Abstract
Controversy surrounds the use of adjunctive corticosteroids in severe community acquired pneumonia (CAP) as current guidelines either do not address or discourage their use. Double-blind, placebo-controlled, randomized controlled trials examining systemic corticosteroids in the treatment of severe CAP were summarized and their impacts on patient-important outcomes assessed. Four trials describing systemic corticosteroid use in adults with severe CAP were identified. One trial had a significant mortality difference favoring corticosteroids. However, this may be the result of a CAP severity imbalance within the trial and the mortality benefit was not confirmed in a larger trial conducted in a similar critical care setting. Pneumonia severity, mortality assessment timing, comorbidities, corticosteroid and antibiotic choice and timing in the CAP disease course, and bias risks varied across the four trials. Because of the clinical heterogeneity of available studies and the unknowns pertaining to clinical efficacy and safety, we do not recommend the use of adjunctive corticosteroids in severe CAP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lower respiratory tract infections are the sixth leading cause of death in high-income countries [1]. Community-acquired pneumonia (CAP), defined as an infection of the lung parenchyma in patients not hospitalized or living in a long-term care facility during the 2 weeks preceding symptom onset, accounts for a significant proportion of lower respiratory tract infections [2, 3]. Approximately 4 million ambulatory care visits by US children and adults in 2006 were attributed to pneumonia, with the majority of visits by adults [2, 3]. Between 2002 and 2009, pneumonia was the third most prevalent emergency department diagnosis leading to intensive care unit (ICU) admission and was the leading infectious disease-related cause of ICU admission [4]. Mortality associated with CAP varies depending on risk factors, severity of illness, and mortality outcome definition, and ranges from 0 to 18 % [2]. A recent study revealed that 52 % of Canadian and American patients diagnosed with CAP had a severe presentation, as defined by a pneumonia severity index (PSI) risk class of IV and V [5]. Despite advances in the understanding of administering early appropriate antimicrobial therapy for CAP, mortality remains approximately 20 % in patients with severe CAP [5, 6].
Therapeutic strategies for the treatment of severe CAP in adults include systematically assessing pneumonia severity and the need for ICU admission, initiating timely appropriate antibiotic therapy, initiating a lung protective ventilation strategy that delivers low tidal volumes, implementing institutional quality improvement initiatives such as a CAP bundle, and providing influenza and pneumococcal immunization where appropriate [7, 8]. One adjunct strategy that remains to be clearly defined is the use of systemic corticosteroids. Severe CAP is associated with a significant increase in pulmonary and circulatory inflammatory cytokines that have been linked to bacteremia, radiographic severity, need for mechanical ventilation, and severity of illness [7, 9–12]. Glucocorticoids suppress inflammatory gene expression from inflammatory structural cells, which predominantly explains their antiinflammatory effects [13]. Mechanistically, glucocorticoids show promise in the adjunctive treatment of severe CAP. However, international adult CAP clinical practice guidelines either do not make specific recommendations for using systemic corticosteroids or strongly recommend against their use for severe CAP [8, 14]. As these guidelines are more than 4 years old, it is necessary to evaluate current published evidence examining systemic corticosteroids for the treatment of severe CAP. The objectives of this review were to summarize recent evidence addressing the role of systemic corticosteroids for adults with severe CAP and to describe their impact on patient-important outcomes.
Methods
The two authors independently performed a literature search to identify published English language studies evaluating systemic corticosteroid use in adults with severe CAP. PubMed, MEDLINE, EMBASE, International Pharmaceutical Abstracts, and Cumulative Index to Nursing and Allied Health Literature were searched from inception to November 2013 using the following terms: pneumonia, community-acquired infections, glucocorticoids, corticosteroid, cortisone, dexamethasone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Citations in retrieved articles were manually reviewed to identify relevant articles not captured in the database search.
