Abstract
Purpose of Review
This review describes the discovery and development of ACE inhibitors as antihypertensive agents, compares their efficacy, tolerability, and safety to ARBs, and highlights the contemporary issues surrounding ACE inhibitor use for HTN.
Recent Findings
Angiotensin-converting enzyme (ACE) inhibitors are commonly prescribed medications for the management of hypertension (HTN) and other chronic conditions including heart failure and chronic kidney disease. These agents inhibit ACE, the enzyme that is responsible for converting angiotensin (AT) I to AT II. Inhibiting the synthesis of AT II causes arterial and venous vasodilation, natriuresis, and a decrease in sympathetic activity, resulting in the reduction of blood pressure. ACE inhibitors are first-line therapy in HTN management along with thiazide diuretics, calcium channel blockers, and angiotensin receptor blockers (ARB). Along with inhibiting AT II synthesis, inhibition of ACE causes accumulation of bradykinin, increasing the risk of bradykinin-mediated side effects like angioedema and cough. Since ARBs do not work on ACE in the renin-angiotensin system, the risk of angioedema and cough are lower with ARBs. Recent evidence has also suggested ARBs may have neuroprotective effects compared to other antihypertensives, including ACE inhibitors; however, this warrants further study.
Summary
Currently, ACE inhibitors and ARBs have an equal class of recommendation for first-line treatment for the management of HTN. Recent evidence has shown ARBs to be just as effective as ACE inhibitors for HTN but with improved tolerability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiotensin converting enzyme (ACE) inhibitors have been commercially available since 1981 and are one of the most frequently prescribed classes of medications in the United States [1]. The ACE inhibitor, lisinopril, was the fourth most frequently prescribed drug in 2020 [2]. This is likely because ACE inhibitors are generically available, affordable, and recommended as a first-line option for the management of highly prevalent chronic conditions, such as hypertension (HTN), heart failure with reduced ejection fraction (HFrEF), and chronic kidney disease (CKD) [3,4,5]. ACE inhibitors are, however, associated with a modest, but significant, risk of bradykinin-mediated angioedema, acute kidney injury (AKI), hyperkalemia, and chronic cough.
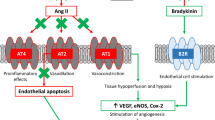
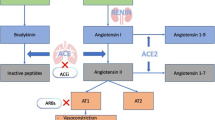
The angiotensin (AT) II receptor blockers (ARBs) were developed as an alternative to ACE inhibitors that work downstream from ACE in the renin-angiotensin system (RAS) and block the binding of AT II to the AT I receptor (Fig. 1) [6]. This results in a reduced risk of bradykinin-mediated angioedema and cough, although similar risks remain for AKI and hyperkalemia. Although ARBs were initially more expensive than ACE inhibitors, both classes of medications are now generically available and similarly affordable. This has raised questions about whether ARBs should preferentially be used instead of ACE inhibitors for HTN given their more favorable safety profile and similar antihypertensive effectiveness.
Mechanism of Action for Inhibitors of the Renin-Angiotensin System (RAS). The renin-angiotensin system regulates blood pressure by balancing sodium and water absorption and vascular tone. Angiotensinogen is hepatically synthesized, a renin substrate, and the precursor for angiotensin I, which is converted by ACE to angiotensin II. ACE inhibition, therefore, reduces the available angiotensin II and subsequent binding to ATR1. Alternatively, angiotensin receptor blockers prevent angiotensin II from binding to the ATR1 receptor and promoting deleterious effects on blood pressure. Parts of this figure were drawn by using images from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/)
In this review, we aim to describe the discovery and development of ACE inhibitors as antihypertensive agents, compare their efficacy, tolerability, and safety to ARBs, and highlight the contemporary issues surrounding ACE inhibitor use for HTN.
