Abstract
Purpose of Review
To summarize research from the past 2 years on the association between insomnia, short sleep duration, and hypertension and provide a critical analysis of the evidence and suggestions for future directions in this field.
Recent Findings
Evidence indicates that the association between insomnia and elevated blood pressure (BP) or stage 1 and 2 hypertension is stronger in those with chronic insomnia, as compared to those with isolated insomnia symptoms, and primarily found in those with the insomnia with objective short sleep duration phenotype. There is a key gap in ambulatory BP monitoring across the sleep-wake cycle as well as in randomized clinical trials testing the effectiveness of pharmacological or cognitive-behavioral insomnia therapies in lowering BP.
Summary
Insomnia is a strong candidate to join the list of risk factors for hypertension along with obstructive sleep apnea. In the meantime, chronic insomnia should become part of the routine assessment of patients with elevated BP and should be a source for referral, diagnostic evaluation, and treatment, rather than regarded as a symptom of the underlying medical disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
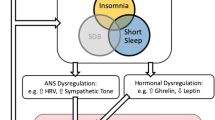
Sleep is an essential physiologic need; therefore, sleep disorders should have adverse consequences on health. Mounting evidence suggests that sleep disorders are associated with increased cardiovascular and cerebrovascular risk. Sleep disordered breathing (SDB), particularly obstructive sleep apnea (OSA), is a well-established risk factor for elevated blood pressure (BP) and clinical hypertension [1, 2]. In contrast, chronic insomnia has not reached this status yet, despite the fact that it is more prevalent than OSA in the general population and clinical practice [3, 4]. Indeed, about 20–30% of the general population reports insomnia symptoms, defined as the occurrence of difficulty falling asleep, difficulty staying asleep, early morning awakening, or nonrestorative sleep at any given time without any chronicity or daytime impairment criteria [3, 4]. Another 8–10% of the population meets criteria for a chronic insomnia disorder, defined as insomnia symptoms occurring at least three nights per week, for at least 3 months, and associated with daytime functioning impairment [3, 4]. Both insomnia symptoms and chronic insomnia are associated with impaired quality of life, adverse occupational outcomes, and are well-established correlates and risk factors for mental health problems, such as depression, anxiety, and other psychiatric disorders [5].
Despite seminal in-lab studies on the association of insomnia with autonomic nervous system activation [6] and clinical observations of its comorbidity with clinical hypertension [7, 8], insomnia is in its infancy of being recognized as a modifiable risk factor for elevated BP and hypertension [1, 9•]. However, emerging evidence over the past decade has refueled the interest of the association of insomnia with hypertension. When discussing the current landscape of insomnia, short sleep duration, and elevated BP, we must consider recently published reviews and meta-analyses [10•, 11•, 12•, 13,14,15], findings that support the existence of a more severe insomnia phenotype [16, 17•], and the most recent evidence of these proposed associations [18,19,20,21,22,23,24,25,26,27,28, 29•]. In the following sections, we briefly review the advances made in the past 2 years on the relationship between insomnia and elevated BP and hypertension. Specifically, we summarize completed research that focused on the association between (1) insomnia and hypertension, (2) short sleep duration and hypertension, and (3) insomnia with short sleep duration and hypertension. This review also presents a discussion of putative mechanisms through which insomnia phenotypes may relate to elevated or dysregulated BP and hypertension. We provide a critical analysis of our knowledge to date and provide suggestions for future directions needed to advance the state of the science in this field, including longitudinal studies with robust and detailed assessments and clinical trials examining the impact of insomnia treatment in reducing its downstream consequences on BP outcomes.
Most Recent Evidence
Insomnia and Hypertension
Among the studies that focused on insomnia and hypertension over the past 2 years, it was most common for researchers to have access to self-reported insomnia symptoms. This is likely due to feasibility issues, as many of the studies were population-based, epidemiologic cohorts. Although information about corresponding daytime functioning impairment is required for an insomnia diagnosis [3, 4], most studies had access to only a few insomnia-related questions focusing on symptom experience and/or frequency [18,19,20,21], with the exception of two studies that captured both insomnia symptoms and daytime difficulties [23, 24]. Additionally, only one study presented evidence based on an ICD-9 diagnosis of insomnia [25].