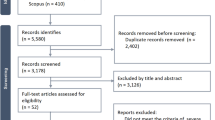
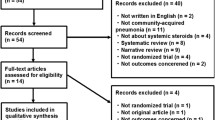
Published double-blind (DB), placebo-controlled (PC), randomized controlled trials (RCTs) describing systemic corticosteroid use in severe CAP were included. Articles assessing systemic corticosteroid use in pediatric patients, fungal pneumonia, or viral pneumonia were excluded. Trials describing CAP of all severity levels without a severe CAP subgroup analysis were also excluded.
Results
Seven studies were initially identified and four met the inclusion criteria (Table 1) [15••, 16••, 17••, 18••, 19–21]. Of the three studies excluded, one described Pneumocystis jiroveci (P. carinii) pneumonia [19], one described viral pneumonia in a pediatric population [20], and one described mild to severe CAP without a severe CAP subgroup analysis [21].
Fernández-Serrano and colleagues conducted a single-center trial assessing methylprednisolone in 56 patients hospitalized with severe CAP [15••]. CAP was defined as any two of: fever, purulent expectoration, pleuritic chest pain, or leukocytosis. Inclusion criteria consisted of lung radiographic opacity affecting at least two lobes and respiratory failure (PaO2/FiO2 < 300). Notable exclusion criteria were steroid treatment or mechanical ventilation prior to study inclusion.
Methylprednisolone was administered as a 200-mg intravenous bolus 30 min before antibiotic administration, followed by a tapering regimen over 9 days. Co-therapy consisted of omeprazole, insulin as needed, and intravenous ceftriaxone for 9 days in addition to intravenous levofloxacin for 5 days, and oral levofloxacin for at least 20 days.
Baseline characteristics were similar between groups with the exception of a significantly higher white blood cell count in the methylprednisolone group (13.5 vs. 10.2 × 103/mm3, p = 0.01). Four patients in the methylprednisolone group and two in the placebo group had chronic obstructive pulmonary disease (COPD, not significantly different). Adrenal insufficiency and asthma were not specifically excluded or described at baseline. There were no differences between groups in the primary endpoint of patients requiring conventional mechanical ventilation or noninvasive positive pressure during the 9 days of corticosteroid administration (4.3 % vs. 22.7 %, not significantly different). There also were no differences seen in mortality, hospital length of stay (LOS), and ICU LOS. Steroid-related complications included one gastrointestinal bleed in the methylprednisolone group and zero in the placebo group (4.3 % vs. 0 %, p value not reported) and one patient on methylprednisolone required insulin compared to none of the patients in the placebo group (4.3 % vs. 0 %, p value not reported).
Details surrounding randomization, including sequence generation, allocation concealment, and implementation, were lacking. Blinding was not described except to say that the physical appearance of the placebo was similar to that of methylprednisolone. Therefore, selection, performance, and detection bias cannot be ruled out with respect to confounding the results. Attrition bias may also have been present as 56 patients were randomized but only 45 were assessed for outcomes.
Sabry and El-Din Omar conducted a multicenter trial comparing 7 days of hydrocortisone intravenous infusion to placebo in 80 patients admitted to the ICU with severe CAP [16••]. Co-therapy consisted of empiric antibiotics. Comorbidities, hydrocortisone timing, and antibiotic administration timing in the CAP disease course were not described. Inclusion required two minor or one major criterion for severe pneumonia as per the 2007 American Thoracic Society (ATS) criteria. Relevant exclusion criteria included immunosuppression, concomitant infections, malignancy, and major gastrointestinal bleed within 3 months of hospitalization. The numbers of patients on mechanical ventilation at baseline were not significantly different between the groups (hydrocortisone 65 % vs. placebo 85 %, p = 0.144). Patients with alternate indications for steroid therapy, such as COPD, were not excluded.