Discovery and Development of ACE Inhibitors
In the early 1960s, banana plantation workers in southwestern Brazil were discovered to collapse and die after being bitten by the pit viper Bothrops jaraca. The cause of death was attributed to severe hypotension from the snake venom. From this discovery, researcher Maurio Rocha e Silva and his student, Sergio Ferreira, at the University of Sao Paulo, began to study the snake venom and identified a peptide in the venom, bradykinin potentiating factor (BPF), that caused the blood pressure (BP) lowering effects [7]. Ferreria then took the peptide to Sir John Vane at the Royal College of Surgeons in London, where the inhibitory effects of BPF on the ACE was determined. Based on this finding, Vane proposed a research program in collaboration with Squibb Institute for Medical Research to develop the first orally active synthetic ACE inhibitor [8, 9•]. After years of research and development, captopril, the first ACE inhibitor, was commercially released in 1981. Over the next 15 years, other competitor ACE inhibitors entered the market and today 10 agents are commercially available (Table 1) [1].
Antihypertensive Effects and Clinical Indications for ACE Inhibitors
ACE inhibitors are considered first-line therapy in the management of HTN along with ARBs, thiazide diuretics, and calcium channel blockers (CCBs) [3]. ACE inhibitors work in the RAS by inhibiting ACE, which is an enzyme that converts AT I to AT II [6]. Decrease in AT II synthesis results in arterial and venous vasodilation, natriuresis, and a decrease in sympathetic activity. A systematic review analyzed 92 randomized clinical trials that randomly assigned participants to an ACE inhibitor or placebo and evaluated the BP lowering effects of different ACE inhibitors in almost 13,000 participants [10]. Results demonstrated ACE inhibitors reduce systolic BP ~ 8 mm Hg and diastolic BP ~ 5 mm Hg. Importantly, 60–70% of the antihypertensive effects associated with ACE inhibitors was found to be achievable with the lowest dose, which may minimize the risk of adverse effects [10]. Systematic reviews analyzing alternative first-line therapies have shown similar BP lowering effects with ACE inhibitors, thiazide diuretics (~ 9/4 mmHg), and ARBs (~ 8/5 mmHg) [11, 12].
The ALLHAT trial remains the largest head-to-head comparison of first-line therapies in patients with HTN [13]. It was designed to determine whether treatment with a CCB or an ACE inhibitor lowered the incidence of coronary heart disease (CHD) or cardiovascular disease (CVD) events versus treatment with a thiazide diuretic. The results demonstrated no significant difference between lisinopril and chlorthalidone for the primary outcome (Hazard Ratio, 0.99; 95% confidence interval, 0.91–1.08) or for the secondary outcomes of all-cause mortality, combined CHD, peripheral arterial disease, cancer, or end stage kidney disease [13]. Since thiazide diuretics, CCBs, ACE inhibitors, and ARBs share a similar class of recommendation for initial management in HTN, compelling indications should be a primary consideration when selecting first-line antihypertensive therapy. ACE inhibitors and ARBs, for example, are also indicated for the management of HFrEF, CKD, and diabetes mellitus (DM) with presence of albuminuria, and should be prioritized in these patient populations [3,4,5].
Tolerability and Safety of ACE Inhibitors
ACE inhibitors are generally well tolerated by most patients, but there are well established adverse effects attributed to ACE inhibitor use. Chronic cough, hyperkalemia, acute kidney injury, and angioedema are expected potential adverse effects with ACE inhibitor use. Additionally, hypotension and dizziness can occur with this drug class, as is the case with all antihypertensive therapies. Safety concerns related to a purported increased risk of cancer, particularly lung cancer, has also been reported in the literature.
