]. 7-T MRI however is not widely available, and care must be taken to quality control the protocols for the potential of field distortion. Indeed, improved 3 T sequences and even low Tesla (e.g., 0.5 T) acquisition with high contrast may ultimately prove dominant due to accessibility, accuracy, and ease of use.
Furthermore, machine learning can be coupled with MRI to improve STN visualization. A 7Tesla MRI study found that machine learning-coupled location was significantly closer to the ground truth than atlas-based location in 80 patients [100]. Deep learning has also been used to improve GPi and globus pallidus externa segmentation. The results were positive and may help accurately locate this other DBS target [101]. STN also benefits from this process through preoperative microelectrode recording prediction via deep learning [102].
Targeting thanks to Tractography and Connectivity
DBS has extensively relied on nucleus targeting, but there is a recent trend in focusing on tracts stimulation. For instance, the DRTT is a key pathway whose disruption is associated with tremor [103], and it comprises the PSA. A study compared DRTT tractography-guided PSA-DBS to conventional landmarks targeting in ET and tremor dominant PD. It found better tremor and quality-of-life outcomes at 6 months and 60 months in the tractography group [104]. In addition to this, combining DRTT tractography and electric field simulations delivered by the DBS may help tailor the current in the wanted direction [105].
Not only structural but also functional connectivity can help in refining or even discovering DBS targets. For instance, Corp et al. have used resting-state functional MRI connectivity in cervical dystonia to unravel a common network that links different lesion locations that cause this disease [106•]. The results show that positive connectivity to the cerebellum and negative connectivity to the somatosensory cortex are specific markers for cervical dystonia, whether being lesional or idiopathic. There was also a correlation between GPi-DBS leads location and the network’s regions of interest. This kind of study may lead to the discovery of new DBS targets within the disease’s functional network.
The same reasoning applies to ET, for which structural and functional MRI connectivity models were significantly predictive of post-DBS tremor improvement [107]. This multimodal connectivity model helped find a sweet spot located in the PSA, intersecting with cerebello-thalamic fibers.
Combined Targeting
Targeting multiple areas to get additional stimulation options may be useful in intractable tremor, assuming that reaching the second site does not compromise the first one [108].
Some pathologies may consider multiple leads up-front. An example is the multiple sclerosis refractory tremor, for which the Vim-ventralis oralis posterior nucleus border and the ventralis oralis anterior-ventralis oralis posterior nucleus border have been targeted with two distinct leads [109]. Patients had one or another lead activated for 3 months and then had both for three more. The authors did not provide a 3-month statistical analysis, but tremor was significantly reduced with a similar effect in both groups [109].
Moreover, using DBS leads with 8 plots allows a broader range to stimulate multiple targets with a single lead. This was used in dual Vim and zona incerta DBS in one ET patient, whose tremor was effectively reduced when stimulating either Vim or zona incerta [110].
A retrospective study focused on 5 tremor-dominant-PD patients who had dual Vim and STN via parietal approach. PD symptoms were significantly reduced in the first 6 months of continuous stimulation and remained stable thereafter. Quality of life and tremor were also improved [111].
Neuromodulation and Neuroprotection
DBS is mostly used in neurodegenerative diseases in neurology. Despite some clues in PD animal models, there is no current evidence that DBS has a neuroprotective (aside from modifying disrupted circuits and local neuronal action) effect in humans, mostly because of a lack of biomarkers [112]. Nevertheless, recent work revealed how changing DBS parameters in STN and substantia nigra had an influence on their neuronal firing patterns and quite probably on their inhibitory synaptic plasticity [113].
DBS in the future may be combined with gene therapy approaches to temporally and spatially modulate the expression of a viral vector. A proof-of-concept study demonstrated that adenovirus vectors in the hippocampus could be modulated at distance by DBS in the medial septal nucleus [114]. Such “electrogenetic” hybrid approaches could be a gateway to modulate growth factors and other therapeutics in the basal ganglia. The effects of stimulation on molecular pathology are not yet fully understood, and it is possible that DBS could be optimized to improve neurodegeneration or enhance neuroprotection.
Another potential link between neuromodulation and neuroprotection has been demonstrated as proof of concept by Iwasa et al., to use DBS to direct stem cell migration and potentially enhance tissue restoration or synaptic plasticity [115•]. The applications of using DBS, which is established as safe and effective, to modulate neuropathology or enhance spatial temporal control of therapeutics or induce activation of repair mechanisms may be potential future out-of-the box solutions that leverage DBS in completely new ways.