Primary outcomes included PaO2:FiO2 > 300 or ≥ 100 increase from study entry, mean sequential organ failure assessment (SOFA) score, and delayed septic shock assessed on day 8. All primary outcomes showed a statistically significant benefit in favor of hydrocortisone (Table 1). The need for mechanical ventilation on day 8 was also lower in the hydrocortisone group (25 % vs. 65 %, p = 0.011). However, survival on day 8 was not significantly different (95 % vs. 85 %, p = 0.633). Equal numbers of upper gastrointestinal bleeds occurred in the two groups. The authors stated that the risks of superinfection, bleeding, and neuromuscular weakness were not increased in the treatment group, but hypernatremia and hyperglycemia were more common in the hydrocortisone group. However, outcome definitions, prevalence, and statistics were not supplied. Selection bias could not be excluded because randomization information was not provided. Other than describing the placebo as an equal volume of intravenous normal saline solution to the volume of hydrocortisone infused, other blinding characteristics were not provided making performance and detection bias impossible to rule out. Attrition bias was low because all 80 patients recruited were analyzed for primary outcomes.
Snijders and colleagues performed a single center trial in 213 hospitalized patients with CAP of any severity and evaluated prednisolone 40 mg daily versus placebo for seven days in addition to antimicrobial therapy [17••]. Although patients with severe CAP were not specifically assessed, planned subgroup analysis of patients with severe CAP was conducted in those with PSI IV to V or CURB-65 scores 3 to 5. Prednisolone was initially provided intravenously and changed to oral administration when antibiotics were stepped down to oral administration. The timing of prednisolone and antimicrobial initiation in the CAP disease course was not described. Eligibility included clinical symptoms suggestive of CAP, new consolidations on chest radiographs, and being more than 18 years of age. Clinical symptoms suggestive of CAP were cough, fever, pleuritic chest pain, and dyspnea. Exclusion criteria included conditions requiring corticosteroids, immunosuppression, malignancy, and the use of macrolides or steroids for more than 24 h. The top three combining to account for 99% of the antibiotics used, based on Netherlands’ national guidelines were amoxicillin, moxifloxacin, and amoxicillin/clavulanic acid. COPD (18.4 % vs. 22.0 %) and asthma (7.9 % vs. 9.5 %) appeared balanced at enrolment between the prednisolone and placebo groups, respectively. Adrenal insufficiency was not assessed.
The primary outcome was clinical cure, defined as resolution or improvement in symptoms and signs related to pneumonia without the need for additional or alternative therapy on day 7. There were no differences in clinical cure rates on day 7 in the subgroup of patients with severe CAP, which was consistent with the analysis of patients with all severities of CAP. In this subgroup, 30-day mortality was also not significantly different between groups. There were no differences between groups in the incidence of hyperglycemia requiring additional therapy or of superinfection, but small numbers were reported and outcome definitions were not provided. Selection bias was minimized by using a computer-generated random allocation sequence and one-on-one allocation using prenumbered containers containing seven vials and seven capsules. Details surrounding randomization restriction were not specified. Performance and detection bias could not be assessed, as intervention similarity and blinding details were not provided. Attrition bias was unlikely as the 213 participants randomized were included in the intention-to-treat analysis.
Confalonieri and colleagues performed a multicenter trial in 48 patients with severe CAP admitted to the ICU or Respiratory Intermediate Unit (RIU) [18••]. Patients with clinical and radiographic evidence of pneumonia were included if they met the 1993 ATS criteria for severe pneumonia. Notable exclusion criteria included severe immunosuppression and conditions requiring more than 0.5 mg/kg/day of prednisone equivalent. Prespecified exit criteria included active gastrointestinal bleeding requiring transfusion, recovery of Candida spp. from multiple sites, or development of a condition requiring prolonged glucocorticoid administration. Hydrocortisone was administered using an intravenous infusion and compared to placebo of normal saline for 7 days. Steroid and antimicrobial timing in the CAP disease course were not specified. Protocol-guided antibiotics were administered based on 1993 ATS adult CAP guidelines and consisted of macrolides, third or fourth generation cephalosporins, fluoroquinolones, antipseudomonal penicillins, aminoglycosides, and glycopeptides.