Chronic cough is a well-documented class effect of ACE inhibitors with a reported incidence of 5% to 35% [14, 15]. Since ACE degrades bradykinin, inhibition of ACE results in increases in bradykinin levels, which is problematic because the lungs are a major site of ACE expression. The accumulation of bradykinin in the upper respiratory tract and lungs results in bronchial irritation and cough [15, 16]. The cough is often described as dry and persistent and can interfere with daily activities and even disrupt sleep. The cough can occur within hours of the first dose or may have a delayed onset of weeks to months after initiation [15, 17]. It usually resolves within a couple days of discontinuation of the ACE inhibitor but can take weeks in some cases [15, 17]. Chronic cough often recurs with rechallenge either with the same or a different ACE inhibitor [15]. Since ARBs do not play a role in inhibition of ACE and have a lower incidence of cough, it is appropriate to switch therapy to an ARB after discontinuation of the ACE inhibitor [17].
Angioedema is a rare but potentially lethal side effect of ACE inhibitor use [18]. The reported overall incidence is 0.1 to 0.7% with higher rates in African Americans compared to Caucasians [19,20,21,22]. Similar to the cough associated with ACE inhibitors, the mechanism of ACE inhibitor-induced angioedema is primarily mediated by bradykinin. Inhibition of bradykinin degradation by ACE inhibitors results in prominent vasodilation and plasma extravasation into the submucosal tissue leading to angioedema [18]. Clinical manifestations consist of swelling of face, lips, tongue, uvula, and upper airways. In cases of airway compromise, tracheal intubation is necessary until swelling resolves. Antihistamines, steroids, and epinephrine are often used but have shown minimal efficacy [18]. Additionally, therapies used for the treatment of hereditary angioedema have shown some benefit in ACE inhibitor-induced angioedema, but evidence is conflicting [23,24,25]. Angioedema usually resolves within 24 to 72 h [18]. Future use of ACE inhibitors is contraindicated in patients with a history of angioedema, but ARBs may be considered when RAS inhibition is necessary [18]. However, the risk versus benefit must be considered, especially given there are multiple other antihypertensives available.
A related concern with ACE inhibitor (and ARB) use is the potential risk of lung cancer. The RAS is well known to be involved in cancer biology and tumor development [26]. Numerous observational studies and meta-analyses of ACE inhibitors, ARBs, and other antihypertensives and risk of cancer and/or lung cancer have shown conflicting results [27,28,29,30,31,32]. In studies showing an increased risk of lung cancer with ACE inhibitors, the absolute risk is quite small; however, this could be problematic given the millions of individuals around the world who use ACE inhibitors. Some studies have also suggested that this risk may be dose and duration dependent [27, 29]. One of the most robust analyses was a network meta-analysis and trial sequential analysis of over 320,000 participants from 70 RCTs [33]. The authors found no relative increase in risk of cancer or cancer-related mortality with ACE inhibitors, ARBs, beta-blockers, diuretics, and calcium channel blockers. Even if a small risk does exist, the benefit of ACE inhibitors in reducing BP appears to outweigh the hypothetical risk of lung cancer for most patients.
ACE inhibitors along with other antihypertensives that affect the RAS can cause AKI and hyperkalemia [34]. It is important to monitor serum creatinine, glomerular filtration rate (GFR), and potassium levels within 10–14 days after ACE inhibitor initiation or dose escalation, and when clinically indicated thereafter [35]. The initial reduction in GFR is typically modest but acute decreases in GFR can occur, although this may not necessitate withholding therapy [34]. The decline in GFR appears to be caused by a decrease in intraglomerular pressure due to vasodilation of the efferent arteriole in the kidney [36]. A creatinine rise > 30% or drop in GFR > 25% warrants further investigation to identify other potential causes (e.g., other medications, diet, renal artery stenosis) and may require a 50% dose reduction followed by repeat laboratory tests in 10–14 days. Patients with bilateral renal artery stenosis, CKD, HFrEF, dehydration are at the highest risk of AKI [34, 35]. In most instances, AKI in association with ACE inhibitor use can often be reversed with discontinuation of the agent or fluid repletion. ACE inhibitors may be retried after resolution of AKI if RAS inhibition is clinically indicated [35]. There does not appear to be any clinically appreciable difference in the rates of AKI between ACE inhibitors and ARBs.