Conclusions
The infancy of DBS is long gone. DBS is now a common therapy in many movement disorders, psychiatric diseases, and others. Research has been increasingly opening new possibilities for both patients and clinicians. For instance, aDBS is designed to tailor delivering electrical current according to the patient’s symptoms. It is of great interest in PD because of motor fluctuations and, hopefully, could critically impact PD patients’ quality of life. More and more DBS targets are studied, allowing us to better understand pathological mechanisms in movement disorders in both the brain (MLR, SNr, NBM, cerebellum) and the spinal cord. Neurosurgeons’ practice is rapidly evolving, thanks to more accurate tools designed to better target basal ganglia or, more recently, critical networks disrupted in movement disorders.
DBS has much more effect in our brain than we initially thought, on the molecular, cellular, connectivity, and higher-order oscillations. It is critical that the field continues to not only make investments in advancing new technologies to improve current paradigms of stimulation but look to the possibility of using DBS to ultimately modify disease progression.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Krack P, Volkmann J, Tinkhauser G, Deuschl G. Deep brain stimulation in movement disorders: from experimental surgery to evidence-based therapy. Mov Disord. 2019;34:1795–810.
Krack P, Martinez-Fernandez R, del Alamo M, Obeso JA. Current applications and limitations of surgical treatments for movement disorders: surgical treatments for movement disorders. Mov Disord. 2017;32:36–52.
Bove F, Fraix V, Cavallieri F, et al. Dementia and subthalamic deep brain stimulation in Parkinson disease: a long-term overview. Neurology. 2020;95:e384–92.
Bove F, Mulas D, Cavallieri F, et al. Long-term outcomes (15 years) after subthalamic nucleus deep brain stimulation in patients with Parkinson disease. Neurology. 2021;97:e254–62. This study shows the sustained effect of STN DBS in PD at 15 years. Both motor effects and quality of life remain significantly improved when compared to baseline.
Dafsari HS, Silverdale M, Strack M, et al. Nonmotor symptoms evolution during 24 months of bilateral subthalamic stimulation in Parkinson’s disease: 24 months nonmotor effects of STN-DBS in PD. Mov Disord. 2018;33:421–30.
Klepitskaya O, Liu Y, Sharma S, Sillau SH, Tsai J, Walters AS. Deep brain stimulation improves restless legs syndrome in patients with Parkinson disease. Neurology. 2018;91:e1013–21.
Lachenmayer ML, Mürset M, Antih N, et al. Subthalamic and pallidal deep brain stimulation for Parkinson’s disease—meta-analysis of outcomes. Npj Park Dis. 2021;7:77.
Wong JK, Cauraugh JH, Ho KWD, et al. STN vs. GPi deep brain stimulation for tremor suppression in Parkinson disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2019;58:56–62.
Wong JK, Viswanathan VT, Nozile-Firth KS, Eisinger RS, Leone EL, Desai AM, Foote KD, Ramirez-Zamora A, Okun MS, Wagle Shukla A. STN versus GPi deep brain stimulation for action and rest tremor in Parkinson’s disease. Front Hum Neurosci. 2020;14:578615.
Chandra V, Hilliard JD, Foote KD. Deep brain stimulation for the treatment of tremor. J Neurol Sci. 2022;435:120190. This review gives a thorough insight of DBS in tremor (targets, indications, outcomes) and surgical techniques.
Schuepbach WMM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med. 2013;368:610–22.
Lhommée E, Wojtecki L, Czernecki V, et al. Behavioural outcomes of subthalamic stimulation and medical therapy versus medical therapy alone for Parkinson’s disease with early motor complications (EARLYSTIM trial): secondary analysis of an open-label randomised trial. Lancet Neurol. 2018;17:223–31.
Barbe MT, Tonder L, Krack P, et al. deep brain stimulation for freezing of gait in Parkinson’s disease with early motor complications. Mov Disord. 2020;35:82–90.
Stoker V, Krack P, Tonder L, et al. Deep brain stimulation impact on social and occupational functioning in Parkinson’s disease with early motor complications. Mov Disord Clin Pract. 2020;7:672–80.