At baseline, mechanically ventilated patients in the hydrocortisone group appeared to have less severe respiratory failure, as more had noninvasive positive pressure ventilation than in the placebo group (53 % vs. 16 %, p = 0.03). Two patients in the hydrocortisone group and one in the placebo group had COPD. Adrenal insufficiency and asthma were not assessed at baseline or specifically excluded. Patients in the hydrocortisone group experienced significant improvement in the three primary outcomes of PaO2: FiO2 ratio ≥300 or increase in PaO2:FiO2 ratio ≥100, multiple organ dysfunction syndrome score, and delayed septic shock by study day 8 (Table 1). This group also showed significantly reduced 60-day mortality (0 % vs. 38 %, p = 0.001), hospital mortality (0 % vs. 30 %, p = 0.009), mechanical ventilation duration (4 days vs. 10 days, p = 0.007), and hospital LOS (13 days vs. 21 days, p = 0.03).
Although not defined, the incidence of upper gastrointestinal bleeds was not different between groups. No difference in nosocomial infections were observed, but this analysis was likely under-powered.
Selection bias was minimized as randomization schemes were generated in blocks of ten for each participating site by a central randomization center and allocation concealment was achieved by providing randomization assignment to the recruiting center in sealed envelopes. Patients and investigators were blinded to treatment assignment, and equal volumes of normal saline and study drug were administered to reduce performance and detection bias. All randomized patients were accounted for, with the exception of two who met prespecified exit criteria, reducing attrition bias.
Discussion
Conflicting results were found in the four DB PC RCTs analyzing the use of systemic corticosteroids in the treatment of severe CAP. Significant clinical heterogeneity existed across studies with respect to pneumonia severity, mortality assessment timing, included comorbidities, corticosteroid and antibiotic choice, dosing and timing in the CAP disease course, and bias risks.
The spectrum of CAP severity across trials ranged from excluding mechanically ventilated patients [15••] to specifically assessing critical care patients [16••, 18••]. One of the two trials with critical care patients was the only trial showing a significant mortality difference favoring corticosteroids [18••]. This may have been the result of a CAP severity imbalance within the trial, as mechanically ventilated patients in the hydrocortisone group appeared to have less severe respiratory failure. Interestingly, the larger of the two ICU trials did not confirm these results [16••]. The timing of mortality assessment may also explain the variable results. For example, there was no difference in mortality assessed on day 8 in one study, but another study showed significant differences in hospital mortality and day-60 mortality between groups [16••, 18••]. As corticosteroids are hypothesized to prevent rebound inflammation complications, it may take longer than 8 days to see the impact of corticosteroids on mortality, if an impact truly exists.
None of the trials excluded patients with COPD, asthma, or adrenal insufficiency and the patients’ baseline cortisol concentrations were not determined for the assessment of the presence of adrenal insufficiency. As a result, the response may have reflected improvements in these concomitant diseases known to respond to corticosteroids rather than an improvement in CAP. Ideally these potentially confounding conditions would be excluded in future trials.
Corticosteroid administration timing in the CAP disease course and the corticosteroid timing in relation to adjunctive antibiotics differed among the four trials, which may be important. Administration of a corticosteroid prior to antibiotics may attenuate the heightened inflammatory response that is seen after antibiotic initiation. This concept has been shown to be clinically relevant in meningitis [22]. Fernández-Serrano and colleagues administered corticosteroids at the time of CAP diagnosis and 30 min before antibiotic administration [15••] and in the other three trials the timing of corticosteroid administration was not specified [16••, 17••, 18••]. Not specifying corticosteroid timing with respect to CAP disease course and antimicrobial administration limits trial reproducibility and may affect CAP treatment response.
Timely use of antimicrobial therapy is also crucial to ensure optimal outcomes in severe CAP [6]. Details regarding antibiotic administration with respect to CAP onset were not specified in the included trials and is a significant limitation [15••, 16••, 17••, 18••]. The choice of antimicrobial therapy also differed between trials. A combination of ceftriaxone and levofloxacin for at least 20 days was recommended in one trial [15••]. This antibiotic combination and duration are not recommended for the empiric treatment of CAP in North American guidelines [8]. Another trial conducted in the Netherlands used amoxicillin or amoxicillin/clavulanic acid monotherapy [17••], also differing from North American empiric CAP treatment recommendations, limiting generalizability to North American patients [8].