The studies outlined in Table 1 suggest that adults with self-reported insomnia symptoms [18,19,20,21, 23] or an ICD-9 diagnosis of insomnia [25] have a 15–41% increased odds for hypertension [18,19,20,21] and 52% increased odds for elevated BP [23] compared to those without insomnia symptoms (see Table 1 for risk ratio breakdown). The Center for Cardiometabolic Risk Reduction in South Asia (CARRS; N = 16,287) reported a 34–41% increased risk for hypertension if individuals had insomnia symptoms over the past month [18]. However, the lack of data to control for SDB was problematic, since OSA has long been associated with hypertension (see Ahmad et al. [2] for a review). Cross-sectional findings with older Chinese adults (N = 3176, all ≥ 60 years old) suggested that experiencing insomnia symptoms over the past month was associated with a 29% increased risk of reporting a history of hypertension after controlling for covariates including depression and breathing problems [19]. In a different cross-sectional sample of Chinese adults (N = 8017), insomnia was associated with a 52% increased risk for hypertension (systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg) in adults ages 40–59 years old after controlling for relevant covariates including salt intake and BMI [23]. However, since the authors did not control for SDB or other sleep disorders, it remains unclear how much of this association was truly due to insomnia alone. Longitudinal research from the Finnish Public Sector Study (FPSS; N = 45,647) determined that onset of insomnia symptoms was associated with a 22% higher risk of initiating hypertension medication within 8 years of follow-up compared to those with undisturbed sleep [20]. Use of participants’ electronic medical records allowed researchers the ability to exclude individuals with specific sleep (e.g., OSA) or medical disorders (e.g., coronary heart disease, stroke) as well as control for a variety of other important potential confounders (e.g., smoking, BMI, depression, anxiety). However, their results only reflected individuals who were initiated on antihypertensive medication and not necessarily those who had elevated BP. An Australian prospective study following elderly women across a 3- to 15-year follow-up (N = 10,721, ages 70–75 at baseline) used repeated measures latent class analysis to identify four groups: persistent insomnia symptoms (i.e., having early morning awakening, poor sleep quality, sleep onset difficulties, and sleep maintenance concerns), persistent early morning awakening only, persistent sleep onset difficulties only, and those without any persistent insomnia symptoms. Participants with persistent insomnia symptoms and persistent early morning awakening were 35 and 15% more likely, respectively, to report a history of hypertension than a control group without persistent insomnia symptoms [21]. Unfortunately, this study did not control for SDB or its symptoms, so it is unknown what influence SDB would have had on the associations. Lastly, in a retrospective cohort study using Taiwan’s National Health Insurance Research Database (NHIRD) [25], authors stratified individuals with an ICD-9 diagnosed sleep disorder (n = 14,853) into four categories (insomnia, sleep disturbance, OSA, and others) and compared them to adults without any recorded sleep disorder (n = 29,706). After controlling for the presence of comorbidities, individuals with insomnia had a 21% increased risk of being diagnosed with hypertension than those without a diagnosed sleep disorder [25]. In sum, many of these studies found larger associations between insomnia and hypertension compared to previous meta-analysis (RR = 1.05 [1.01–1.08]) [10•]; however, several studies did not have data to control for SDB, which may have accounted for at least some portion of the association.
Importantly, there were two studies that either found no association [24] or an inverse association [22] between insomnia and hypertension. A Hispanic community sample (N = 2148) found no significant association between self-reported insomnia symptoms and hypertension after controlling for SDB using participants’ apnea-hypopnea index (AHI) derived from home polysomnography (PSG) [24]. Given OSA’s previously established link with hypertension [2], and a recent review suggesting that Hispanics may have a higher prevalence of OSA than non-Hispanic whites [34], it is possible that this sample contained more individuals with at least mild-to-moderate OSA, which accounted for a significant proportion of variance associated with hypertension risk. An inverse relationship between insomnia and BP was found in cross-sectional data from Norway’s Nord-Trøndelag Health Study (HUNT-3; N = 50,806). Self-reported insomnia symptoms were actually associated with lower systolic and diastolic BP after adjusting for established cardiovascular disease risk factors and breathing/snoring problems [22]. The authors proposed that insomnia might offer a protective effect in some subgroups, but that actual differences in BP between those with and without insomnia complaints were small and of little clinical significance. Of note, this is the only study in the literature showing such inverse relationship between insomnia symptoms and BP levels.
In summary, most studies over the past 2 years examining the relationship between insomnia and hypertension used self-reported insomnia symptoms without frequency, chronicity, or daytime impairment data allowing the identification of chronic insomnia disorder [18,19,20,21,22], yet found a 15–41% increased odds for hypertension [18,19,20,21] and 52% increased odds for elevated BP [23] compared to those without insomnia symptoms (see Table 1 for risk ratio breakdown). The magnitude of these associations was much larger than the 5% increased hypertension risk reported in a recent meta-analysis of hypertension incidence in prospective cohort studies [10•]. While most studies were able to control for SDB or its symptoms [19,20,21,22, 24], there were a handful that did not [18, 23, 25], which may have biased the degree of the association detected.
Short Sleep Duration and Hypertension
There were five studies over the past 2 years looking at the relationship between short sleep duration and hypertension [18, 20, 24, 26, 27]. Sleep duration was commonly measured as self-reported habitual total sleep time [18, 20, 26, 27], with the exception of two studies using actigraphy [24] or PSG [27]. The majority of studies found no significant relationship between short sleep duration and increased hypertension risk, with the exception of a large prospective study [26] and a small in-lab experimental study [27] (see Table 1 for risk ratios and associations). The Taiwan-based prospective study recruited and followed healthy adults from 1996 to 2014 (N = 162,121) [26]. Self-reported short sleepers (< 6 h) were 8% more likely to develop elevated BP (systolic BP ≥ 130 mmHg or diastolic BP ≥ 85 mmHg) compared to normal duration sleepers (≥ 6 h) [26], but there was no data to control for SDB, which may have accounted for at least some, if not all, of the association. It is unknown whether the association would have persisted or been stronger for levels of systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg. A small Canadian experimental study (N = 30) using total sleep time derived from sleep diaries and PSG found that, across both subjective and objective reports of sleep, having shorter sleep duration was correlated with increased morning BP [27].