Moro E, LeReun C, Krauss JK, Albanese A, Lin J-P, Walleser Autiero S, Brionne TC, Vidailhet M. Efficacy of pallidal stimulation in isolated dystonia: a systematic review and meta-analysis. Eur J Neurol. 2017;24:552–60.
Tsuboi T, Jabarkheel Z, Zeilman PR, Barabas MJ, Foote KD, Okun MS, Wagle Shukla A. Longitudinal follow-up with VIM thalamic deep brain stimulation for dystonic or essential tremor. Neurology. 2020;94:e1073–84.
Miterko LN, Baker KB, Beckinghausen J, et al. Consensus paper: experimental neurostimulation of the cerebellum. Cerebellum. 2019;18:1064–97.
Brown EG, Bledsoe IO, Luthra NS, Miocinovic S, Starr PA, Ostrem JL. Cerebellar deep brain stimulation for acquired hemidystonia. Mov Disord Clin Pract. 2020;7:188–93.
Horisawa S, Arai T, Suzuki N, Kawamata T, Taira T. The striking effects of deep cerebellar stimulation on generalized fixed dystonia: case report. J Neurosurg. 2020;132:712–6.
Diniz JM, Cury RG, Iglesio RF, Lepski GA, França CC, Barbosa ER, de Andrade DC, Teixeira MJ, Duarte KP. Dentate nucleus deep brain stimulation: technical note of a novel methodology assisted by tractography. Surg Neurol Int. 2021;12:400.
Wharen RE, Okun MS, Guthrie BL, et al. Thalamic DBS with a constant-current device in essential tremor: a controlled clinical trial. Parkinsonism Relat Disord. 2017;40:18–26.
Dallapiazza RF, Lee DJ, De Vloo P, Fomenko A, Hamani C, Hodaie M, Kalia SK, Fasano A, Lozano AM. Outcomes from stereotactic surgery for essential tremor. J Neurol Neurosurg Psychiatry. 2019;90:474–82.
Cury RG, Fraix V, Castrioto A, Pérez Fernández MA, Krack P, Chabardes S, Seigneuret E, Alho EJL, Benabid A-L, Moro E. Thalamic deep brain stimulation for tremor in Parkinson disease, essential tremor, and dystonia. Neurology. 2017;89:1416–23.
Giordano M, Caccavella VM, Zaed I, Foglia Manzillo L, Montano N, Olivi A, Polli FM. Comparison between deep brain stimulation and magnetic resonance-guided focused ultrasound in the treatment of essential tremor: a systematic review and pooled analysis of functional outcomes. J Neurol Neurosurg Psych. 2020;91:1270–8.
Paschen S, Forstenpointner J, Becktepe J, Heinzel S, Hellriegel H, Witt K, Helmers A-K, Deuschl G. Long-term efficacy of deep brain stimulation for essential tremor: an observer-blinded study. Neurology. 2019;92:e1378–86.
Martinez-Ramirez D, Jimenez-Shahed J, Leckman JF, et al. Efficacy and safety of deep brain stimulation in Tourette syndrome: the International Tourette Syndrome Deep Brain Stimulation Public Database and Registry. JAMA Neurol. 2018;75:353.
Johnson KA, Fletcher PT, Servello D, et al. Image-based analysis and long-term clinical outcomes of deep brain stimulation for Tourette syndrome: a multisite study. J Neurol Neurosurg Psychiatry. 2019;90:1078–90. This study confirms the efficacy of GPi DBS in Tourette syndrome and strongly suggests that superior or medial GPi locations may be better in controlling OCD.
Welter M-L, Houeto J-L, Worbe Y, et al. Long-term effects of anterior pallidal deep brain stimulation for Tourette’s syndrome: letters: new observations. Mov Disord. 2019;34:586–8.
Krauss JK, Lipsman N, Aziz T, et al. Technology of deep brain stimulation: current status and future directions. Nat Rev Neurol. 2021;17:75–87.
Choe C, Hidding U, Schaper M, Gulberti A, Köppen J, Buhmann C, Gerloff C, Moll CKE, Hamel W, Pötter-Nerger M. Thalamic short pulse stimulation diminishes adverse effects in essential tremor patients. Neurology. 2018;91:e704–13.
Kroneberg D, Ewert S, Meyer A-C, Kühn AA. Shorter pulse width reduces gait disturbances following deep brain stimulation for essential tremor. J Neurol Neurosurg Psychiatry. 2019;90:1046–50.