Although our review included studies of high methodological quality, some of the included studies did not provide enough information about randomization, blinding, and participant flow to rule out selection, performance, detection, and attrition bias. Randomization details were inadequate to rule out selection bias in two trials [15••, 16••]. Performance and detection bias could not be assessed, as there was insufficient blinding information in three trials [15••, 16••, 17••]. Attrition bias could not be ruled out in one trial because several patients were excluded from analysis for protocol violation [15••].
It should be noted that five meta-analyses have been published assessing the role of corticosteroids in adult patients with bacterial CAP [23•, 24–27]. The most recent meta-analysis assessed high-quality trials in patients with severe CAP [23•]. The primary outcome of mortality was found to be significantly lower in the corticosteroid group (Peto odds ratio 0.39, 95 % confidence interval 0.17–0.90). There was moderate heterogeneity among the four RCTs with respect to mortality results (p = 0.14, I 2 = 46 %). Based on the results of a sensitivity analysis, the mortality pooled estimate was downgraded for inconsistency and imprecision. The results of these meta-analyses should be interpreted with caution due to the clinical heterogeneity of the individual trials.
A trial to watch for is the Extended Steroid in CAP(e) (ESCAPe) trial (NCT01283009). It is estimated that this study will be completed in 2017–2018, and will dramatically contribute to the literature on severe CAP, as the estimated enrolment is 1,450 subjects. It is a DB PC RCT in critically ill patients with severe CAP assessing 20 days of methylprednisolone versus placebo with the primary outcome of 60-day all-cause mortality.
Conclusions
Due to the clinical heterogeneity of available studies and unknowns with respect to efficacy and safety, the use of adjunctive corticosteroids in severe CAP cannot be recommended at this time. Large multicenter DB PC RCTs in patients with severe pneumonia assessing clinical efficacy and safety outcomes are needed to clarify the role of corticosteroids in CAP. Ideally patients with confounding concomitant conditions, including asthma, COPD, and adrenal insufficiency, would be excluded. Sufficient details about randomization, blinding, and participant flow should also be provided so that bias can be ruled out. Finally, extending the study assessment period for clinically relevant mortality and morbidity primary outcomes from days to months, when the impact of corticosteroids on the second wave of inflammation in severe CAP is thought to occur, is also needed.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
WHO. The top 10 causes of death: the 10 leading causes of death by income group. Geneva:. World Health Organization. 2011. http://www.who.int/mediacentre/factsheets/fs310/en/index1.html. Accessed 28 Mar 2014.
File TM, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130–41.
Shappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2006. Natl Health Stat Rep. 2008;8:1–29.
Mullins PM, Goyal M, Pines JM. National growth in intensive care unit admissions from emergency departments in the United States from 2002 to 2009. Acad Emerg Med. 2013;20:479–86.
Arnold FW, Wiemken TL, Peyrani P, Ramirez JA, Brock GN. Mortality differences among hospitalized patients with community-acquired pneumonia in three world regions: results from the community-acquired pneumonia organization (CAPO) international cohort study. Respir Med. 2013;107:1101–11.
Houck PM, Bratzler DW, Nsa W, Ma A, Bartlett JG. Timing of antibiotic administration outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med. 2004;164:637–44.
Restrepo MI, Anzueto A. Severe community-acquired pneumonia. Infect Dis Clin N Am. 2009;23:503–20.
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Disease Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–72.
Puren AJ, Feldman C, Savage N, Becker PJ, Smith C. Patterns of cytokine expression in community-acquired pneumonia. Chest. 1995;107:1342–9.
