Abstract
Purpose of Review
This review discusses the recent literature on subjectively and objectively assessed sleep duration in relation to hypertension risk and out-of-clinic blood pressure (BP) measures and highlights critical areas for future research.
Recent Findings
Sleep duration, particularly short sleep, may influence BP through disturbed autonomic balance, hormonal imbalances, increased adiposity and metabolic dysfunction, and disrupted circadian rhythms. Observational studies indicate that short and long sleep are associated with hypertension risk, reduced nocturnal dipping, and elevated morning BP, but evidence is stronger for short sleep. Experimental sleep restriction increases BP, while sleep extension may lower BP in prehypertensive individuals. Women and racial/ethnic minorities are more prone to the detrimental effects of short sleep on BP.
Summary
Additional studies are warranted to clarify the association of objectively assessed sleep with BP level and diurnal pattern and to determine the sex- and race-specific effects of sleep restriction and extension on BP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep serves critical roles in cardiovascular health (CVH) and is increasingly recognized as an important lifestyle risk factor for cardiovascular disease (CVD) [1••]. Several dimensions of sleep including duration, quality, regularity, and timing as well as the presence of sleep disorders can alter cardiovascular risk. Given that sleep duration is the most straightforward, simple measure of sleep habits, it is the most widely studied in relation to health [2]. The ubiquity of insufficient sleep has led to a growing literature on the potential effects of short sleep on health. According to the National Heart, Lung, and Blood Institute, approximately one third of US adults have habitually insufficient sleep [2]. Further, sleep duration shows substantial intra- and inter-individual variation [3]. A large proportion of inter-individual variability in sleep is likely dictated by psychosocial, behavioral, genetic, cultural, and environmental factors, which can also lead to sex and racial/ethnic disparities in the associations of sleep duration with cardiovascular risk [3].
Sleep duration plays a role in CVD etiology through its influence on CVD risk factors, including blood pressure (BP) [1••]. Experimental sleep deprivation studies and population-based epidemiological studies indicate that sleep duration may play an important role in hypertension’s etiology [4]. Much of the early studies on sleep and BP relied on self-reported sleep duration. Recently, there has been increasing interest in gathering objective sleep data, and several population-based cohorts now have ancillary sleep studies that measure sleep phenotypes with polysomnography (PSG) and/or wrist actigraphy. Furthermore, given that the most recent American Heart Association/American College of Cardiology (AHA/ACC) statement on BP guidelines indicates that measures of out-of-office BP may be more strongly related to cardiovascular morbidity and mortality than clinic BP [5••], an emerging area of scientific interest is investigating the influence of sleep duration on measures of the diurnal pattern of BP from out-of-office BP monitoring including ambulatory BP monitoring (ABPM), the reference standard for out-of-office BP and home BP monitoring (HBPM) [6••].
In this review, we discuss literature from the past 5 years in addition to landmark studies that examine short and long sleep duration in relation to hypertension risk and BP level and variability among adults. We will also review the evidence on hypothesized underlying mechanisms and sex and racial/ethnic disparities in these relations. The papers and topic areas are discussed with an emphasis on knowledge gaps that may guide future research and the clinical and public health implications of the existing literature.
Definitions of Short, Normal, and Long Sleep in the Literature
Studies of twins demonstrate that sleep duration heritability can range between 31% and 55% suggesting that the need for sleep may vary substantially among individuals [7, 8]. In 2015, the American Academy of Sleep Medicine (AASM) and Sleep Research Society (SRS) released a Joint Consensus Statement that addresses the recommended amount of sleep to promote optimal general, cardiovascular, metabolic, mental, and immunologic heath in adults based on the existing literature [3]. At the time, much of the reviewed evidence was based on studies that examined self-reported sleep duration. The statement concluded that sleeping ≤ 6 h/night is associated with sub-optimal health and is therefore considered short sleep, while normal sleep was defined as 7–9 h/night for adults aged 18–60 years [3]. However, the appropriateness of ≥ 9 h/night and 6–7 h/night of sleep for optimal health could not be ascertained with certainty.
Consistently with the AASM/SRS joint consensus statement [3], the National Sleep Foundation currently recommends a sleep duration of 7–9 h/night for adults aged 18–64 years and a sleep duration of 7–8 h/night for older adults aged ≥ 65 years based on a systematic review of studies published between 2004 and 2014 [9]. Importantly, the published statement emphasized that some individuals who fall outside of the recommended ranges for sleep duration may have no adverse health effects but cautioned that intentionally restricting sleep over a prolonged period may compromise health and well-being. More recently, in an AHA statement [1••], short sleep was defined as < 7 h/night and long sleep as ≥ 9 h/night, unless otherwise noted, given that detrimental effects on cardiometabolic risk were observed using these definitions. The AHA statement concluded with a call for health organizations to include evidence-based sleep recommendations in their lifestyle guidelines for optimal health, akin to recommendations for other lifestyle behaviors such as diet and physical activity.
Prevalence of Short and Long Sleep in the Population
According to the Centers for Disease Control and Prevention, one third of US adults are not getting the recommended 7 h of sleep per night [10] and > 50% indicate that they do not get enough sleep on workdays [11]. Self-reported sleep data from the 2014 Behavioral Risk Factor Surveillance System (BRFSS) indicate that 35.2% of US adults sleep < 7 h/night [12]. Long sleep duration was less prevalent, as only 4.4% reported sleeping 9 h and 3.6% reported sleeping ≥ 10 h. Similar to other health behaviors, significant sex and racial/ethnic differences in sleep duration exist in the US population. The BRFSS showed that the prevalence of short sleep was similar in men and women (35.5% and 34.8%, respectively). However, there is emerging evidence that women may be more prone to short sleep and that sleep duration varies across the lifespan [13]. A cross-sectional telephone study of 19,136 US adults showed that self-reported sleep latency, the amount of time it takes to transition from being fully awake to sleep, is longer in women [14]. Further, women aged < 55 years reported more sleepiness than men, and older women reported sleeping, on average, 20 min less than men [14]. These data indicate that sex differences in sleep epidemiology likely exist, but there are significant research gaps on sex differences in the prevalence of objectively assessed short and long sleep and the physiological mechanisms that may underlie these differences [13].
The prevalence of extremes of sleep duration (i.e., short and long sleep) may be higher among racial/ethnic minorities. In the BRFSS [10], self-reported short sleep was least prevalent among white adults (33.4%), whereas nearly half of African-American adults reported having short sleep (45.8%). The prevalence of short sleep in Hispanic and Asian adults was 34.5% and 37.5%, respectively. Similarly, data from 2007 to 2008 National Health and Nutrition Examination Survey (NHANES) demonstrated that African Americans, Hispanics/Latinos, and Asians were more than twice as likely to report very short (< 5 h) and short sleep duration (5–6 h) compared to whites [15]. Self-reported sleep data from Hispanic Community Health Study/Study of Latinos (HCHS/SOL), the largest population-based cohort in US Hispanics/Latinos, indicate that the prevalence of sleeping < 7 h and > 9 h in this population is 18.6% and 20%, respectively [16].