In the past 2 years, two out of five studies [18, 20, 26, 27, 31] found a significant association between short sleep duration and elevated BP [26, 27]. Meta-analytic results reporting on the association between short sleep and hypertension have also been mixed; two meta-analyses reported between 21 and 23% increased risk for hypertension [10•, 15], and one found no significant association [30]. However, emerging evidence has suggested that the combination of insomnia and short sleep duration (< 6 h) is particularly detrimental for cardiometabolic health (see Fernandez-Mendoza [12•] for a recent review) and its association with elevated BP and hypertension reviewed in the following section.
Insomnia with Short Sleep Duration and Hypertension
Some studies appearing over the past 2 years has sought to replicate Vgontzas and colleagues’ [17•] proposal of a more severe insomnia phenotype, characterized by objective short sleep duration < 6 h, physiologic hyperarousal, a chronic course, and significant medical comorbidities, such as hypertension [16, 17•]. Four recent studies [26, 28, 29•, 33•] corroborated the findings in the Penn State Adult Cohort [17•], with one of those studies only providing further evidence for its chronic course but not for its association with hypertension (see Table 1 for risk ratio breakdown) [33•].
Two recent studies showed that individuals with insomnia and short sleep duration (< 6 h) had more than a threefold increased risk for reporting hypertension as a current problem compared to those with insomnia and normal sleep duration (≥ 6 h) [29•] and over a twofold increased risk for being treated with hypertension medication compared to good sleeping controls [28]. Specifically, in a well-characterized sample of adults with chronic insomnia disorder (N = 255), Bathgate et al. [29•] used average total sleep time across two consecutive nights of PSG to classify individuals with insomnia disorder as having either short sleep duration (< 6 h) or normal sleep duration (≥ 6 h). After controlling for numerous potential confounders, including SDB, high cholesterol, diabetes, and depression, adults with insomnia disorder and short sleep duration were 3.59 times more likely to report hypertension as a current medical problem than those with insomnia disorder and normal sleep duration. This was similar to previous findings in the Penn State Adult Cohort, such that individuals with chronic insomnia and objective short sleep duration were 3.5 to 5.1 times more likely to have hypertension (defined based on systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg and/or antihypertensive medication use) [31] and 3.8 times more likely to report a new-onset of a history of hypertension after 7.5 years of follow-up [32] than normal sleeping controls. Additionally, Bathgate et al. [29•] did not observe a significant hypertension risk (OR = 1.13) using short sleep duration (< 6 h) derived from self-reported 2-week sleep diaries. Later that year, research from a large, diverse community sample (N = 3911, 25% Black) found that individuals with insomnia disorder and self-reported short sleep duration (< 6 h) were 2.13 times more likely to report being treated with antihypertensive medication than good sleeping controls, even after excluding individuals at high risk for OSA [28]. When stratifying analyses by race, Black Americans were more likely to have insomnia disorder with self-reported short sleep duration (< 6 h) compared to their non-Hispanic White counterparts [28]. Most importantly, this study highlighted that short sleepers with either current or remitted insomnia disorder had higher prevalence rates of hypertension compared to those with normal sleep duration (current or remitted insomnia) and those who never had insomnia (regardless of sleep duration). Taken together, these data suggested that having the combination of short sleep duration (< 6 h) and insomnia at any point might negatively influence hypertension risk. Moreover, in a large prospective study from Taiwan that was previously described in the “short sleep duration and hypertension” section above (N = 162,121), the authors stratified their analyses by the presence of self-reported insomnia symptoms [26]. Although insomnia symptoms did not significantly modify the association between self-reported short sleep duration and hypertension, the risk of hypertension was significantly elevated for individuals reporting insomnia symptoms and short sleep duration as compared to those reporting insomnia symptoms and normal sleep duration (HR = 1.06). However, the presence of insomnia symptoms was assessed using only one multiple choice question and authors did not have any data on symptoms frequency, chronicity, daytime consequences, or SDB.
Contrary to the three studies above, there was one study that did not find a significant association between insomnia with objective short sleep duration and hypertension. In a German sample of 328 clinically referred insomnia patients previously evaluated at a sleep lab, Johann et al. [33•] found no difference of hypertension risk among those with chronic insomnia disorder and objective short sleep (< 6 h) and those with chronic insomnia disorder and normal sleep duration (≥ 6 h). However, there was a significant difference between the two insomnia subgroups in their insomnia chronicity; those with insomnia and short sleep duration during their first night of PSG reported having insomnia 3.7 years longer than those with insomnia and normal sleep duration [33•], a finding replicating a previous study in the Penn State Adult Cohort [17•]. There were methodological issues that need to be considered when interpreting these data. Even though the authors had access to two consecutive nights of PSG, they analyzed each night independently. As a result, short sleepers from night 1 (n = 148) were a different sample than short sleepers from night 2 (n = 64) when using the same 6-h criterion, and no analyses were conducted by averaging the two consecutive nights. The latter approach may have provided a better marker of in-lab sleep duration than factors in inter-individual and intra-individual variability across nights [29•]. Importantly, the method by which BP was ascertained changed over time and differed across subjects, which may have significantly impacted the estimation of BP levels and any potential inter-individual differences. In fact, the sample had fewer individuals with insomnia and hypertension (31.5%) [33•] compared to the Penn State Adult Cohort (52%); however, the Bathgate et al. study [29•] had 25.5% of people with insomnia and hypertension and did find insomnia with objective short sleep duration to be associated with increased hypertension risk as compared to insomnia with normal sleep duration. Interestingly, previous population-based studies in Germany have suggested a downward trend in BP over the past decade among middle age and older adults [35], which is contrary to the upward trend in the USA [36]. The sleep lab’s archival database of clinically referred patients provided no indication of the original potential referral source that may have been present when patients were referred to and underwent their sleep study. In fact, together with those suffering from SDB and depression, many other individuals were excluded from the analyses such as those with “serious medical conditions,” which may have included those naturally co-occurring with hypertension in those with a more severe form of insomnia. In other words, the Johann et al. sample may have been a more selective group of individuals attending a specialist setting (a psychiatry-based sleep clinic) than those from the Penn State Adult Cohort [17•], which was a randomly selected population-based sample, or the Bathgate et al. sample [29•], which was recruited for a diagnostic reliability study from multiple sleep centers with diverse specialties (e.g., pulmonary, psychiatry, neurology) [37]. Overall, there appears to be methodologically strong evidence that supports the association between insomnia with short sleep duration (< 6 h) and hypertension. Nevertheless, more studies are needed to examine the synergistic effect of having both insomnia disorder and objective short sleep duration on hypertension risk using large, diverse cohorts with a longitudinal design.