Rammo RA, Ozinga SJ, White A, Nagel SJ, Machado AG, Pallavaram S, et al. Directional stimulation in Parkinson’s disease and essential tremor: the Cleveland Clinic experience. Neuromodul Technol Neural Interf. 2021. https://doi.org/10.1111/ner.13374.
Pintér D, Járdaházi E, Balás I, Harmat M, Makó T, Juhász A, et al. Antiparkinsonian drug reduction after directional versus omnidirectional bilateral subthalamic deep brain stimulation. Neuromodul Technol Neural Interf. 2022. https://doi.org/10.1016/j.neurom.2022.01.006.
Vitek JL, Jain R, Chen L, et al. Subthalamic nucleus deep brain stimulation with a multiple independent constant current-controlled device in Parkinson’s disease (INTREPID): a multicentre, double-blind, randomised, sham-controlled study. Lancet Neurol. 2020;19:491–501. This work provides class 1 evidence that new MICC STN-DBS is safe and effective. The MICC technology allows to set each plot with specific parameters, rather than a defined setting for the entire lead.
Tass PA, Qin L, Hauptmann C, Dovero S, Bezard E, Boraud T, Meissner WG. Coordinated reset has sustained aftereffects in Parkinsonian monkeys. Ann Neurol. 2012;72:816–20.
Arlotti M, Rosa M, Marceglia S, Barbieri S, Priori A. The adaptive deep brain stimulation challenge. Parkinsonism Relat Disord. 2016;28:12–7.
Marceglia S, Guidetti M, Harmsen IE, Loh A, Meoni S, Foffani G, Lozano AM, Volkmann J, Moro E, Priori A. Deep brain stimulation: is it time to change gears by closing the loop? J Neural Eng. 2021;18:061001.
Goyal A, Goetz S, Stanslaski S, Oh Y, Rusheen AE, Klassen B, Miller K, Blaha CD, Bennet KE, Lee K. The development of an implantable deep brain stimulation device with simultaneous chronic electrophysiological recording and stimulation in humans. Biosens Bioelectron. 2021;176:112888.
Arlotti M, Colombo M, Bonfanti A, et al. A new implantable closed-loop clinical neural interface: first application in Parkinson’s disease. Front Neurosci. 2021;15:763235.
Priori A, Foffani G, Pesenti A, Tamma F, Bianchi A, Pellegrini M, Locatelli M, Moxon K, Villani R. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson’s disease. Exp Neurol. 2004;189:369–79.
Brown P, Williams D. Basal ganglia local field potential activity: character and functional significance in the human. Clin Neurophysiol. 2005;116:2510–9.
Kühn AA, Kupsch A, Schneider G-H, Brown P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease: STN activity and motor improvement. Eur J Neurosci. 2006;23:1956–60.
Ray NJ, Jenkinson N, Wang S, Holland P, Brittain JS, Joint C, Stein JF, Aziz T. Local field potential beta activity in the subthalamic nucleus of patients with Parkinson’s disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp Neurol. 2008;213:108–13.
Arlotti M, Marceglia S, Foffani G, et al. Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neurology. 2018;90:e971–6.
Arlotti M, Palmisano C, Minafra B, et al. Monitoring subthalamic oscillations for 24 hours in a freely moving Parkinson’s disease patient. Mov Disord. 2019;34:757–9.
Little S, Beudel M, Zrinzo L, et al. Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2016;87:717–21.
Cagnan H, Mallet N, Moll CKE, et al. Temporal evolution of beta bursts in the parkinsonian cortical and basal ganglia network. Proc Natl Acad Sci. 2019;116:16095–104. This work describes the changes in beta oscillations in both STN and the cortex that correlate with PD motor signs. These findings provide insights into the network dynamics of beta activity that can guide novel strategies to interfere with their generation and maintenance in PD.
Swann NC, de Hemptinne C, Thompson MC, Miocinovic S, Miller AM, Gilron R, Ostrem JL, Chizeck HJ, Starr PA. Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J Neural Eng. 2018;15:046006.
He S, Baig F, Mostofi A, Pogosyan A, Debarros J, Green AL, Aziz TZ, Pereira E, Brown P, Tan H. Closed-loop deep brain stimulation for essential tremor based on thalamic local field potentials. Mov Disord. 2021;36:863–73.