Studies of objectively assessed sleep also demonstrate significant racial/ethnic disparities in the prevalence of short and long sleep and are suggestive that the prevalence of short sleep among racial/ethnic minorities may be higher than estimated from self-reported data. In the Coronary Artery Risk Development in Young Adults (CARDIA) sleep study, the mean sleep duration from wrist actigraphy among 612 African-American and white adults was similar at 6.1 h, but African-American men and women had higher prevalence of sleeping < 6 h (76% and 56% vs. 37% and 18%, respectively) [17]. In the HCHS/SOL, 1 week of wrist actigraphy indicates that Hispanic/Latino adults sleep, on average, 6.7 h [18•]. Furthermore, the prevalence of sleeping < 6 h was 22% and that of sleeping ≥ 8 h was 10.3% [19•], suggesting that previous self-reported sleep data from this cohort may have overestimated the prevalence of long sleep and underestimated the prevalence of short sleep. There is also evidence that increased acculturation to the US lifestyle is associated with worse sleep habits in Hispanics/Latinos, particularly with shorter sleep duration [20].
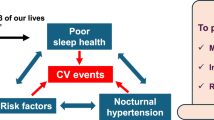
Potential Mechanisms Underlying the Association of Sleep Duration with Hypertension Risk and Abnormalities in the Diurnal Pattern of Blood Pressure
Sleep duration, particularly short sleep, may influence BP through disturbed autonomic balance, hormonal imbalances, increased adiposity and metabolic dysfunction, and disrupted circadian rhythmicity (Fig. 1) [4]. Compromised sleep is associated with disrupted autonomic balance, particularly elevated sympathetic nervous system activity and reduced parasympathetic dominance during sleep [4]. Insomnia patients with short sleep duration have significantly dampened parasympathetic activation and increased sympathovagal imbalance [21]. Similarly, in the Multi-Ethnic Study of Atherosclerosis (MESA), short sleep (< 6 h) was associated with markers of autonomic tone that indicate lower levels of cardiac parasympathetic tone and/or higher levels of sympathetic tone including higher heart rate, greater heart rate orthostatic reactivity, and lower high-frequency heart rate variability [22]. Autonomic dysfunction, particularly sympathetic activation, has in turn been linked to hypertension development and progression and to reduced nocturnal dipping [4, 23, 24].
Short sleep may also increase hypertension risk through mechanisms related to adiposity and metabolic dysfunction [4]. In experimental studies, sleep restriction decreased leptin levels, increased ghrelin levels, and elevated hunger and appetite with particular cravings for sweets, starch, and salty snacks [25]. Short sleep has also been linked to poor diet quality in observational studies [19•]. Collectively, these changes can lead to a positive energy balance and increased risk for overweight and obesity. In fact, short sleep duration has been linked to higher incidence of obesity and type 2 diabetes in epidemiologic studies [1••], and type 2 diabetes and obesity have been shown to act as partial mediators of the association between short sleep and the incidence of hypertension [26, 27]. These findings indicate that the metabolic alterations associated with obesity and diabetes are likely in the causal pathway linking short sleep duration to hypertension. Long sleep, to a lesser extent than short sleep, has also been linked to increased obesity, metabolic syndrome, and type 2 diabetes risk [1••], suggesting that excess adiposity and metabolic dysfunction may also underlie the association between long sleep and BP, but these associations warrant further investigation.
Finally, habitually short sleep may increase hypertension risk and abnormalities in the diurnal pattern of BP by leading to circadian misalignment, a state of desynchrony between behaviors and physiological functions controlled by central and peripheral biological clocks [4]. Circadian rhythms are thought to have evolved as an adaptation to 24-h light/dark cycles to ensure that physiologic functions are performed at optimal times and are controlled by a master clock in the suprachiasmatic nucleus of the brain [28, 29]. In addition, multiple organs have peripheral clocks that regulate their function and are synchronized to the master clock. Chronically restricted sleep is associated with prolonged exposure to physical and psychological stressors and with engaging in eating and activity at unconventional circadian times leading to a desynchrony between the master clock in the brain and the peripheral clocks in the organs [4, 30]. Collectively, this disrupts the circadian rhythmicity with which physiological processes occur, including diurnal variation of BP [4, 31], leading to increased hypertension risk, having reduced nocturnal dipping, a shifting of the daily BP profile to higher values, and increased BP variability [4, 32, 33].
Observational Studies of the Association Between Short Sleep and Hypertension
The association between short sleep and hypertension risk has been investigated in a number of landmark US cohorts. In the Sleep and Heart Health Study (n = 5910), self-reported sleep < 6 h and 6–7 h versus 7–8 h was associated with 66% and 19% higher odds for hypertension (OR (95% CI) 1.66 (1.35–2.04) and 1.19 (1.02–1.39)), respectively [34]. Similarly, in the 2007–2009 National Health Interview Surveys (NHIS) (n = 71,455), the odds of hypertension were increased among adults who reported sleeping < 6 h (OR (95% CI) 1.49 (1.34–1.64)) or 6 h (OR (95% CI) 1.15 (1.08–1.23)) compared to 8 h [35]. Longitudinal analyses of the first NHANES (n = 4810) also showed that self-reported short sleep, defined as ≤ 5 h, was associated with 32% increased hypertension risk over 8–10 years of follow-up [26]. Cohort studies that used objectively assessed sleep also demonstrated strong associations between short sleep and hypertension risk. In CARDIA [36], shorter actigraphy-assessed sleep duration predicted significantly higher systolic BP (SBP) and diastolic BP (DBP) levels cross-sectionally and adverse changes in SBP and DBP levels over 5 years. Short sleep was also associated with 37% increased odds of incident hypertension (OR (95% CI) 1.37 (1.05–1.7)).
Given the plethora of studies that have examined the role of sleep in hypertension etiology over the past decade, the evidence was summarized in a 2016 AHA statement on sleep and cardiometabolic health [1••], which concluded that there is strong epidemiological evidence that short sleep is a risk factor for hypertension. It is notable that most of the reviewed studies relied on self-reported sleep duration and that different cut-offs were used to define short sleep (≤ 5 h, ≤ 6 h, or ≤ 7 h). Two recent meta-analyses showed that short sleep duration (≤ 5 h or ≤ 6 h), assessed mostly from self-report, is associated with hypertension without evidence of heterogeneity [37, 38]. In one meta-analysis, based on six prospective studies, short sleep was associated with 21% higher hypertension risk (RR (95% CI) 1.21 (1.05–1.40)) [37]. Similarly, in another meta-analysis, the pooled results from cross-sectional and prospective studies showed that short sleep is associated with 21% and 23% higher hypertension risk, respectively (OR (95% CI) 1.21 (1.09–1.34) and RR (95% CI) 1.23 (1.06–1.42)) [38].
In contrast, a third meta-analysis showed that while short sleep is associated with an increased risk of prevalent hypertension in cross-sectional studies (OR (95% CI) 1.20 (1.09–1.32)), there is no association with incident hypertension based on six prospective studies (RR (95% CI) 1.11 (0.84–1.47)), with evidence of heterogeneity between studies [39]. This meta-analysis also showed that the risk for hypertension among short sleepers was increased among adults aged < 65 years but not those aged ≥ 65 years [39]. Indeed, in French cross-sectional studies, self-reported sleep < 6 h was not associated with hypertension prevalence in elderly adults [40], but sleeping ≤ 5 h vs. 7 h, as measured by self-report, was associated with 80% higher odds for hypertension in middle-aged adults [41].
More recently, a 2018 review [42•] summarized the evidence from the past 2 years (2016–2018) on short sleep in relation to hypertension risk. A total of four new observational studies were identified [43,44,45,46•], out of which only one relied on objectively assessed sleep duration [46•]. While two Asian and Finnish studies that relied on self-reported sleep reported a null association [44, 45], a prospective cohort of 162,121 Taiwanese adults [43] showed that self-reported short sleep (< 6 h) increased the risk for high BP (≥ 130/85 mmHg) by 8% (HR (95% CI) 1.08 (1.04–1.13)) over follow-up from 1996 to 2014. In contrast, in a cross-sectional analysis of the HCHS/SOL study, there was no association between actigraphy-assessed sleep duration and BP or HTN risk in n = 2252 participants aged 18–64 years [46•].