Summary of Most Recent Findings
The past years have been replete with international research examining the relationships between insomnia, short sleep duration, and hypertension. Evidence from these recent studies estimated that adults with insomnia have a 15–41% increased odds for hypertension [18,19,20,21] and a 52% increased odds for elevated BP [23] compared to those without insomnia symptoms. This risk was much higher than the 5% increased risk previously found in a 2013 meta-analysis of insomnia and hypertension incidence [10•]. The relationship between short sleep duration and hypertension was weaker than previously reported. Shorter sleep duration was correlated with increased BP [27], and a study that was unable to control for SDB found that short sleep duration increased hypertension risk by 8% [26]. These results diverge from previous meta-analytic findings that short sleep duration was associated with a 21–23% increased risk of hypertension [10•, 15]. Recent evidence tended to support the existence of a more severe insomnia phenotype characterized by short sleep duration and increased hypertension risk. Individuals with both insomnia and short sleep duration (< 6 h) had over a threefold increased risk for reporting hypertension as a current problem compared to those with insomnia and normal sleep duration (≥ 6 h) [29•] and over a twofold increased risk for being treated with hypertension medication compared to good sleeping controls [28]. This corroborates the Penn State Adult Cohort findings, where short sleeping adults with chronic insomnia were 3.5 to 5.1 times more likely to report hypertension [31] and 3.8 times more likely to have incident hypertension [32] than normal sleeping controls. Kalmbach et al. [28] also reported that the prevalence of high BP was highest among short sleeping individuals with current or remitted insomnia, suggesting that having the combination of insomnia with short sleep at any time is a risk factor for hypertension. In contrast, Johann et al. [33•] did not replicate the Vgontzas et al. [17•], Bathgate et al. [29•], and Kalmbach et al. [28] findings but did provide further support to the persistent course of insomnia disorder with objective short sleep duration previously reported.
Given that much of the recent evidence has come from population-based studies, precise measurements of insomnia disorder and sleep duration were not always present. The majority of studies that we have reviewed used self-reported insomnia symptoms with no data on corresponding frequency, chronicity, or daytime functional impairment, which is required to diagnose chronic insomnia [3, 4]. Similarly, it was more common for studies to use self-reported usual sleep duration rather than objective data from actigraphy or PSG. Perhaps the lack of detected associations between short sleep duration and hypertension was the result of using subjectively determined total sleep time, as previous research has suggested that objectively determined total sleep time can better detect hypertension risk than subjective estimates [29•]. It might also reflect previous findings that short sleep duration alone may not warrant clinically significant hypertension risk but that the combination of insomnia with short sleep duration will [31]. Given the well-established link between OSA and hypertension [2], it was advantageous that many studies included SDB as a covariate or exclusionary criteria, particularly those studying the insomnia with short sleep duration phenotype. However, a handful of studies did not have access to this data [18, 21, 26] or did not provide clear evidence of using it as a covariate [25], which challenges the strength of those observed associations. With regard to BP and hypertension measurement, most studies used self-report, daytime BP, or information from medical records. Only one study collected bedtime BP and no studies used ambulatory BP measurements. Previous studies have noted that nocturnal BP is a better predictor of cardiovascular outcomes than daytime BP [38, 39]; therefore, increased use of ambulatory BP measurements, rather than conventional in-office BP, might better capture this phenomenon.
Insomnia Phenotypes
Insomnia is a heterogeneous disorder and the field has tirelessly tried to describe multiple subtypes or phenotypes. However, the diagnostic insomnia subtypes proposed in the past demonstrated such poor reliability and validity that current nosology has abandoned them in favor of a unitary “insomnia disorder” diagnosis [3, 4]. Not surprisingly, self-reported insomnia symptoms of difficulty falling asleep, difficulty staying asleep, or early morning awakening have also not provided reliable and valid subtyping linked to specific underlying pathophysiology or clinical outcomes. One of the reasons for the lack of reliable, valid, and clinically useful insomnia phenotypes is the lack of biomarkers that are specific or more likely to be present in certain but not all individuals with the disorder, which is at odds with other sleep disorders. While PSG, the multiple sleep latency test (MSLT), or actigraphy is indicated for the evaluation and subtyping of patients with SDB, narcolepsy, movement-related disorders, or circadian disorders, they are not required for the assessment and diagnosis of insomnia [3, 4], which may be, in part, because insomnia patients tend to demonstrate a small magnitude of impaired sleep on objective tests such as PSG [40].