Gilron R, Little S, Perrone R, et al. Long-term wireless streaming of neural recordings for circuit discovery and adaptive stimulation in individuals with Parkinson’s disease. Nat Biotechnol. 2021;39:1078–85. This study reports on aDBS using wireless streaming of neural recordings in ecological conditions in five PD patients.
Virtual Clinic | Abbott NeurosphereTM. https://neurosphere.abbott/virtual-clinic/. Accessed 18 May 2022.
Deer TR, Esposito MF, Cornidez EG, Okaro U, Fahey ME, Chapman KB. Teleprogramming service provides safe and remote stimulation options for patients with DRG-S and SCS implants. J Pain Res. 2021;14:3259–65.
Mohammed A, Bayford R, Demosthenous A. A framework for adapting deep brain stimulation using Parkinsonian state estimates. Front Neurosci. 2020;14:499.
Meoni S, Cury RG, Moro E. New players in basal ganglia dysfunction in Parkinson’s disease. In: Prog Brain Res. Amsterdam: Elsevier; 2020. p. 307–27.
Cury RG, Pavese N, Aziz TZ, Krauss JK, Moro E, the neuromodulation of gait study group from Movement Disorders Society. gaps and roadmap of novel neuromodulation targets for treatment of gait in Parkinson’s disease. Npj Park Dis. 2022;8:8.
Thevathasan W, Debu B, Aziz T, et al. Pedunculopontine nucleus deep brain stimulation in Parkinson’s disease: a clinical review: clinical review of PPN DBS. Mov Disord. 2018;33:10–20.
Bourilhon J, Olivier C, You H, et al. Pedunculopontine and cuneiform nuclei deep brain stimulation for severe gait and balance disorders in Parkinson’s disease: interim results from a randomized double-blind clinical trial. J Park Dis. 2022;12:639–53.
Perera T, Tan JL, Cole MH, et al. Balance control systems in Parkinson’s disease and the impact of pedunculopontine area stimulation. Brain. 2018;141:3009–22.
Weiss D, Walach M, Meisner C, et al. Nigral stimulation for resistant axial motor impairment in Parkinson’s disease? A randomized controlled trial. Brain. 2013;136:2098–108.
Heilbronn M, Scholten M, Schlenstedt C, Mancini M, Schöllmann A, Cebi I, Pötter-Nerger M, Gharabaghi A, Weiss D. Anticipatory postural adjustments are modulated by substantia nigra stimulation in people with Parkinson’s disease and freezing of gait. Parkinsonism Relat Disord. 2019;66:34–9.
Valldeoriola F, Muñoz E, Rumià J, Roldán P, Cámara A, Compta Y, Martí MJ, Tolosa E. Simultaneous low-frequency deep brain stimulation of the substantia nigra pars reticulata and high-frequency stimulation of the subthalamic nucleus to treat levodopa unresponsive freezing of gait in Parkinson’s disease: a pilot study. Parkinsonism Relat Disord. 2019;60:153–7.
Weiss D, Milosevic L, Gharabaghi A. Deep brain stimulation of the substantia nigra for freezing of gait in Parkinson’s disease: is it about stimulation frequency? Parkinsonism Relat Disord. 2019;63:229–30.
Li H, McConnell GC. Intraoperative microelectrode recordings in substantia nigra pars reticulata in anesthetized rats. Front Neurosci. 2020;14:367.
Yadav AP, Nicolelis MAL. Electrical stimulation of the dorsal columns of the spinal cord for Parkinson’s disease: spinal cord stimulation for PD. Mov Disord. 2017;32:820–32.
Cury RG, Moro E. New developments for spinal cord stimulation. In: Int Rev Neurobiol. Amsterdam: Elsevier; 2021. p. 129–51.
Cai Y, Reddy RD, Varshney V, Chakravarthy KV. Spinal cord stimulation in Parkinson’s disease: a review of the preclinical and clinical data and future prospects. Bioelectron Med. 2020;6:5. This review reports on relevant data concerning SCS in PD and its effectiveness in axial signs like freezing of gait.
Samotus O, Parrent A, Jog M. Spinal cord stimulation therapy for gait dysfunction in advanced Parkinson’s disease patients: spinal cord stimulation for gait in PD. Mov Disord. 2018;33:783–92.
Prasad S, Aguirre-Padilla DH, Poon Y, Kalsi-Ryan S, Lozano AM, Fasano A. Spinal cord stimulation for very advanced Parkinson’s disease: a 1-year prospective trial. Mov Disord. 2020;35:1082–3.