Another effect of AT II is the release of aldosterone from the adrenal glands, causing increased reabsorption of sodium and water and excretion of potassium. ACE inhibitor inhibition of AT II synthesis (or ARB blockade of the AT II receptor) interferes with the secretion of aldosterone from the adrenal gland [37]. This leads to the reduction of potassium excretion, thereby increasing serum levels of potassium. Overall incidence of hyperkalemia varies depending on contributing comorbidities and medications [37]. Risk of hyperkalemia increases with renal insufficiency, HFrEF, DM, and concurrent use of medications that increase potassium retention, such as aldosterone antagonists [34, 37]. Management of hyperkalemia depends on potassium levels, changes in electrocardiogram, and kidney function. For comorbidities such as CKD and HFrEF where RAS inhibition is most beneficial, it may be helpful to add on potassium-wasting diuretics or cation exchanging agents that reduce RAS inhibitor-associated hyperkalemia [37]. Similarly to AKI, the incidence of hyperkalemia appears to be similar between ACE inhibitors and ARBs.
Contemporary Considerations
ACE inhibitors and ARBs effectively lower BP by a similar degree through RAS inhibition [10, 11]. Current clinical practice guidelines consider both drug classes to have an equivalent class of recommendation as first-line therapy for the management of HTN [3]. However, for decades, ACE inhibitors have been more commonly prescribed than ARBs. Early trials of ACE inhibitors demonstrated a clear benefit of morbidity and mortality compared with placebo while ARBs did not consistently demonstrate a mortality benefit compared with placebo [38,39,40]. This led to the conclusion that ACE inhibitors possess cardioprotective properties that ARBs do not. It is important to note that ARB trials were conducted over a decade after ACE inhibitor trials. In that time period, strategies for cardiovascular prevention had greatly improved, including increased use of concomitant statin therapy in ARB trials compared to ACE inhibitor trials. As a result, cardiovascular risk is considerably lower in the ARB trials, which may be affecting overall results [41]. Additionally, since the first ACE inhibitors were approved over a decade before ARBs, generically available ACE inhibitors became available earlier, leading to greater utilization over ARBs. However, both ACE inhibitors and ARBs are generically available today and a recent systematic review of cost-effectiveness analyses favored ARBs over other first-line antihypertensives [42].
Head-to-head trials comparing the antihypertensive effectiveness of ACE inhibitors and ARBs remain limited [43,44,45,46]. The ONTARGET trial was one of the first large head-to-head comparison studies of ACE inhibitors and ARBs [43]. It was designed to show whether telmisartan is noninferior to ramipril to prevent vascular events in high-risk patients who had CVD or DM but did not have HFrEF. The study results demonstrated that telmisartan was equivalent to ramipril in patients with vascular disease or high-risk DM and was associated with less angioedema and cough [43]. Overall, systematic reviews and meta-analyses have concluded that ARBs are an equally effective alternative to ACE inhibitors with a better safety profile [47,48,49].
Most of the evidence comparing ACE inhibitors and ARBs has come from high-risk populations, which may limit application to the majority patients initiating antihypertensives [43,44,45,46]. As a result, a large-scale observational study of over 3 millions patients worldwide was designed to compare the effectiveness and safety of ACE inhibitors and ARBs when initiated as first-line treatment for HTN [50••]. Consistent with previous evidence, the study results showed no statistically significant difference in the effectiveness of ACE inhibitors versus ARBs on acute myocardial infarction, HFrEF, stroke, or composite cardiovascular events. However, ARBs demonstrated a better safety profile with lower risk of pancreatitis, angioedema, cough, and gastrointestinal bleeding. The study authors concluded that based on these results, ARBs should be selected over ACE inhibitors as first-line therapy for the management of HTN [50••].