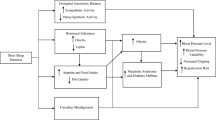
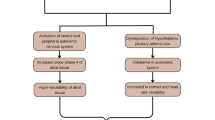
As mentioned above, the hypothesis that insomnia with objective short sleep duration may represent a more biologically severe phenotype of the disorder associated with adverse health outcomes through biological changes (e.g., stress system) was put forward by Vgontzas et al. [17•]. Fernandez-Mendoza [12•] recently identified further support that the association of chronic insomnia with physiologic hyperarousal, cardiometabolic morbidity, and subclinical markers of cardiovascular risk is primarily found in specific phenotypes defined by objective sleep measures. The association of insomnia with subclinical measures such as BP dipping, resting heart rate (HR), HR variability (HRV), or pre-ejection period has been examined primarily in in-lab studies (see Nano et al. [41•] for a recent systematic review). For example, studies have found that individuals with insomnia exhibit increased nighttime systolic BP, morning-evening and day-to-day ambulatory BP variability, and lower systolic BP dipping [11•, 41•]. Furthermore, multiple studies have shown an elevated resting HR or impaired HRV in patients with insomnia, with the latter suggesting a shift of the sympathovagal balance toward a predominance of sympathetic modulation during both wake and nighttime [11•, 41•]. Importantly, in those studies reporting a positive association with impaired HRV, insomnia patients were carefully defined and presented with objective sleep disturbances, particularly short sleep duration [12•, 41•]. However, no study to date has used HRV data to phenotype insomnia and link it to increased cardiometabolic risk. An obvious downside of HRV as a potential phenotyping marker is its lack of specificity to insomnia disorder, as compared to objective/physiologic nighttime sleep or daytime alertness measures. Given that increased daytime sleep latency on the MSLT is primarily found in individuals with insomnia and objective short sleep duration [17•], Li et al. [42] recently showed that insomnia patients with an MSLT greater than 14 min and those with an MSLT greater than 17 min were associated with a 3.3-fold and 4.3-fold increased odds of hypertension, respectively, while insomnia patients with an MSLT lower than 14 min were not significantly associated with hypertension (OR = 1.17). A similar approach has been used to demonstrate neurocognitive deficits in chronic insomnia with increased MSLT latencies but not in those with low or normal MSLT [43]. These studies exemplify how markers of physiologic hyperarousal, such as short nighttime sleep duration or increased daytime alertness, may be useful in phenotyping insomnia and assessing its biological severity.
Thus, a growing literature suggests that the association of insomnia with cardiovascular risk is more pronounced when insomnia is associated with objectively measured short sleep duration or other measures of physiologic arousal such as longer latencies in MSLT [11•]. However, insomnia phenotypes cannot be based solely on their association with clinical outcomes. The insomnia with objective short sleep duration phenotype, as compared to other proposed biomarkers (e.g., MSLT, HRV), has been characterized by the presence of physiologic hyperarousal, as measured by hypothalamic-pituitary-adrenal (HPA) axis and sympatho-adrenal-medullary (SAM) axis activation, increased low-grade inflammation, a chronic natural course, increased cardiometabolic morbidity and mortality, neurocognitive deficits, and increased psychiatric risk independent of cognitive-emotional factors [12•, 17•]. In contrast, the insomnia with objective normal sleep duration phenotype, despite being associated with sleep misperception, anxious-ruminative, depressive traits, and maladaptive coping skills, which play a role in its increased psychiatric risk [44], is not associated with significant HPA/SAM axes activation, cardiometabolic morbidity, mortality or neurocognitive deficits [12].
In summary, data continues to support that specific insomnia phenotypes, particularly insomnia with objective short sleep duration, are at increased cardiometabolic risk, including hypertension. Additionally, evidence suggests that stress system activation, impaired cardiac autonomic modulation, and chronic low-grade inflammation may be underlying mechanisms leading to adverse health outcomes in this insomnia phenotype [11•, 12•].
Future Directions
As reviewed above, evidence shows a relationship between insomnia, elevated BP, and other types of BP dysregulation. The direct translational implication of these findings is that insomnia should become part of the routine assessment of patients with hypertension and should be a source for referral, evaluation, and treatment. However, further research needs to be pursued to move the field forward and answer key questions remaining in the literature.