Pinto de Souza C, Hamani C, Oliveira Souza C, Lopez Contreras WO, dos Santos Ghilardi MG, Cury RG, Reis Barbosa E, Jacobsen Teixeira M, Talamoni Fonoff E. Spinal cord stimulation improves gait in patients with Parkinson’s disease previously treated with deep brain stimulation: spinal cord stimulation in PD. Mov Disord. 2017;32:278–82.
Akiyama H, Nukui S, Akamatu M, Hasegawa Y, Nishikido O, Inoue S. Effectiveness of spinal cord stimulation for painful camptocormia with Pisa syndrome in Parkinson’s disease: a case report. BMC Neurol. 2017;17:148.
Kobayashi R, Kenji S, Taketomi A, Murakami H, Ono K, Otake H. New mode of burst spinal cord stimulation improved mental status as well as motor function in a patient with Parkinson’s disease. Parkinsonism Relat Disord. 2018;57:82–3.
Mazzone P, Viselli F, Ferraina S, Giamundo M, Marano M, Paoloni M, Masedu F, Capozzo A, Scarnati E. High cervical spinal cord stimulation: a one year follow-up study on motor and non-motor functions in Parkinson’s disease. Brain Sci. 2019;9:78.
Samotus O, Parrent A, Jog M. Long-term update of the effect of spinal cord stimulation in advanced Parkinson’s disease patients. Brain Stimulat. 2020;13:1196–7. The effectiveness of SCS in freezing of gait in four PD patients at 3-year follow-up is here described.
Furusawa Y, Matsui A, Kobayashi-Noami K, et al. Burst spinal cord stimulation for pain and motor function in Parkinson’s disease: a case series. Clin Park Relat Disord. 2020;3:100043.
Cury RG, Carra RB, Capato TTC, Teixeira MJ, Barbosa ER. Spinal cord stimulation for Parkinson’s disease: dynamic habituation as a mechanism of failure? Mov Disord. 2020;35:1882–3.
Kübler D, Wellmann SK, Kaminski J, Skowronek C, Schneider G-H, Neumann W-J, Ritter K, Kühn A. Nucleus basalis of Meynert predicts cognition after deep brain stimulation in Parkinson’s disease. Parkinsonism Relat Disord. 2022;94:89–95.
Bohnen NI, Yarnall AJ, Weil RS, Moro E, Moehle MS, Borghammer P, Bedard M-A, Albin RL. Cholinergic system changes in Parkinson’s disease: emerging therapeutic approaches. Lancet Neurol. 2022;21:381–92.
Nazmuddin M, Philippens IHCHM, van Laar T. Electrical stimulation of the nucleus basalis of Meynert: a systematic review of preclinical and clinical data. Sci Rep. 2021;11:11751.
Gratwicke J, Zrinzo L, Kahan J, et al. Bilateral nucleus basalis of Meynert deep brain stimulation for dementia with Lewy bodies: a randomised clinical trial. Brain Stimulat. 2020;13:1031–9.
Gratwicke J, Zrinzo L, Kahan J, et al. Bilateral deep brain stimulation of the nucleus basalis of Meynert for Parkinson disease dementia: a randomized clinical trial. JAMA Neurol. 2018;75:169.
Maltête D, Wallon D, Bourilhon J, et al. Nucleus basalis of Meynert stimulation for Lewy body dementia: a phase i randomized clinical trial. Neurology. 2021;96:e684–97.
Sasikumar S, Cohn M, Harmsen IE, et al. Single-trajectory multiple-target deep brain stimulation for Parkinsonian mobility and cognition. Mov Disord. 2022;37:635–40.
Klockgether T, Mariotti C, Paulson HL. Spinocerebellar ataxia. Nat Rev Dis Primer. 2019;5:24.
França C, de Andrade DC, Teixeira MJ, Galhardoni R, Silva V, Barbosa ER, Cury RG. Effects of cerebellar neuromodulation in movement disorders: a systematic review. Brain Stimulat. 2018;11:249–60.
França C, de Andrade DC, Silva V, Galhardoni R, Barbosa ER, Teixeira MJ, Cury RG. Effects of cerebellar transcranial magnetic stimulation on ataxias: a randomized trial. Parkinsonism Relat Disord. 2020;80:1–6.