Hypertension is also associated with increased risk of cognitive decline and dementia [51]. This association has sparked research investigating the role of antihypertensives and their effects on brain health. Contemporary evidence suggests that ARBs may be superior to other antihypertensives (including ACE inhibitors) at reducing the progression of cognitive decline and dementia. An observational study has shown a small reduction in dementia risk for ARBs compared with ACE inhibitors within the first 12 months of follow-up [52]. Other studies have demonstrated that ARBs cause slower cognitive decline compared to ACE inhibitors [53, 54•, 55]. The mechanism is still not fully understood, but some studies have suggested that ARBs propensity to cross the blood–brain barrier may possess more cognitive benefit [53, 56••]. Overall, large prospective, randomized, controlled studies are needed to further investigate the role of ARBs in reducing progression of cognitive decline and dementia compared to other antihypertensives.
Conclusion
ACE inhibitors are effective antihypertensives for the management of HTN and provide important clinical benefits for patients with compelling chronic conditions (e.g., DM, HFrEF). Since the class discovery in the 1980s, ACE inhibitors have been among the most prescribed medications in the United States. However, ACE inhibitors are associated with dry cough, angioedema, hyperkalemia, GFR reduction, and hypotension. Chronic cough and angioedema occur at higher rates with ACE inhibitors than other antihypertensive agents, specifically ARBs. Currently, ACE inhibitors and ARBs are equally guideline-recommended first-line therapy in the management of HTN; however, recent evidence suggests ARBs to be just as effective as ACE inhibitors but with improved tolerability. Clinicians may consider ARBs over ACE inhibitors in patients newly starting on RAS inhibitor therapy.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sica DA. The evolution of renin-angiotensin blockade: angiotensin-converting enzyme inhibitors as the starting point. Curr Hypertens Rep. 2010;12(2):67–73. https://doi.org/10.1007/s11906-010-0091-9.
Leading 20 U.S. pharma products by total prescriptions in 2020. Statista. Sept 2022. Accessed 5 Apr 2023. https://www.statista.com/statistics/233986/top-us-pharma-products-by-prescriptions/.
Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in Hypertension. 2018 Jun;71(6):e140–e144]. Hypertension. 2018;71(6):e13–e115. https://doi.org/10.1161/HYP.0000000000000065.
Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021;99(3S):S1–S87. https://doi.org/10.1016/j.kint.2020.11.003.
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263–421. https://doi.org/10.1016/j.jacc.2021.12.012.
Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13(8 Suppl B):9–20. https://doi.org/10.18553/jmcp.2007.13.s8-b.9.
Ferreira SH. Bradykinin-potentiating factor (BPF) present in the venom of bothrops jararca. Br J Pharmacol Chemother. 1965;24(1):163–9. https://doi.org/10.1111/j.1476-5381.1965.tb02091.x.
Cushman DW, Ondetti MA. History of the design of captopril and related inhibitors of angiotensin converting enzyme. Hypertension. 1991;17(4):589–92. https://doi.org/10.1161/01.hyp.17.4.589.
• Gavras H, Brunner HR, Turini GA, et al. Antihypertensive effect of the oral angiotensin converting-enzyme inhibitor SQ 14225 in man. N Engl J Med. 1978;298(18):991–5. https://doi.org/10.1056/NEJM197805042981803. First publication to report the potential of ACE inhibition for hypertension in humans.
Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database Syst Rev. 2008;2008(4):CD003823. Published 2008. https://doi.org/10.1002/14651858.CD003823.pub2.
Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev. 2008;2008(4):CD003822. Published 2008. https://doi.org/10.1002/14651858.CD003822.pub2.
Musini VM, Nazer M, Bassett K, Wright JM. Blood pressure-lowering efficacy of monotherapy with thiazide diuretics for primary hypertension. Cochrane Database Syst Rev. 2014;(5):CD003824. Published 2014. https://doi.org/10.1002/14651858.CD003824.pub2.
ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981–2997. https://doi.org/10.1001/jama.288.23.2981.
Ravid D, Lishner M, Lang R, Ravid M. Angiotensin-converting enzyme inhibitors and cough: a prospective evaluation in hypertension and in congestive heart failure. J Clin Pharmacol. 1994;34(11):1116–20. https://doi.org/10.1002/j.1552-4604.1994.tb01989.x.
Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med. 1992;117(3):234–242. https://doi.org/10.7326/0003-4819-117-3-234.
Fox AJ, Lalloo UG, Belvisi MG, Bernareggi M, Chung KF, Barnes PJ. Bradykinin-evoked sensitization of airway sensory nerves: a mechanism for ACE-inhibitor cough. Nat Med. 1996;2(7):814–7. https://doi.org/10.1038/nm0796-814.
Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):169S–173S. https://doi.org/10.1378/chest.129.1_suppl.169S.
Kostis WJ, Shetty M, Chowdhury YS, Kostis JB. ACE Inhibitor-Induced Angioedema: a Review. Curr Hypertens Rep. 2018;20(7):55. Published 2018. https://doi.org/10.1007/s11906-018-0859-x.
Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005;165(14):1637–42. https://doi.org/10.1001/archinte.165.14.1637.
Slater EE, Merrill DD, Guess HA, et al. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA. 1988;260(7):967–70.
Miller DR, Oliveria SA, Berlowitz DR, Fincke BG, Stang P, Lillienfeld DE. Angioedema incidence in US veterans initiating angiotensin-converting enzyme inhibitors. Hypertension. 2008;51(6):1624–30. https://doi.org/10.1161/HYPERTENSIONAHA.108.110270.
Messerli FH, Nussberger J. Vasopeptidase inhibition and angio-oedema. Lancet. 2000;356(9230):608–9. https://doi.org/10.1016/S0140-6736(00)02596-4.
Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372(5):418–25. https://doi.org/10.1056/NEJMoa1312524.
Sinert R, Levy P, Bernstein JA, et al. Randomized Trial of Icatibant for Angiotensin-Converting Enzyme Inhibitor-Induced Upper Airway Angioedema. J Allergy Clin Immunol Pract. 2017;5(5):1402–1409.e3. https://doi.org/10.1016/j.jaip.2017.03.003.
Lewis LM, Graffeo C, Crosley P, et al. Ecallantide for the acute treatment of angiotensin-converting enzyme inhibitor-induced angioedema: a multicenter, randomized, controlled trial. Ann Emerg Med. 2015;65(2):204–13. https://doi.org/10.1016/j.annemergmed.2014.07.014.
Vallejo Ardila DL, Walsh KA, Fifis T, et al. Immunomodulatory effects of renin-angiotensin system inhibitors on T lymphocytes in mice with colorectal liver metastases. J Immunother Cancer. 2020;8(1):e000487. https://doi.org/10.1136/jitc-2019-000487.
Hicks BM, Filion KB, Yin H, Sakr L, Udell JA, Azoulay L. Angiotensin converting enzyme inhibitors and risk of lung cancer: population based cohort study. BMJ. 2018;363:k4209. Published 2018 Oct 24. https://doi.org/10.1136/bmj.k4209.
Lin SY, Lin CL, Lin CC, et al. Association between Angiotensin-Converting Enzyme Inhibitors and Lung Cancer-A Nationwide, Population-Based, Propensity Score-Matched Cohort Study. Cancers (Basel). 2020;12(3):747. Published 2020. https://doi.org/10.3390/cancers12030747.
Kristensen KB, Hicks B, Azoulay L, Pottegård A. Use of ACE (Angiotensin-Converting Enzyme) Inhibitors and Risk of Lung Cancer: A Nationwide Nested Case-Control Study. Circ Cardiovasc Qual Outcomes. 2021;14(1):e006687. https://doi.org/10.1161/CIRCOUTCOMES.120.006687.
Wang Z, Wei L, Yin C, Li W, Wan B. Angiotensin Receptor Blocker Associated with a Decreased Risk of Lung Cancer: An Updated Meta-Analysis. J Pers Med. 2023;13(2):243. Published 2023. https://doi.org/10.3390/jpm13020243.