Both experimental, in-lab studies and large, prospective studies need to improve their assessments of insomnia and BP and subject recruitment. Studies should focus on chronic insomnia disorder based on diagnostic criteria including frequency, chronicity, and daytime impact [3, 4]. However, studies should also consider examining individuals with insomnia symptoms at risk of developing chronic insomnia [45], as such subclinical forms may help elucidate the temporal relationship between the natural evolution of chronic insomnia, BP abnormalities, and the development of hypertension in longitudinal designs. Similarly, future studies should apply current BP guidelines to examine all levels of elevated BP starting at 120 mmHg for systolic BP and clearly identify stage 1 and 2 hypertension [1]. Studies are also needed to disentangle the differences in findings when hypertension is defined based on BP levels vs. self-report of a history of hypertension or use of antihypertensive medication. Given that hypertension does not appear to be a consistent and reliable risk factor for insomnia [45, 46], a plausible explanation for the stronger association between insomnia and self-reported hypertension (i.e., self-report or the use of antihypertensive medication vs. solely BP levels) may be that elevated BP is routinely identified by primary care physicians before patients report insomnia symptoms as a chief complaint, which may be captured in cross-sectional, observational studies. For this purpose, large population-based cohorts using objective measures are needed rather than highly selective volunteers or individuals seeking treatment through specialty routes. Furthermore, in-lab studies should include multiple nights of objective sleep (i.e., PSG), measures of relevant biomarkers of underlying mechanisms (i.e., sympathetic activity, as measured by overnight noradrenergic release or microneurography, HPA axis activity, as measured by plasma, urinary, hair or salivary cortisol levels), and both psychosocial (i.e., perceived stress, anxiety, personality traits) and lifestyle factors (i.e., alcohol, tobacco, diet, physical activity). Moreover, further data needs to be established on the association of insomnia with BP dipping and BP reactivity. With current available methods, in-lab studies may be replaced by more ecologically valid designs including ambulatory monitoring of BP coupled with ambulatory monitoring of sleep via objective sleep testing (e.g., type II home sleep testing or newer actigraphy devices) and standardized sleep diaries. Finally, there is a clear need for longitudinal studies to include enough follow-up time points to allow for closer causal inferences.
Regardless of these research needs, chronic insomnia disorder has become the focus of many professional health organizations. The American College of Physicians (ACP) has recently released a clinical guideline recommending that “all adult patients receive cognitive-behavioral therapy for insomnia (CBT-I) as the initial treatment for chronic insomnia disorder” and that “clinicians use a shared decision-making approach, including a discussion of the benefits, harms, and costs of short-term use of [sleep] medications, to decide whether to add pharmacological therapy in adults with chronic insomnia disorder in whom CBT-I alone was unsuccessful” [47]. This clinical guideline represents a shift in the current treatment of insomnia and disseminates CBT-I as a well-established, first-line, effective treatment. However, the introduction of pharmacological therapy is based on failure to respond to CBT-I, rather than based on matching insomnia treatment modalities to specific phenotypes. This issue applies similarly when studying the relationship between insomnia and elevated BP and hypertension.
There is very limited evidence that insomnia treatments improve BP levels or lower the risk of hypertension. Surprisingly, no RCT to date has assessed whether the most widely used hypnotic medications (e.g., zolpidem, trazodone) lower BP levels. In a recent clinical trial, the addition of the intermediate-acting benzodiazepine estazolam to usual antihypertensive treatment in individuals with insomnia produced significantly greater decreases in systolic BP and diastolic BP levels (− 10.5 and − 8.1 mmHg, respectively) as compared to placebo with antihypertensive treatment as usual (− 3.4 and − 2.7 mmHg, respectively) [48]. The literature on the effects of the current first-line treatment CBT-I on BP levels is equally limited. A recent clinical trial examined the effect of 8 weeks of CBT-I, administered via a web platform (SLEEPIO), on 24-h ambulatory systolic BP levels [49]. Researchers found no statistically significant or clinically meaningful changes as compared to standard care, which consisted of education on vascular risk factors (− 0.9 vs. 0.8 mmHg, respectively) [49]. Interestingly, Bathgate et al. [50] recently reported that individuals with chronic insomnia and objective short sleep duration have a blunted response to CBT-I, while individuals with chronic insomnia and objective normal sleep duration show high response and remission rates with CBT-I. Future RCTs should be designed to test the effectiveness of pharmacological and behavioral therapies for insomnia in reducing BP levels, particularly in individuals with insomnia and elevated BP and focusing on the insomnia with objective short sleep duration phenotype. It is likely that combined CBT-I plus pharmacological treatment, particularly those agents that downregulate the arousal and stress systems (e.g., trazodone, doxepin, suvorexant), in the most severe insomnia phenotype would show the most significant and clinically relevant BP effects; however, this hypothesis needs yet to be tested.
Conclusions
Evidence has continued to accumulate supporting the association of insomnia with elevated BP or stage 1 and 2 hypertension and that this association is stronger or primarily found in the objective short sleep duration phenotype. Beyond OSA, an established risk factor for elevated BP and hypertension, insomnia should become part of the routine assessment of patients with elevated BP and should be a reason for referral, diagnostic evaluation, and targeted treatment. In-lab and large, longitudinal studies should examine the association between insomnia and BP using objective ambulatory measures of sleep and BP throughout the 24-h sleep-wake cycle, including BP dipping. Future studies should follow-up individuals with insomnia and elevated BP or other subclinical BP levels (e.g., non-dipping, hypertensive reactivity) and study their long-term cardiovascular outcomes. RCTs on individuals with insomnia and hypertension, including elevated BP as well as stage 1 and 2 hypertension, are needed to test the effectiveness of insomnia treatments on lowering BP levels, particularly in those with insomnia and objective short sleep duration. These next steps will most likely lead to the use of objective measures of sleep in the assessment, phenotyping, and treatment-matching of patients with insomnia in order to provide targeted treatments that expand beyond sleep outcomes, such as cardiovascular health.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017; https://doi.org/10.1016/j.jacc.2017.11.006.