Over the past year, a number of additional original research studies on sleep and BP have been published and have reported conflicting results. Two cross-sectional studies, a Finnish study (n = 6462) that relied on self-report [47] and a Japanese study (n = 7051) that relied on wrist actigraphy [48], reported null associations between sleep duration and hypertension risk. Similarly, a cross-sectional study among 323 women in New York City who participated in the ongoing AHA Go Red for Women Strategically Focused Research Network (mean age = 39 ± 17 years) showed no association between self-reported sleep duration and BP [49•]. In contrast to these findings, aggregated data from the 2013 BRFSS (n = 433,386) and the combined 2007–2016 NHIS (n = 295,331) demonstrate higher odds for hypertension among those sleeping ≤ 4 h (OR = 1.86, p < 0.001), 5 h (OR = 1.56, p < 0.001), and 6 h (OR = 1.27, p < 0.001) compared to 7 h [50••]. Similarly, in a study of 27,034 active duty military and Coast Guard personnel, self-reported sleeping < 5 h vs. 7–8 h was associated with > 2-fold higher odds for hypertension (OR (95% CI) 2.22 (1.89–2.61)) [51]. On balance, the majority of the studies summarized herein indicate that short sleep is associated with higher hypertension risk. Discrepant findings may be attributed to the measurement modality of sleep (self-reported versus objectively assessed), varying definitions of short sleep across the studies, and potential differential associations by race/ethnicity.
Observational Studies of the Association Between Long Sleep and Hypertension
The role of long sleep in hypertension etiology is less clear but increasingly of interest. It is notable that most studies on long sleep and BP have relied on self-reported sleep duration. Studies in non-US populations are indicative of a null association between self-reported long sleep and hypertension risk. In a cross-sectional study in south Asia, there was no association between self-reported long sleep (≥ 9 h) and hypertension (OR (95% CI) 1.02 (0.86–1.21)) [45]. In a Finnish prospective cohort study, self-reported long sleep (≥ 9 h) was not related to hypertension incidence over 8 years of follow-up (HR (95% CI) 0.95 (0.61–1.49)) [44]. Similarly, in a Taiwanese study, self-reported sleeping > 8 h was not related to hypertension incidence (HR (95% CI) 1.02 (0.95–1.09)) [43], but results were not reported for a sleep duration ≥ 9 h.
In contrast, studies in US populations are indicative that self-reported long sleep may increase the risk for hypertension. Aggregated data from the 2013 BRFSS (n = 433,386) and the combined 2007–2016 NHIS (n = 295,331) showed that long sleepers had up to 41% higher odds of hypertension (9 h vs. 7 h, OR = 1.19, p < 0.001; and ≥ 10 h vs. 7 h, OR = 1.41, p < 0.001), but this association was significantly attenuated after accounting for illness indices and may represent confounding by health status [50••]. Data from 71,455 participants in 2007–2009 NHIS also showed that sleeping ≥ 10 h vs. 8 h is associated with greater odds for hypertension (OR (95% CI) 1.20 (1.05–1.37)) [35]. Similarly, in the Sleep and Heart Health Study (n = 5910), sleeping between 8 and 9 h and ≥ 9 h vs. 7–8 h was associated with 19% and 30% higher odds for hypertension, respectively [34].
In a meta-analysis of cross-sectional studies, long sleep duration, self-reported and defined as ≥ 9 h in most studies, was associated with increased risk of prevalent hypertension with moderate evidence of heterogeneity (OR (95% CI) 1.11 (1.05–1.17)), but not with risk of incident hypertension [39]. Similarly, in two other meta-analyses, long sleep duration, ascertained mainly by self-report, was not associated with the development of hypertension [37, 38]. Furthermore, although meta-analyses have shown that associations between short sleep and hypertension risk are more pronounced among adults aged < 65 years, no differences by age group have been observed for the association between long sleep and hypertension [39]. In fact, younger men and women typically display little risk from sleep durations > 7 h. It is hypothesized that associations between self-reported long sleep and hypertension may represent confounding by health status, as those with functional limitations due to various illnesses tend to spend more time resting in bed which could be misreported as sleep [50••]. Studies with objectively assessed long sleep are needed to confirm these associations.
Effect of Sleep Restriction and Extension on Blood Pressure Levels
Intervention studies that examine the effect of restricting sleep on BP are limited, particularly among US adults. In a Canadian intervention study of healthy normotensive adults, young (age 20–28 years; n = 8) and elderly participants (age 60–69 years; n = 8) underwent a night of sleep and 24.5 h of sleep deprivation in a cross-over counterbalanced design [52]. Sleep deprivation induced a significant increase in SBP and DBP in elderly but not young adults. An attenuation of the SBP orthostatic response was observed in both age groups. However, in a French study of 12 men, 2 and 6 days of sleep restriction (4-h sleep, 02:00 am–06:00 am) did not affect BP [53].
To date, there are limited published studies that investigate the effect of mild to severe sleep restriction on BP among US adults. In one randomized controlled trial, 45 healthy adults (mean age 32 years; 23 women) underwent either repeated sleep restriction (4 h of sleep from 03:00 am to 07:00 am for three nights followed by recovery sleep of 8 h, repeated four times in succession) or a sleep duration of 8 h/night from 11:00 pm to 07:00 am [54•]. In the sleep-restricted group, SBP was significantly increased for the whole day during the first block of sleep restriction, and DBP was significantly increased for the whole day during the first, second, and fourth block of sleep restriction (p < 0.01) suggesting that repeated exposure to shortened sleep is associated with elevated SBP and DBP levels. In another study with a randomized cross-over design, the effect of psychological stress tasks and sleep deprivation on BP in 20 healthy adults was evaluated [55]. Findings demonstrated a significant interaction between sleep deprivation and stress on SBP (p = 0.02), as sleep deprivation amplified SBP increases due to psychological stress, suggesting that short sleep may increase BP via mechanisms related to the dysregulation of stress physiology [55].
Only one US study examined the effect of extending sleep duration on BP. In a 6-week intervention study, 22 participants with prehypertension or stage 1 hypertension and habitual sleep durations of ≤ 7 h were randomized to a sleep extension group aiming to increase bedtime by 1 h daily over a 6-week period or to a sleep maintenance group aiming to maintain habitual bedtimes [56•]. Participants in the sleep extension group increased their actigraphically assessed daily sleep duration by 35 ± 9 min, while subjects in the sleep maintenance condition also increased their sleep duration slightly by 4 ± 9 min (p = 0.03). A significant decrease in 24-h beat-to-beat SBP (14 ± 3 mmHg) and DBP (8 ± 3 mmHg) was observed from the pre- to post-intervention visit in the sleep extension group (p < 0.05). A non-significant reduction in SBP and DBP of 7 ± 5 mmHg and 3 ± 4 mmHg was observed in the sleep maintenance group. These findings indicate that sleep extension may have a clinically significant impact on 24-h BP that warrants further investigation in larger samples and other populations.