Batais M, Almigbal T, Alotaibi K, et al. Angiotensin converting enzyme inhibitors and risk of lung cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2021;100(17):e25714. https://doi.org/10.1097/MD.0000000000025714.
Wu Z, Yao T, Wang Z, et al. Association between angiotensin-converting enzyme inhibitors and the risk of lung cancer: a systematic review and meta-analysis. Br J Cancer. 2023;128(2):168–76. https://doi.org/10.1038/s41416-022-02029-5.
Bangalore S, Kumar S, Kjeldsen SE, et al. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol. 2011;12(1):65–82. https://doi.org/10.1016/S1470-2045(10)70260-6.
Momoniat T, Ilyas D, Bhandari S. ACE inhibitors and ARBs: Managing potassium and renal function. Cleve Clin J Med. 2019;86(9):601–7. https://doi.org/10.3949/ccjm.86a.18024.
Schoolwerth AC, Sica DA, Ballermann BJ, Wilcox CS; Council on the Kidney in Cardiovascular Disease and the Council for High Blood Pressure Research of the American Heart Association. Renal considerations in angiotensin converting enzyme inhibitor therapy: a statement for healthcare professionals from the Council on the Kidney in Cardiovascular Disease and the Council for High Blood Pressure Research of the American Heart Association. Circulation. 2001;104(16):1985–1991. https://doi.org/10.1161/hc4101.096153.
Hirsch S. Pre-renal success. Kidney Int. 2012;81(6):596–7. https://doi.org/10.1038/ki.2011.418.
Hundemer GL, Sood MM. Hyperkalemia with RAAS inhibition: Mechanism, clinical significance, and management. Pharmacol Res. 2021;172:105835. https://doi.org/10.1016/j.phrs.2021.105835.
Fox KM. EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet. 2003;362(9386):782–788. https://doi.org/10.1016/s0140-6736(03)14286-9.
Heart Outcomes Prevention Evaluation Study Investigators, Yusuf S, Sleight P, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients [published correction appears in 2000 May 4;342(18):1376] [published correction appears in N Engl J Med 2000 Mar 9;342(10):748]. N Engl J Med. 2000;342(3):145–153. https://doi.org/10.1056/NEJM200001203420301.
Yusuf S, Diener HC, Sacco RL, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359(12):1225–37. https://doi.org/10.1056/NEJMoa0804593.
Messerli FH, Bavishi C, Bangalore S. Why Are We Still Prescribing Angiotensin-Converting Enzyme Inhibitors? Circulation. 2022;145(6):413–5. https://doi.org/10.1161/CIRCULATIONAHA.121.057835.
Park C, Wang G, Durthaler JM, Fang J. Cost-effectiveness Analyses of Antihypertensive Medicines: A Systematic Review. Am J Prev Med. 2017;53(6S2):S131–S142. https://doi.org/10.1016/j.amepre.2017.06.020.
ONTARGET Investigators, Yusuf S, Teo KK, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–1559. https://doi.org/10.1056/NEJMoa0801317.
Bremner AD, Baur M, Oddou-Stock P, Bodin F. Valsartan: long-term efficacy and tolerability compared to lisinopril in elderly patients with essential hypertension. Clin Exp Hypertens. 1997;19(8):1263–85. https://doi.org/10.3109/10641969709083217.
Barnett AH, Bain SC, Bouter P, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy [published correction appears in N Engl J Med. 2005 Apr 21;352(16)1731]. N Engl J Med. 2004;351(19):1952–1961. https://doi.org/10.1056/NEJMoa042274.
Spinar J, Vítovec J, Souček M, Dušek L, Pavlík T; CORD investigators. CORD: COmparison of Recommended Doses of ace inhibitors and angiotensin II receptor blockers. Int J Cardiol. 2010;144(2):293–294. https://doi.org/10.1016/j.ijcard.2009.02.022.