Ahmad M, Makati D, Akbar S. Review of and updates on hypertension in obstructive sleep apnea. Int J Hypertens. 2017;2017:1848375:1–13. https://doi.org/10.1155/2017/1848375.
American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien: Author; 2014.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: Author; 2013.
Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28(11):1457–64.
Monroe LJ. Psychological and physiological differences between good and poor sleepers. J Abnorm Psychol. 1967;72(3):255–64.
Kales A, Kales JD. Evaluation and treatment of iInsomnia. New York: Oxford University Press; 1984.
Taylor DJ, Lichstein KL, Durrence HH. Insomnia as a health risk factor. Behav Sleep Med. 2003;1(4):227–47. https://doi.org/10.1207/s15402010bsm0104_5.
• St-Onge MP, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134(18):e367–e86. https://doi.org/10.1161/cir.0000000000000444. This scientific statement supports sleep quality, including insomnia, and short sleep duration as potential risk factors for cardiometabolic health, including hypertension.
• Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens Res. 2013;36(11):985–95. This is the only available meta-analysis to date that has examined the association of insomnia with hypertension
• Hall M, Fernandez-Mendoza J, Kline CN, Vgontzas A. Insomnia and health. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 6th ed. New York: Elsevier Academic Press; 2016. p. 794–803. This is a comprehensive review in a handbook of references for clinicians and researchers on the association of insomnia with adverse cardiovascular and metabolic health outcomes.
• Fernandez-Mendoza J. The insomnia with short sleep duration phenotype: an update on it's importance for health and prevention. Curr Opin Psychiatry. 2017;30(1):56–63. https://doi.org/10.1097/yco.0000000000000292. This paper brielfy reviews the 2013-2017 evidence, beyond cardiometabolic outcomes, on the insomnia with objective short sleep duration phenotype
Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol. 2014;21(1):57–64. https://doi.org/10.1177/2047487312460020.
Thomas SJ, Calhoun D. Sleep, insomnia, and hypertension: current findings and future directions. J Am Soc Hypertens: JASH. 2017;11(2):122–9. https://doi.org/10.1016/j.jash.2016.11.008.
Guo X, Zheng L, Wang J, Zhang X, Zhang X, Li J, et al. Epidemiological evidence for the link between sleep duration and high blood pressure: a systematic review and meta-analysis. Sleep Med. 2013;14(4):324–32. https://doi.org/10.1016/j.sleep.2012.12.001.
Vgontzas AN, Fernandez-Mendoza J. Insomnia with short sleep duration: nosological, diagnostic, and treatment implications. Sleep Med Clin. 2013;8(3):309–22. https://doi.org/10.1016/j.jsmc.2013.04.009.
• Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17(4):241–54. This review first established the underlying pathophysiologic and phenotyping model of insomnia with objective short sleep duration
Shivashankar R, Kondal D, Ali MK, Gupta R, Pradeepa R, Mohan V, Kadir MM, Narayan KMV, Tandon N, Prabhakaran D, Peasey A Associations of sleep duration and disturbances with hypertension in metropolitan cities of Delhi, Chennai, and Karachi in South Asia: cross-sectional analysis of the CARRS study. Sleep 2017;40(9). https://doi.org/10.1093/sleep/zsx119.
Wang YM, Song M, Wang R, Shi L, He J, Fan TT, et al. Insomnia and multimorbidity in the community elderly in China. J Clin Sleep Med. 2017;13(4):591–7. https://doi.org/10.5664/jcsm.6550.
Clark AJ, Salo P, Lange T, Jennum P, Virtanen M, Pentti J, et al. Onset of impaired sleep and cardiovascular disease risk factors: a longitudinal study. Sleep. 2016;39(9):1709–18. https://doi.org/10.5665/sleep.6098.
Leigh L, Hudson IL, Byles JE. Sleep difficulty and disease in a cohort of very old women. J Aging Health. 2016;28(6):1090–104. https://doi.org/10.1177/0898264315624907.
Hauan M, Strand LB, Laugsand LE. Associations of insomnia symptoms with blood pressure and resting heart rate: the HUNT study in Norway. Behav Sleep Med. 2016;1–21. https://doi.org/10.1080/15402002.2016.1228651.
Wang Y, Jiang T, Wang X, Zhao J, Kang J, Chen M, et al. Association between insomnia and metabolic syndrome in a Chinese Han population: a cross-sectional study. Sci Rep. 2017;7(1):10893. https://doi.org/10.1038/s41598-017-11431-6.
Ramos AR, Weng J, Wallace DM, Petrov MR, Wohlgemuth WK, Sotres-Alvarez D, et al. Sleep patterns and hypertension using actigraphy in the Hispanic community health study/study of Latinos. Chest. 2018;153(1):87–93. https://doi.org/10.1016/j.chest.2017.09.028.
Lin CL, Liu TC, Lin FH, Chung CH, Chien WC. Association between sleep disorders and hypertension in Taiwan: a nationwide population-based retrospective cohort study. J Hum Hypertens. 2017;31(3):220–4. https://doi.org/10.1038/jhh.2016.55.
Deng HB, Tam T, Zee BC, Chung RY, Su X, Jin L et al. Short sleep duration increases metabolic impact in healthy adults: a population-based cohort study. Sleep 2017;40(10). https://doi.org/10.1093/sleep/zsx130.