Sleep Duration and the Diurnal Pattern of Blood Pressure
Observational Studies
Given that abnormalities in 24-h BP diurnal patterns, as measured using ABPM, are associated with elevated cardiovascular risk, independent of clinic BP levels, and are a stronger predicator of CVD morbidity and mortality [5, 33], understanding the influence of lifestyle behaviors on out-of-office BP is critical for developing effective prevention approaches. Recent observational and intervention studies [40, 57,58,59,60,61,62,63,64] have examined both self-reported and actigraphically assessed sleep duration, primarily focusing on short sleep, in relation to morning BP and nocturnal dipping, assessed using ABPM and HBPM. Findings generally indicate that a sleep deficit or surfeit may be associated with reduced nocturnal dipping and an elevated morning surge [40, 57,58,59,60,61,62,63,64]. Although existing studies provide a possible link for the heightened risk of CVD associated with disturbances in diurnal BP pattern and the extremes of sleep quantity, most observational studies have limited sample sizes, and there is a paucity of evidence from US populations.
In 1908 Finnish study participants, aged 41–74 years, self-reported sleep duration was examined in relation to home BP, assessed using an automatic oscillometric device [64]. Morning–evening, day-by-day, and morning day-by-day variables of home SBP variability were significantly higher in long sleepers. Further, morning day-by-day SBP, day-by-day DBP, and first–second measurement of home DBP variability variables were higher in short sleepers than in the reference group.
In a Japanese study of 478 adults, morning SBP and DBP on HBPM were compared among those who reported sleeping 7–8 h (mean 7.3 ± 0.3 h) and < 7 h (mean 5.7 ± 4.9 h) [63]. Morning SBP was higher among self-reported short sleepers (117.7 ± 14.9 mmHg vs. 116.9 ± 14.9 mmHg, p < 0.01). However, there was no difference in morning DBP. In a study among 500 elderly French adults, there were no significant differences in 24-h, diurnal, and nocturnal SBP on ABPM by self-reported sleep duration, but sleeping < 6 h and ≥ 8 h compared to 6–8 h was associated with lower prevalence of SBP dippers (58% and 64% vs. 68%, respectively, p = 0.07) [40]. Similarly, findings from a pilot study of Korean-American women demonstrated that those with reduced nocturnal dipping on ABPM were more likely to have a shorter self-reported sleep duration [62]. Consistent with these findings, a Canadian study of 108 normotensive and 417 hypertensive subjects showed that a 1-h decrease in sleep duration was associated with 12% higher odds for reduced nocturnal dipping (< 10% nocturnal SBP fall) (p = 0.04) and 15% higher odds with age per 5-year increment (p = 0.0003) [61]. In this study, each 1-h increment in sleep duration was also associated with 13% higher odds for an elevated morning surge (≥ 18.0 mmHg) (p = 0.02).
In the USA, post hoc analysis of actigraphy and 24-h ABPM data from the Lifestyle Modification in Blood Pressure Lowering Study (LIMBS) of adults, aged 18–80 years, and the Penn Icelandic Sleep Apnea Study (PISA) of adults, aged 40–65 years, was used to evaluate sleep duration in relation to ABPM [60•]. The 24-h mean SBP was 12.7 mmHg higher in LIMBS (p < 0.001; n = 66) and 4.7 mmHg higher in PISA (p = 0.005; n = 153) among participants with self-reported sleep < 7 h vs. ≥ 7 h. In LIMBS, the greatest difference by sleep duration was observed for daytime SBP (148.1 vs. 136.0 mmHg, p < 0.001), although there was also a significant difference in nocturnal SBP (133.4 vs. 124.1 mmHg, p = 0.029), but this was not the case in PISA. In multivariable adjusted models, although there was no significant association between short sleep and nocturnal dipping, a shorter sleep duration was strongly associated with higher 24-h SBP, independent of nocturnal BP and in-office BP.
Intervention Studies
Few studies have examined the effect of sleep restriction or deprivation on out-of-office BP. One of the early reports on sleep deprivation in relation to the morning BP surge was an intervention study in 18 normotensive subjects [59]. Restricting sleep duration to 5 h from 2:00 am to 7:00 am versus sleeping from 11:00 pm to 7:00 am was associated with a decrease in SBP and DBP at dawn before awakening [59]. However, during the morning after the recovery sleep period, SBP significantly increased. While these findings may not be intuitive, the authors explained this decrease in SBP and DBP at dawn as being due to a potential reorganization of the sleep phases in the restricted sleep regimen. The increase in SBP after awakening was explained as a possible consequence of greater sympathetic activation in response to the stress of sleep restriction. Similarly, in a recent Canadian intervention study (n = 30, mean age 26.7 years) that sought to examine acute stress-induced arousal and its association with nocturnal sleep, subjective and objective short sleep duration (< 6 h) derived from sleep diaries and PSG, respectively, were correlated with increased morning BP (r = − 0.45, p < 0.05 and r = − 0.55, p < 0.01, respectively) [58].
Intervention studies also indicate that sleep restriction or deprivation may reduce nocturnal dipping. In a randomized controlled trial of 45 healthy adults, restricting sleep to 4 h/night from 03:00 am to 07:00 am for three nights followed by recovery sleep of 8 h repeated four times in succession resulted in blunted sleep-associated DBP dipping during all four blocks of sleep restriction (p = 0.002), despite intermittent catch-up sleep [54•]. In a randomized cross-over trial in 36 never-treated mild to moderate hypertensive patients, sleep deprivation was associated with higher mean 24-h BP, and this difference was most pronounced at night [57]. Experimental sleep deprivation was associated with reduced nocturnal dipping. BP also significantly increased in the morning after a sleep-insufficient night suggesting that lack of sleep in hypertensive patients may increase sympathetic nervous activity during the night and the following morning leading to elevated BP. Long-term studies are needed to determine how chronic sleep deprivation, restriction, and extension contribute to changes in out-of-office BP.
Sex Differences in Associations of Sleep Duration with Blood Pressure
Sex differences in the influence of sleep duration on BP have been demonstrated and may account for gender differences in CVD risk and outcomes [65•]. Short sleep, assessed primarily by self-report and defined as 5–6 h, appears to be more strongly related to hypertension risk among women [66]. In a meta-analysis by Wang et al., short sleep (≤ 5 h vs. 7 h) was associated with 68% higher risk for hypertension among women (OR (95% CI) 1.68 (1.39–2.03)) but not among men (OR (95% CI) 1.30 (0.93–1.83)) [67]. Similarly, in a meta-analysis of cross-sectional studies by Guo et al. [38], short sleep (≤ 5 h or ≤ 6 h) was associated with 36% higher odds of hypertension among women only (p < 0.001). In contrast, long sleep (≥ 9 h), assessed mostly from self-report, was associated with 12% higher odds for prevalent hypertension in men (p = 0.036) but not in women (p = 0.243) [38].
Emerging evidence from Asian cohorts corroborates findings that short sleep may be more detrimental in women, while long sleep may be more detrimental among men. In the prospective Kangbuk Samsung Health Study of Korean adults [68•], both a decrease in self-reported sleep duration over time and persistently short sleep were associated with an elevated risk of hypertension in women, while an increase in sleep duration was associated with hypertension among men. In particular, a decrease of ≥ 2 h of sleep and an increase of ≥ 2 h of sleep compared to no change in sleep duration were associated with a higher risk of incident hypertension in women (HR (95% CI) 1.46 (1.08–1.98)) and men (HR (95% CI) 1.31 (1.10–1.56)), respectively [68•]. Women with persistently shorter sleep durations compared with those who maintained 7 h of sleep were at greater risk of developing hypertension during the follow-up period. Consistently with these findings, in a rural Chinese cohort, men with the longest sleep duration (≥ 10 h) were at increased risk for hypertension (HR (95% CI) 1.52 (1.25–1.84)), but this association was weaker and only borderline significant in women (HR (95% CI) 1.21 (1.00–1.45)) [69]. In contrast, in a South African study of ~ 1300 adults, longer sleep duration was associated with higher DBP (β = 0.005, p < 0.01) and SBP (β = 0.003, p < 0.05) in females only [70].