Anderson CJ, Figueroa KP, Dorval AD, Pulst SM. Deep cerebellar stimulation reduces ataxic motor symptoms in the shaker rat. Ann Neurol. 2019;85:681–90.
Cury RG, França C, Silva V, Barbosa ER, Capato TTC, Lepski G, Duarte KP, Teixeira MJ, Ciampi de Andrade D. Effects of dentate nucleus stimulation in spinocerebellar ataxia type 3. Parkinsonism Relat Disord. 2019;69:91–3.
Cury RG, França C, Duarte KP, et al. Safety and outcomes of dentate nucleus deep brain stimulation for cerebellar ataxia. Cerebellum. 2021. https://doi.org/10.1007/s12311-021-01326-8.
Welton T, Cardoso F, Carr JA, Chan L-L, Deuschl G, Jankovic J, Tan E-K. Essential tremor. Nat Rev Dis Primer. 2021;7:83.
Kremer NI, Pauwels RWJ, Pozzi NG, Lange F, Roothans J, Volkmann J, Reich MM. Deep brain stimulation for tremor: update on long-term outcomes, target considerations and future directions. J Clin Med. 2021;10:3468.
Barbe MT, Reker P, Hamacher S, et al. DBS of the PSA and the VIM in essential tremor: a randomized, double-blind, crossover trial. Neurology. 2018;91:e543–50.
Dembek TA, Petry-Schmelzer JN, Reker P, et al. PSA and VIM DBS efficiency in essential tremor depends on distance to the dentatorubrothalamic tract. NeuroImage Clin. 2020;26:102235.
Coenen VA, Sajonz B, Prokop T, Reisert M, Piroth T, Urbach H, Jenkner C, Reinacher PC. The dentato-rubro-thalamic tract as the potential common deep brain stimulation target for tremor of various origin: an observational case series. Acta Neurochir (Wien). 2020;162:1053–66.
Kvernmo N, Konglund AE, Reich MM, Roothans J, Pripp AH, Dietrichs E, Volkmann J, Skogseid IM. Deep brain stimulation for arm tremor: a randomized trial comparing two targets. Ann Neurol. 2022;91:585–601.
Neudorfer C, Hunsche S, Hellmich M, El Majdoub F, Maarouf M. Comparative study of robot-assisted versus conventional frame-based deep brain stimulation stereotactic neurosurgery. Stereotact Funct Neurosurg. 2018;96:327–34.
Philipp LR, Matias CM, Thalheimer S, Mehta SH, Sharan A, Wu C. Robot-assisted stereotaxy reduces target error: a meta-analysis and meta-regression of 6056 trajectories. Neurosurgery. 2021;88:222–33. This review includes significant number of DBS trajectories. Robot assistance for stereotactic electrode implantation is independently associated with improved accuracy and reduced target error and so, better outcomes.
Patriat R, Cooper SE, Duchin Y, Niederer J, Lenglet C, Aman J, Park MC, Vitek JL, Harel N. Individualized tractography-based parcellation of the globus pallidus pars interna using 7T MRI in movement disorder patients prior to DBS surgery. Neuroimage. 2018;178:198–209.
Aman JE, Johnson LA, Sanabria DE, et al. Directional deep brain stimulation leads reveal spatially distinct oscillatory activity in the globus pallidus internus of Parkinson’s disease patients. Neurobiol Dis. 2020;139:104819.
Plantinga BR, Temel Y, Duchin Y, et al. Individualized parcellation of the subthalamic nucleus in patients with Parkinson’s disease with 7T MRI. Neuroimage. 2018;168:403–11.
Kim J, Duchin Y, Shamir RR, Patriat R, Vitek J, Harel N, Sapiro G. Automatic localization of the subthalamic nucleus on patient-specific clinical MRI by incorporating 7 T MRI and machine learning: application in deep brain stimulation. Hum Brain Mapp. 2019;40:679–98.
Solomon O, Palnitkar T, Patriat R, Braun H, Aman J, Park MC, Vitek J, Sapiro G, Harel N. Deep-learning based fully automatic segmentation of the globus pallidus interna and externa using ultra-high 7 Tesla MRI. Hum Brain Mapp. 2021;42:2862–79.
Shamir RR, Duchin Y, Kim J, et al. Microelectrode recordings validate the clinical visualization of subthalamic-nucleus based on 7t magnetic resonance imaging and machine learning for deep brain stimulation surgery. Neurosurgery. 2019;84:749–57.