Bangalore S, Fakheri R, Toklu B, Ogedegbe G, Weintraub H, Messerli FH. Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers in Patients Without Heart Failure? Insights From 254,301 Patients From Randomized Trials. Mayo Clin Proc. 2016;91(1):51–60. https://doi.org/10.1016/j.mayocp.2015.10.019.
Li EC, Heran BS, Wright JM. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev. 2014;2014(8):CD009096. Published 2014 Aug 22. https://doi.org/10.1002/14651858.CD009096.pub2.
Sanders GD, Coeytaux R, Dolor RJ, et al. Angiotensin-Converting Enzyme Inhibitors (ACEIs), Angiotensin II Receptor Antagonists (ARBs), and Direct Renin Inhibitors for Treating Essential Hypertension: An Update. Rockville (MD): Agency for Healthcare Research and Quality (US); June 2011.
•• Chen R, Suchard MA, Krumholz HM, et al. Comparative First-Line Effectiveness and Safety of ACE (Angiotensin-Converting Enzyme) Inhibitors and Angiotensin Receptor Blockers: A Multinational Cohort Study. Hypertension. 2021;78(3):591–603. https://doi.org/10.1161/HYPERTENSIONAHA.120.166. ARBs have similar antihypertensive effectiveness as ACE inhibitors but with a better safety profile.
Walker KA, Power MC, Gottesman RF. Defining the Relationship Between Hypertension, Cognitive Decline, and Dementia: a Review. Curr Hypertens Rep. 2017;19(3):24. https://doi.org/10.1007/s11906-017-0724-3.
Goh KL, Bhaskaran K, Minassian C, Evans SJ, Smeeth L, Douglas IJ. Angiotensin receptor blockers and risk of dementia: cohort study in UK Clinical Practice Research Datalink. Br J Clin Pharmacol. 2015;79(2):337–50. https://doi.org/10.1111/bcp.12511.
Ho JK, Nation DA. Alzheimer’s Disease Neuroimaging Initiative. Memory is preserved in older adults taking AT1 receptor blockers. Alzheimers Res Ther. 2017;9(1):33. Published 2017. https://doi.org/10.1186/s13195-017-0255-9.
• Hajjar I, Okafor M, McDaniel D, et al. Effects of Candesartan vs Lisinopril on Neurocognitive Function in Older Adults With Executive Mild Cognitive Impairment: A Randomized Clinical Trial. JAMA Netw Open. 2020;3(8):e2012252. Published 2020. https://doi.org/10.1001/jamanetworkopen.2020.12252. Randomized trial demonstrating potential neurocognitive benefits with candesartan compared to lisinopril.
Deng Z, Jiang J, Wang J, et al. Angiotensin Receptor Blockers Are Associated With a Lower Risk of Progression From Mild Cognitive Impairment to Dementia. Hypertension. 2022;79(10):2159–69. https://doi.org/10.1161/HYPERTENSIONAHA.122.19378.
•• Ho JK, Moriarty F, Manly JJ, et al. Blood-Brain Barrier Crossing Renin-Angiotensin Drugs and Cognition in the Elderly: A Meta-Analysis. Hypertension. 2021;78(3):629–43. https://doi.org/10.1161/HYPERTENSIONAHA.121.17049. Suggests possible links between blood-brain barrier crossing renin-angiotensin drugs and less memory decline.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Anarug Menta—Research Grant from VCU Health Pauley Heart Center. Benjamin VanTassell—Novo Nordisk Research Grant, Novartis Research Grant, Kiniksa Pharmaceuticals Consulting Fees, and Implicit Bioscience Consulting Fees. Dave Dixon—Boehringer Ingelheim Research Grant. Stacey Cutrell, Ibrahim S. Alhomoud, and Azita Talasaz declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cutrell, S., Alhomoud, I.S., Mehta, A. et al. ACE-Inhibitors in Hypertension: A Historical Perspective and Current Insights. Curr Hypertens Rep 25, 243–250 (2023). https://doi.org/10.1007/s11906-023-01248-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-023-01248-2