In the USA, no differences by sex were observed for the associations of short and long sleep with hypertension risk in the CARDIA study [36], the Sleep and Heart Health Study [34], or the HCHS/SOL sleep study [46•]. However, data from the BRFSS and NHIS [50••] demonstrate that women are more at risk for hypertension associated with self-reported short sleep throughout every decade of life. Women were also at greater risk (~ 50%) for hypertension than men due to self-reported long sleep (≥ 10 h) in the 35–49 and 50–64 year age categories. Other landmark US cohort studies also demonstrate that short sleep may have a detrimental impact on BP in women. In the Nurses’ Health Study (NHS)-I (n = 71,658) and the NHS-II (n = 84,674), women in all age categories who slept ≤ 5 h or 6 h compared to 7 h had up to 25% higher odds for hypertension [27]. However, shorter self-reported sleep duration of ≤ 5 h was associated with a higher incidence of hypertension only in younger women (HR (95% CI) 1.20 (1.09–1.31)) aged < 50 years [27]. Furthermore, up to 21% higher odds for hypertension were observed in middle-aged to older women who reported long sleep. In the Western New York Study (n = 3027; 56.5% female), sleeping < 6 h vs. 6–8 h was associated with 66% higher odds for hypertension in women only [71]. Similarly to the NHS, this association was stronger among pre-menopausal women, in whom short sleep was associated with > 3-fold higher odds for hypertension versus 49% higher risk observed in postmenopausal women who were short sleepers.
Taken together, these findings indicate that women may be more prone to the effects of extremes in sleep duration on hypertension risk and that there may be critical periods over the female life course during which these associations are most pronounced. Additional research is warranted to confirm these associations in studies with objectively assessed sleep and to elucidate the biological mechanisms underlying the observed sex differences.
Racial Differences in Associations of Sleep Duration with Blood Pressure
Sleep deficiency has been proposed as a potential contributor to racial disparities in CVH [72•, 73••]. In a recent AHA statement on CVH in African Americans [73••], insufficient sleep was acknowledged as an adverse health behavior that contributes to elevated cardiovascular risk in African Americans, especially that African Americans may be more vulnerable to short sleep and to its hypertensive effects. In fact, data from the NHIS indicate that African Americans are 41% and 62% more likely than whites to report being short and long sleepers [74]. Similarly, objectively assessed sleep duration using wrist actigraphy was approximately 48 min shorter among African-American adults compared to white adults in the Chicago Area Sleep Study [75•].
In the 2009 NHIS, there was a significant interaction between race/ethnicity and sleep quantity in relation to hypertension risk [76]. While both white and African-American adults with short sleep had greater odds for hypertension compared to those who reported sleeping 6–8 h, African Americans who slept < 6 h and > 8 h were 34% and 37% more likely to report hypertension than their white counterparts, respectively (p < 0.01) [76]. Further, among African Americans with metabolic syndrome (n = 1035; mean age 62 ± 14 years; female 69.2%), those who have resistant hypertension have a higher prevalence of short sleep (26.8% vs. 14.9%, p < 0.001) and 2-fold greater likelihood of being short sleepers [77]. In the longitudinal CARDIA study, objectively assessed sleep duration appeared to mediate the difference between African Americans and whites in DBP change over time [36], and controlling for sleep duration explained 84% of the excess change in DBP exhibited by African-American men compared to white women.
However, in a 2018 study that used data from the BRFSS and NHIS [50••], the association of self-reported short sleep with higher odds for hypertension was consistent across all racial/ethnic groups. On the other hand, the finding that long sleep is associated with higher odds for hypertension was less pronounced among African-American and Other/Multiracial groups.
The mechanisms underlying the association between sleep duration and BP are known to be implicated in the racial disparities in hypertension [72•], although studies of mechanisms have not necessarily examined race-specific effects on biological functions that affect BP level and variability. Potentiated reactivity to stressors, such as sleep deprivation, is likely a main contributor to racial disparities in hypertension [72•]. It has been hypothesized that African Americans have greater cardiovascular reactivity to physical and mental stressors, and due to allostatic load, the cardiovascular hyper-reactivity induced by sleep restriction may be amplified thereby predisposing African Americans to hypertension [72•]. However, in a recent paper, white women exhibited decreased vagally mediated control of the heart during sleep compared to African-American women [78]. Furthermore, the influence of short and long sleep on known hypertension risk factors, including obesity and type 2 diabetes, is also more pronounced among African Americans [1, 79•] [80].
Finally, it is noteworthy that other racial/ethnic minorities may also be more vulnerable to the effects of short and long sleep duration on BP. For instance, data from the 2014 NHIS indicate that very short sleep (< 5 h) was associated with 63% higher risk for hypertension in Native Hawaiian/Pacific Islanders [81], who report having shorter sleep than whites. Additional studies are needed to identify those who are at highest risk for hypertension associated with short or prolonged sleep duration and to understand the psychosocial, behavioral, and biological factors that contribute to racial/ethnic disparities in these relations.
Limitations of Existing Studies and Future Research Directions
The reviewed studies are highly heterogeneous in terms of predictor and outcome assessment and reporting. Although several cohorts now have ancillary sleep studies with subjective and objective measures of sleep, much of the evidence base on sleep duration in relation to BP is derived from studies that relied on subjective sleep duration. However, self-reported sleep data are only moderately correlated with objective sleep and are systematically biased [82]. Further, self-reported measures of sleep were not standardized or assessed similarly across studies, which also limits comparability of findings among studies. Another important dimension to consider is the varying definitions of short and long sleep and of the reference group across the reviewed studies. Some studies considered 6 h, 7 h, or 8 h of sleep as a reference group, while others used ranges such as 6–7 h, 7–8 h, or 6–8 h. Therefore, future studies should collect both objective sleep data with PSG and/or actigraphy and subjective measures of sleep using validated questionnaires and should use definitions for short and long sleep that are consistent with the most up-to-date scientific consensus statements [1, 3].
Another major caveat of the reviewed studies is the varying definitions for high BP and hypertension, which makes comparisons across studies and the interpretation of results difficult. While a number of the studies discussed herein have been published in the past year, the cut-offs used to define hypertension and high clinic BP are not necessarily consistent with the most recent AHA/ACC guidelines [5••]. Most studies used the previous definition of hypertension (SBP/DBP ≥ 140/90 mmHg). Therefore, it is unclear whether associations of short and long sleep with BP would be stronger if the recommended lower cut-off to define hypertension were used. Future studies should examine associations of short and long sleep with hypertension incidence based on the most current definitions. Given that the AHA/ACC statement indicates that out-of-office and self-monitoring of BP measurements may be critical for diagnosing hypertension and assessing long-term cardiovascular risk [5••], prospective studies that collect wrist actigraphy and ABPM data at multiple time points are necessary. These studies would help clarify the association of objective sleep duration with changes in out-of-office BP and the development of abnormal out-of-office BP phenotypes such as masked hypertension or nocturnal hypertension. This would provide essential data that may be used to develop effective prevention and therapeutic approaches.