Haubenberger D, Hallett M. Essential Tremor. N Engl J Med. 2018;378:1802–10.
Low HL, Ismail MN, Bin M, Taqvi A, Deeb J, Fuller C, Misbahuddin A. Comparison of posterior subthalamic area deep brain stimulation for tremor using conventional landmarks versus directly targeting the dentatorubrothalamic tract with tractography. Clin Neurol Neurosurg. 2019;185:105466.
Nordin T, Zsigmond P, Pujol S, Westin C-F, Wårdell K. White matter tracing combined with electric field simulation – a patient-specific approach for deep brain stimulation. NeuroImage Clin. 2019;24:102026.
Corp DT, Joutsa J, Darby RR, et al. Network localization of cervical dystonia based on causal brain lesions. Brain. 2019;142:1660–74. This study found a functional cervical dystonia network and correlated it with classical DBS targets.
Al-Fatly B, Ewert S, Kübler D, Kroneberg D, Horn A, Kühn AA. Connectivity profile of thalamic deep brain stimulation to effectively treat essential tremor. Brain. 2019;142:3086–98.
Iorio-Morin C, Fomenko A, Kalia SK. Deep-brain stimulation for essential tremor and other tremor syndromes: a narrative review of current targets and clinical outcomes. Brain Sci. 2020;10:925.
Oliveria SF, Rodriguez RL, Bowers D, Kantor D, Hilliard JD, Monari EH, Scott BM, Okun MS, Foote KD. Safety and efficacy of dual-lead thalamic deep brain stimulation for patients with treatment-refractory multiple sclerosis tremor: a single-centre, randomised, single-blind, pilot trial. Lancet Neurol. 2017;16:691–700.
Dos Santos Ghilardi MG, Ibarra M, Alho EJL, Reis PR, Lopez Contreras WO, Hamani C, Fonoff ET. Double-target DBS for essential tremor: 8-contact lead for cZI and Vim aligned in the same trajectory. Neurology. 2018;90:476–8.
Neudorfer C, Hinzke M, Hunsche S, El Majdoub F, Lozano A, Maarouf M. Combined deep brain stimulation of subthalamic nucleus and ventral intermediate thalamic nucleus in tremor-dominant Parkinson’s disease using a parietal approach. Neuromodulation Technol Neural Interface. 2019;22:493–502.
McKinnon C, Gros P, Lee DJ, Hamani C, Lozano AM, Kalia LV, Kalia SK. Deep brain stimulation: potential for neuroprotection. Ann Clin Transl Neurol. 2019;6:174–85.
Milosevic L, Kalia SK, Hodaie M, Lozano AM, Fasano A, Popovic MR, Hutchison WD. Neuronal inhibition and synaptic plasticity of basal ganglia neurons in Parkinson’s disease. Brain. 2018;141:177–90.
Fomenko A, Lee DJ, McKinnon C, et al. Deep brain stimulation of the medial septal nucleus induces expression of a virally delivered reporter gene in dentate gyrus. Front Neurosci. 2020;14:463.
Iwasa SN, Shi HH, Hong SH, Chen T, Marquez-Chin M, Iorio-Morin C, Kalia SK, Popovic MR, Naguib HE, Morshead CM. Novel electrode designs for neurostimulation in regenerative medicine: activation of stem cells. Bioelectricity. 2020;2:348–61. This work highlights the ability to guide stem cells via DBS electric currents, with the potential for regenerative medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sina R. Potel declares no competing interests. Sara Marceglia reports personal fees from Newronika SpA, during the conduct of the study. Elena Moro reports personal fees from Medtronic, personal fees from Abbott, personal fees from Kyowa, grants from Ipsen, and grants from Abbott, outside the submitted work. Sara Meoni declares no competing interests. Suneil K. Kalia reports non-financial support from Abbott, Boston, advisory board and speaker honoraria from Medtronic, outside the submitted work. Rubens G. Cury declares no competing interests.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Movement Disorders.
Rights and permissions
About this article
Cite this article
Potel, S.R., Marceglia, S., Meoni, S. et al. Advances in DBS Technology and Novel Applications: Focus on Movement Disorders. Curr Neurol Neurosci Rep 22, 577–588 (2022). https://doi.org/10.1007/s11910-022-01221-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-022-01221-7