Schütte H, Lohmeyer J, Rosseau S, Ziegler S, Siebert C, Kielisch H, et al. Bronchoalveolar and systemic cytokine profiles in patients with ARDS, severe pneumonia and cardiogenic pulmonary oedema. Eur Respir J. 1996;9:1858–67.
Monton C, Torres A, El-Ebiary M, Filella X, Xaubet A, de la Bellacasa JP. Cytokine expression in severe pneumonia: a bronchoalveolar lavage study. Crit Care Med. 1999;27:1745–53.
Fernandez-Serrano S, Dorca J, Coromines M, Carratalà J, Gudiol F, Manresa F. Molecular inflammatory responses measured in blood of patients with severe community-acquired pneumonia. Clin Diagn Lab Immunol. 2003;10:813–20.
Newton R, Leigh R, Giembycz MA. Pharmacological strategies for improving the efficacy and therapeutic ratio of glucocorticoids in inflammatory lung diseases. Pharmacol Ther. 2010;125:286–327.
Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, et al. Guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(Suppl III):iii1–iii55.
Fernández-Serrano S, Dorca J, Garcia-Vidal C, Fernández-Sabé N, Carratalà J, Fernández-Agüera A, et al. Effect of corticosteroids on the clinical course of community-acquired pneumonia: a randomized controlled trial. Crit Care. 2011;15:R96. Most recent randomized control trial assessing methylprednisolone in 56 patients hospitalized with severe CAP.
Sabry NA, El-Din OE. Corticosteroids and ICU course of community acquired pneumonia in Egyptian settings. Pharmacol Pharm. 2011;2:73–81. Critical care patients with severe CAP. Although similar in design, it did not confirm the mortality benefit seen in the trial by Confalonieri et al. [18].
Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med. 2010;181:975–82. Largest RCT evaluating the efficacy corticosteroids in CAP.
Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomised study. Am J Respir Crit Care Med. 2005;171(3):242–8. RCT in severe CAP patients that revealed a significant effect on clinically relevant outcomes favoring hydrocortisone.
Gagnon S, Boota AM, Fischl MA, Baier H, Kirksey OW, La Voie L. Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome: a double-blind, placebo-controlled trial. N Engl J Med. 1990;323:1444–50.
van Woensel JB, van Aalderen WM, de Weerd W, Jansen NJ. Dexamethasone for treatment of patients mechanically ventilated for lower respiratory tract infection caused by respiratory syncytial virus. Thorax. 2003;58:383–7.
Meijvis SC, Hardeman H, Remmelts HH, Heijligenberg R, Rijkers GT, van Velzen-Blad H, et al. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:2023–30.
Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267–84.
Cheng M, Pan ZY, Yang J, Gao YD. Corticosteroid therapy for severe community-acquired pneumonia: a meta-analysis. Respir Care. 2013. doi:10.4187/respcare.02758. Most recent meta-analysis specifically assessing high-quality trials in patients with severe CAP.
Shafiq M, Mansoor MS, Khan AA, Sohail MR, Murad MH. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68–75.
Nie W, Zhang Y, Cheng J, Xiu Q. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
Lamontagne F, Briel M, Guyatt GH, Cook DJ, Bhatnagar N, Meade M. Corticosteroid therapy for acute lung injury, acute respiratory distress syndrome, and severe pneumonia: a meta-analysis of randomized controlled trials. J Crit Care. 2010;25:420–35.
Siempos II, Vardakas KZ, Kopterides P, Falagas ME. Adjunctive therapies for community-acquired pneumonia: a systematic review. J Antimicrob Chemother. 2008;62:661–8.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Sean Gorman declares no conflicts of interest. Tasha Ramsey declares no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by either author.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Respiratory Infections
Rights and permissions
About this article
Cite this article
Ramsey, T.D., Gorman, S.K. Corticosteroids in the Treatment of Severe Community-Acquired Pneumonia. Curr Infect Dis Rep 16, 405 (2014). https://doi.org/10.1007/s11908-014-0405-1
Published:
DOI: https://doi.org/10.1007/s11908-014-0405-1