The influence of sleep on BP level and the diurnal pattern of BP is complex, as several aspects of sleep may play a synergistic role in hypertension etiology. According to the AHA statement on sleep and cardiometabolic health [1••], short sleep, sleep disordered breathing, and insomnia alone or in combination are risk factors for hypertension. Recent evidence from HCHS/SOL suggests that, beyond sleep duration, the timing and regularity of sleep–wake schedules are related to hypertension prevalence and BP level, as lower inter-daily stability and a delayed sleep midpoint were associated with a higher prevalence of hypertension and with higher BP levels [83].
Other identified gaps in the literature on sleep duration in relation to BP pertain to differences in these associations by sex, life stage, and race/ethnicity. The sex- and race-specific effects of sleep curtailment and extension on BP level and the diurnal pattern of BP requires further investigation, as does the assessment of sex- and race-specific variability in associations of sleep with hypertension incidence and ABPM measures in prospective observational studies. A deeper understanding of these associations may provide the basis for sex-specific, racially sensitive, preventive and therapeutic strategies to combat poor sleep habits in order to mitigate the unequal burden of hypertension and CVD in the population. Another important dimension to consider is the role of sleep duration in hypertension etiology across the life course. Emerging studies are suggestive that the adverse cardiovascular consequences of short sleep may start as early as childhood and adolescence [84, 85]. Additional studies, particularly sleep cohort studies that begin in childhood, are warranted to clarify how early life exposure to short or long sleep duration contributes to hypertension risk during adulthood and to identify critical periods across the life course during which interventions targeting sleep habits may be most effective for lowering lifetime cardiovascular risk.
Conclusions
Evidence has continued to accumulate in support of the association between sleep duration and hypertension risk as well as BP level and the diurnal pattern of BP. There are lines of evidence from experimental sleep deprivation studies and population-based epidemiological studies that short sleep is a risk factor for hypertension. Sex, race/ethnicity, and life stage influence the association of sleep duration with hypertension risk. The hypertensive effects of short sleep are most pronounced in women across the life course, but particularly among young women, and in adults of African descent. The role of long sleep in hypertension etiology is less studied, but cross-sectional studies of self-reported sleep duration indicate that long sleep is associated with greater hypertension prevalence. Experimental evidence is limited but suggestive that sleep deprivation increases BP, including out-of-office BP, reduces nocturnal dipping, and increases morning BP, while sleep extension may lower 24-h BP in individuals with prehypertension or stage 1 hypertension.
The observed associations between sleep duration and hypertension indicate that interventions targeting sleep duration could serve as effective primary, secondary, and tertiary preventive measures for hypertension, but critical knowledge gaps remain to be addressed (Table 1). Longitudinal studies that examine the influence of objectively assessed short and long sleep duration on hypertension risk, mean 24-h, awake, and sleep BP, nocturnal dipping, morning BP surge, and night-to-day variability over time are warranted. Furthermore, intervention studies that examine the effects of mild to severe sleep restriction and extension on BP level and the diurnal pattern of BP are needed to establish causality and clarify the sex- and race/ethnicity-specific effects of sleep duration on BP. A better understanding of these relationships will be necessary for developing personalized and public health interventions to address optimal sleep needed to support BP control and ideal CVH.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• St-Onge M, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134(18):e367–86 This statement summarizes the evidence on sleep duration and sleep disorders in relation to cardiometabolic health and indicates that short and long sleep duration are associated with adverse cardiometabolic risk profiles and outcomes.
Institute of Medicine, Committee on Sleep Medicine and Research, Board on Health Sciences Policy. Sleep disorders and sleep deprivation: an unmet public health problem. Washington, DC: National Academies Press; 2006.
Panel CC, Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep. 2015;38(8):1161–83.
Gangwisch JE. A review of evidence for the link between sleep duration and hypertension. Am J Hypert. 2014;27(10):1235–42.
•• Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–248 This guideline statement includes new definitions for hypertension that will influence the ascertainment of hypertension in future epidemiological studies and emphasizes the importance of home blood pressure monitoring and ambulatory blood pressure monitoring for the assessment of cardiovascular risk.
•• Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of ambulatory and home blood pressure monitoring in clinical practice: a narrative review. Ann Intern Med. 2015;163(9):691–700 This review describes the role of ambulatory and home blood pressure monitoring in assessing cardiovascular risk and highlights future areas of research.
de Castro JM. The influence of heredity on self-reported sleep patterns in free-living humans. Physiol Behav. 2002;76(4–5):479–86.
Partinen M, Kaprio J, Koskenvuo M, Putkonen P, Langinvainio H. Genetic and environmental determination of human sleep. Sleep. 1983;6(3):179–85.
National Sleep Foundation. National Sleep Foundation Recommends New Sleep Times. 2015 [cited 2019 Mar 12]. Available from: https://www.sleepfoundation.org/press-release/national-sleep-foundation-recommends-new-sleep-times.
Centers for Disease Control and Prevention. Short Sleep Duration Among US Adults. 2017 [cited 2019 Mar 12]. Available from: https://www.cdc.gov/sleep/data_statistics.html.
National Sleep Foundation. 2010 Sleep in America Poll: Summary of Findings. 2010 [cited 2019 Mar 12]. Available from: https://sleepfoundation.org/sites/default/files/nsaw/NSF%20Sleep%20in%20%20America%20Poll%20-%20Summary%20of%20Findings%20.pdf.
Liu Y. Prevalence of healthy sleep duration among adults—United States, 2014. MMWR. Morbidity and mortality weekly report. 2016;65(6);137–141.
Mallampalli MP, Carter CL. Exploring sex and gender differences in sleep health: a Society for Women’s Health Research report. J Women Health. 2014;23(7):553–62.
Ohayon MM, Reynolds CF III, Dauvilliers Y. Excessive sleep duration and quality of life. Ann Neurol. 2013;73(6):785–94.
Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep. 2014;37(3):601–11.
Patel SR, Sotres-Alvarez D, Castaneda SF, Dudley KA, Gallo LC, Hernandez R, et al. Social and health correlates of sleep duration in a US Hispanic population: results from the Hispanic community health study/study of Latinos. Sleep. 2015;38(10):1515–22.
Lauderdale DS, Knutson KL, Rathouz PJ, Yan LL, Hulley SB, Liu K. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA sleep study. Am J Epidemiol. 2009;170(7):805–13.
• Cespedes EM, Hu FB, Redline S, Rosner B, Alcantara C, Cai J, et al. Comparison of self-reported sleep duration with actigraphy: results from the Hispanic Community Health Study/Study of Latinos Sueño ancillary study. Am J Epidemiol. 2016;183(6):561–73 This study demonstrates that there is a modest correlation between self-reported and objectively assessed sleep in US Hispanics/Latinos.
• Mossavar-Rahmani Y, Weng J, Wang R, Shaw PA, Jung M, Sotres-Alvarez D, et al. Actigraphic sleep measures and diet quality in the Hispanic Community Health Study/Study of Latinos Sueño ancillary study. J Sleep Res. 2017;26(6):739–46 This paper demonstrates that sleep duration is associated with diet quality, highlighting a potential mechanism through which sleep could contribute to hypertension risk.
Loredo JS, Soler X, Bardwell W, Ancoli-Israel S, Dimsdale JE, Palinkas LA. Sleep health in U.S. Hispanic population. Sleep. 2010;33(7):962–7.
Jarrin DC, Ivers H, Lamy M, Chen IY, Harvey AG, Morin CM. Cardiovascular autonomic dysfunction in insomnia patients with objective short sleep duration. J Sleep Res. 2018;27(3):e12663.
Castro-Diehl C, Roux AVD, Redline S, Seeman T, McKinley P, Sloan R, et al. Sleep duration and quality in relation to autonomic nervous system measures: the multi-ethnic study of atherosclerosis (MESA). Sleep. 2016;39(11):1927–40.
Sherwood A, Routledge FS, Wohlgemuth WK, Hinderliter AL, Kuhn CM, Blumenthal JA. Blood pressure dipping: ethnicity, sleep quality, and sympathetic nervous system activity. Am J Hypertens. 2011;24(9):982–8.
Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res. 2014;114(11):1804–14.
Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–50.
Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertens. 2006;47(5):833–9.
Gangwisch JE, Feskanich D, Malaspina D, Shen S, Forman JP. Sleep duration and risk for hypertension in women: results from the nurses’ health study. Am J Hypertens. 2013;26(7):903–11.
Laposky AD, Bass J, Kohsaka A, Turek FW. Sleep and circadian rhythms: key components in the regulation of energy metabolism. FEBS Lett. 2008;582(1):142–51.
Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–54.
St-Onge M, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, et al. Meal timing and frequency: implications for cardiovascular disease prevention a scientific statement from the American Heart Association. Circulation. 2017;135(9):e96–e121.
Goncharuk VD, Van Heerikhuize J, Dai J, Swaab DF, Buijs RM. Neuropeptide changes in the suprachiasmatic nucleus in primary hypertension indicate functional impairment of the biological clock. J Comp Neurol. 2001;431(3):320–30.
Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453–8.
Kawano Y. Diurnal blood pressure variation and related behavioral factors. Hypertens Res. 2011;34(3):281–5.
Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, et al. Association of usual sleep duration with hypertension: the sleep heart health study. Sleep. 2006;29(8):1009–14.
Fang J, Wheaton AG, Keenan NL, Greenlund KJ, Perry GS, Croft JB. Association of sleep duration and hypertension among US adults varies by age and sex. Am J Hypertens. 2012;25(3):335–41.
Knutson KL, Van Cauter E, Rathouz PJ, Yan LL, Hulley SB, Liu K, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med. 2009;169(11):1055–61.
Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens Res. 2013;36(11):985–95.
Guo X, Zheng L, Wang J, Zhang X, Zhang X, Li J, et al. Epidemiological evidence for the link between sleep duration and high blood pressure: a systematic review and meta-analysis. Sleep Med. 2013;14(4):324–32.
Wang Q, Xi B, Liu M, Zhang Y, Fu M. Short sleep duration is associated with hypertension risk among adults: a systematic review and meta-analysis. Hypertens Res. 2012;35(10):1012–8.
Sforza E, Saint Martin M, Barthelemy JC, Roche F. Association of self-reported sleep and hypertension in non-insomniac elderly subjects. J Clin Sleep Med. 2014;10(09):965–71.
Faraut B, Touchette E, Gamble H, Royant-Parola S, Safar ME, Varsat B, et al. Short sleep duration and increased risk of hypertension: a primary care medicine investigation. J Hypertens. 2012;30(7):1354–63.
• Bathgate CJ, Fernandez-Mendoza J. Insomnia, short sleep duration, and high blood pressure: recent evidence and future directions for the prevention and management of hypertension. Curr Hypertens Rep. 2018;20(6):52 This review summarizes the recent evidence on insomnia and blood pressure and indicates that insomnia is a strong candidate to join the list of risk factors for hypertension.
Deng H, Tam T, Zee BC, Chung RY, Su X, Jin L, et al. Short sleep duration increases metabolic impact in healthy adults: a population-based cohort study. Sleep. 2017; 40(10):zsx130
Clark AJ, Salo P, Lange T, Jennum P, Virtanen M, Pentti J, et al. Onset of impaired sleep and cardiovascular disease risk factors: a longitudinal study. Sleep. 2016;39(9):1709–18.
Shivashankar R, Kondal D, Ali MK, Gupta R, Pradeepa R, Mohan V, et al. Associations of sleep duration and disturbances with hypertension in metropolitan cities of Delhi, Chennai, and Karachi in South Asia: cross-sectional analysis of the CARRS Study. Sleep. 2017;40(9).
• Ramos AR, Weng J, Wallace DM, Petrov MR, Wohlgemuth WK, Sotres-Alvarez D, et al. Sleep patterns and hypertension using actigraphy in the Hispanic Community Health Study/Study of Latinos. Chest. 2018;153(1):87–93 This study demonstrates that sleep continuity and daytime napping, but not objectively assessed short sleep duration, are associated with prevalent hypertension in US Hispanics/Latinos.
Basnet S, Merikanto I, Lahti T, Männistö S, Laatikainen T, Vartiainen E, et al. Seasonality, morningness–eveningness, and sleep in common non-communicable medical conditions and chronic diseases in a population. Sleep Sci. 2018;11(2):85–91.
Matsumoto T, Murase K, Tabara Y, Gozal D, Smith D, Minami T, et al. Impact of sleep characteristics and obesity on diabetes and hypertension across genders and menopausal status: the Nagahama study. Sleep. 2018;41.
• Aggarwal B, Makarem N, Shah R, Emin M, Wei Y, St-Onge M, et al. Effects of inadequate sleep on blood pressure and endothelial inflammation in women: findings from the American Heart Association Go Red for Women Strategically Focused Research Network. J Am Heart Assoc. 2018;7(12):e008590 This study demonstrates that prevalent sleep disturbances such as poor sleep quality and insomnia are associated with increased blood pressure and vascular inflammation even in the absence of inadequate sleep duration in women.
•• Grandner M, Mullington JM, Hashmi SD, Redeker NS, Watson NF, Morgenthaler TI. Sleep duration and hypertension: analysis of >700,000 adults by age and sex. J Clin Sleep Med. 2018;14(06):1031–9 This large population-based cohort study provides evidence that both short and long sleep duration are associated with increased hypertension risk across most age groups, but that associations between short sleep and blood pressure were stronger among younger adults and women.
Hruby A, Lieberman HR, Smith TJ. Self-reported health behaviors, including sleep, correlate with doctor-informed medical conditions: data from the 2011 health related behaviors survey of US active duty military personnel. BMC Public Health. 2018;18(1):853.
Robillard R, Lanfranchi PA, Prince F, Filipini D, Carrier J. Sleep deprivation increases blood pressure in healthy normotensive elderly and attenuates the blood pressure response to orthostatic challenge. Sleep. 2011;34(3):335–9.
Sauvet F, Drogou C, Bougard C, Arnal PJ, Dispersyn G, Bourrilhon C, et al. Vascular response to 1 week of sleep restriction in healthy subjects. A metabolic response? Int J Cardiol. 2015;190:246–55.
• Yang H, Haack M, Gautam S, Meier-Ewert HK, Mullington JM. Repetitive exposure to shortened sleep leads to blunted sleep-associated blood pressure dipping. J Hypertens. 2017;35(6):1187–94 This randomized controlled trial indicates that repeated exposure to shortened sleep blunts nocturnal blood pressure dipping, despite intermittent catch-up sleep suggesting that individuals with chronic insufficient sleep may be at increased risk for hypertension due to blunting of sleep-associated blood pressure dipping.
Franzen PL, Gianaros PJ, Marsland AL, Hall MH, Siegle GJ, Dahl RE, et al. Cardiovascular reactivity to acute psychological stress following sleep deprivation. Psychosom Med. 2011;73(8):679–82.
• Haack M, Serrador J, Cohen D, Simpson N, Meier-Ewert H, Mullington JM. Increasing sleep duration to lower beat-to-beat blood pressure: a pilot study. J Sleep Res. 2013;22(3):295–304 This study demonstrates that a 6-week sleep extension intervention among participants with prehypertension or stage 1 hypertension is associated with a decrease in 24-h beat-to-beat systolic blood pressure from pre- to post-intervention visit.
Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am J Hypertens. 1999;12(1 Pt 1):63–8.
Chen IY, Jarrin DC, Ivers H, Morin CM. Investigating psychological and physiological responses to the Trier social stress test in young adults with insomnia. Sleep Med. 2017;40:11–22.
Lusardi P, Mugellini A, Preti P, Zoppi A, Derosa G, Fogari R. Effects of a restricted sleep regimen on ambulatory blood pressure monitoring in normotensive subjects. Am J Hypertens. 1996;9(5):503–5.
• Shulman R, Cohen DL, Grandner MA, Gislason T, Pack AI, Kuna ST, et al. Sleep duration and 24-hour ambulatory blood pressure in adults not on antihypertensive medications. The J Clin Hypertens. 2018;20(12):1712-20 This study demonstrates that short sleep is associated with higher 24-h systolic blood pressure suggesting that adults with shorter sleep duration may benefit from screening with 24-hour ambulatory blood pressure monitoring to promote earlier detection of hypertension.
Friedman O, Shukla Y, Logan AG. Relationship between self-reported sleep duration and changes in circadian blood pressure. Am J Hypertens. 2009;22(11):1205–11.
Suh M, Barksdale DJ, Logan J. Relationships among acculturative stress, sleep, and nondipping blood pressure in Korean American women. Clin Nurs Res. 2013;22(1):112–29.
Kawabe H, Saito I. Does short sleep duration in daily life affect morning home blood pressure? Evaluation in Japanese people. Clin Exp Hypertens. 2008;30(3–4):183–90.
Johansson JK, Kronholm E, Jula AM. Variability in home-measured blood pressure and heart rate: associations with self-reported insomnia and sleep duration. J Hypertens. 2011;29(10):1897–905.
• Makarem N, Aggarwal B. Gender differences in associations between insufficient sleep and cardiovascular disease risk factors and endpoints: a contemporary review. Gender and the Genome. 2017;1(2):80–8 This review summarizes the literature on gender differences in the influence of insufficient sleep on cardiovascular risk factors, including blood pressure.
Dean E, Bloom A, Cirillo M, Hong Q, Jawl B, Jukes J, et al. Association between habitual sleep duration and blood pressure and clinical implications: a systematic review. Blood Press. 2012;21(1):45–57.
Wang Y, Mei H, Jiang YR, Sun WQ, Song YJ, Liu SJ, et al. Relationship between duration of sleep and hypertension in adults: a meta-analysis. J Clin Sleep Med. 2015 Sep 15;11(9):1047–56.
• Kim C, Chang Y, Kang J, Ryu S. Changes in sleep duration and subsequent risk of hypertension in healthy adults. Sleep. 2018;41(11):zsy159 This large population-based study demonstrates that both a decrease in sleep duration and persistently short sleep are associated with greater hypertension risk in young and middle-aged women and men.
Zhang H, Li Y, Mao Z, Liu M, Huo W, Liu R, et al. A dose–response association of night sleep duration with hypertension in a Chinese rural population: the Henan rural cohort study. J Am Soc Hypertens. 2018;12(12):867–79.
Pretorius S, Stewart S, Carrington MJ, Lamont K, Sliwa K, Crowther NJ. Is there an association between sleeping patterns and other environmental factors with obesity and blood pressure in an urban African population? PLoS One. 2015;10(10):e0131081.
Stranges S, Dorn JM, Cappuccio FP, Donahue RP, Rafalson LB, Hovey KM, et al. A population-based study of reduced sleep duration and hypertension: the strongest association may be in premenopausal women. J Hypertens. 2010;28(5):896–902.
• Covassin N, Greene EL, Singh P, Somers VK. Disparities in hypertension among African-Americans: implications of insufficient sleep. Curr Hypertens Rep. 2017;20(7):57 This review summarizes the evidence on the contribution of insufficient sleep to racial disparities in cardiovascular disease and indicates that African Americans may be more vulnerable to the hypertensive effects of sleep deficiency.
•• Carnethon MR, Pu J, Howard G, Albert MA, Anderson CA, Bertoni AG, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136(21):e393–423 This statement reviews racial disparities in cardiovascular health and indicates that there is a growing body of evidence that demonstrates that African Americans are more prone to insufficient sleep and to its adverse effects on cardiovascular risk, including hypertension risk.
Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30(9):1096–103.
• Carnethon MR, De Chavez PJ, Zee PC, Kim KA, Liu K, Goldberger JJ, Ng J, Knutson KL. Disparities in sleep characteristics by race/ethnicity in a population-based sample: Chicago Area Sleep Study. Sleep Med. 2016;18:50–5 This study demonstrates that African Americans are more prone to insufficient sleep compared to whites.
Pandey A, Williams N, Donat M, Ceide M, Brimah P, Ogedegbe G, et al. Linking sleep to hypertension: greater risk for blacks. Int J Hypertens. 2013;2013:1–7.
Rogers A, Necola O, Sexias A, Luka A, Newsome V, Williams S, et al. Resistant hypertension and sleep duration among blacks with metabolic syndrome MetSO. J Sleep Disord Treat Care. 2016;5(4):10.
Hall MH, Middleton K, Thayer JF, Lewis TT, Kline CE, Matthews KA, et al. Racial differences in heart rate variability during sleep in women: the study of women across the nation sleep study. Psychosom Med. 2013;75(8):783–90.
• Jean-Louis G, Youngstedt S, Grandner M, Williams NJ, Sarpong D, Zizi F, et al. Unequal burden of sleep-related obesity among black and white Americans. Sleep Health. 2015;1(3):169–76 This study among US adults demonstrates that African Americans with short sleep duration (≤ 5 h) may be unequally burdened by sleep-related overweight/obesity.
Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep. 2013;36(7):981–90.
Matthews EE, Li C, Long CR, Narcisse M, Martin BC, McElfish PA. Sleep deficiency among native Hawaiian/Pacific Islander, black, and white Americans and the association with cardiometabolic diseases: analysis of the National Health Interview Survey Data. Sleep Health. 2018;4(3):273–83.
Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19(6):838–45.
Abbott SM, Weng J, Reid KJ, Daviglus ML, Gallo LC, Loredo JS, et al. Sleep timing, stability, and BP in the Sueño ancillary study of the Hispanic community health study/study of Latinos. Chest. 2018;155(1):60–8.
Bal C, Ozturk A, Cicek B, Ozdemir A, Zararsiz G, Unalan D, et al. The relationship between blood pressure and sleep duration in Turkish children: a cross-sectional study. J Clin Res Pediatr Endocrinol. 2018;10(1):51–8.
Mezick EJ, Hall M, Matthews KA. Sleep duration and ambulatory blood pressure in black and white adolescents. Hypertension. 2012;59(3):747–52.
Funding
N.M. is supported by an American Heart Association Go Red for Women Strategically Focused Research Network Soter Collaborative Award (grant no. 16SFRN27880000-1). M.A. is supported through 18AMFDP34380732 from the American Heart Association. M.H.H. is supported, in part, through R01 AG047139 from the National Institutes of Aging.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Sleep and Hypertension
Rights and permissions
About this article
Cite this article
Makarem, N., Shechter, A., Carnethon, M.R. et al. Sleep Duration and Blood Pressure: Recent Advances and Future Directions. Curr Hypertens Rep 21, 33 (2019). https://doi.org/10.1007/s11906-019-0938-7
Published:
DOI: https://doi.org/10.1007/s11906-019-0938-7