Chen IY, Jarrin DC, Ivers H, Morin CM. Investigating psychological and physiological responses to the Trier Social Stress Test in young adults with insomnia. Sleep Med. 2017;40:11–22. https://doi.org/10.1016/j.sleep.2017.09.011.
Kalmbach DA, Pillai V, Arnedt JT, Drake CL. DSM-5 insomnia and short sleep: comorbidity landscape and racial disparities. Sleep. 2016;39(12):2101–11. https://doi.org/10.5665/sleep.6306.
• Bathgate CJ, Edinger JD, Wyatt JK, Krystal AD. Objective, but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39(5):1037–45. https://doi.org/10.5665/sleep.5748. This study replicated the finding that insomnia with objective short sleep duration is associated with increased hypertension risk in an independent sample of individuals with chronic insomnia disorder
Wang Q, Xi B, Liu M, Zhang Y, Fu M. Short sleep duration is associated with hypertension risk among adults: a systematic review and meta-analysis. Hypertens Res. 2012;35(10):1012–8. https://doi.org/10.1038/hr.2012.91.
Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–7.
Fernandez-Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela-Bueno A, Basta M, et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60(4):929–35.
• Johann AF, Hertenstein E, Kyle SD, Baglioni C, Feige B, Nissen C, et al. Insomnia with objective short sleep duration is associated with longer duration of insomnia in the Freiburg Insomnia Cohort compared to insomnia with normal sleep duration, but not with hypertension. PLoS One. 2017;12(7):e0180339. https://doi.org/10.1371/journal.pone.0180339. This study could not replicate the finding that insomnia with objective short sleep duration is associated with increased hypertension risk in an independent sample of clinically-referred patients with chronic insomnia disorder
Hnin K, Mukherjee S, Antic NA, Catcheside P, Chai-Coetzer CL, McEvoy RD, et al. The impact of ethnicity on the prevalence and severity of obstructive sleep apnea. Sleep Med Rev. 2018; https://doi.org/10.1016/j.smrv.2018.01.003.
Neuhauser H, Diederichs C, Boeing HB, Felix S, Jünger C, Lorbeer R, et al. Hypertension in Germany: data from seven population-based epidemiological studies (1994–2012). Dtsch Arztebl Int. 2016;113(48):809–15. https://doi.org/10.3238/arztebl.2016.0809.
Benjamin EJ, Virani SS, Callaway CW, Chang AR, Cheng S, Chiuve SE, et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. In: Circulation, vol. 137; 2018. p. e67–e492.
Edinger JD, Wyatt JK, Stepanski EJ, Olsen MK, Stechuchak KM, Carney CE, et al. Testing the reliability and validity of DSM-IV-TR and ICSD-2 insomnia diagnoses. Results of a multitrait-multimethod analysis. Arch Gen Psychiatry. 2011;68(10):992–1002.
Conen D, Bamberg F. Noninvasive 24-h ambulatory blood pressure and cardiovascular disease: a systematic review and meta-analysis. J Hypertens. 2008;26(7):1290–9. https://doi.org/10.1097/HJH.0b013e3282f97854.
Fan HQ, Li Y, Thijs L, Hansen TW, Boggia J, Kikuya M, et al. Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. J Hypertens. 2010;28(10):2036–45. https://doi.org/10.1097/HJH.0b013e32833b49fe.
Baglioni C, Regen W, Teghen A, Spiegelhalder K, Feige B, Nissen C, et al. Sleep changes in the disorder of insomnia: a meta-analysis of polysomnographic studies. Sleep Med Rev. 2014;18(3):195–213. https://doi.org/10.1016/j.smrv.2013.04.001.
• Nano MM, Fonseca P, Vullings R, Aarts RM. Measures of cardiovascular autonomic activity in insomnia disorder: a systematic review. PLoS One. 2017;12(10):e0186716. https://doi.org/10.1371/journal.pone.0186716. This systematic review summarizes the state-of-the-art in the association of insomnia with diverse indices of cardiac autonomic modulation
Li Y, Vgontzas AN, Fernandez-Mendoza J, Bixler EO, Sun Y, Zhou J, et al. Insomnia with physiological hyperarousal is associated with hypertension. Hypertension. 2015;65(3):644–50.
Edinger JD, Means MK, Krystal AD. Does physiological hyperarousal enhance error rates among insomnia sufferers? Sleep. 2013;36(8):1179–86. https://doi.org/10.5665/sleep.2882.
Fernandez-Mendoza J, Shea S, Vgontzas AN, Calhoun SL, Liao D, Bixler EO. Insomnia and incident depression: role of objective sleep duration and natural history. J Sleep Res. 2015;24:390–8. https://doi.org/10.1111/jsr.12285.
Fernandez-Mendoza J, Vgontzas AN, Bixler EO, Singareddy R, Shaffer ML, Calhoun SL, et al. Clinical and polysomnographic predictors of the natural history of poor sleep in the general population. Sleep. 2012;35(5):689–97. https://doi.org/10.5665/sleep.1832.
Singareddy R, Vgontzas AN, Fernandez-Mendoza J, Liao D, Calhoun S, Shaffer ML, et al. Risk factors for incident chronic insomnia: a general population prospective study. Sleep Med. 2012;13(4):346–53. https://doi.org/10.1016/j.sleep.2011.10.033.
Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians management of chronic insomnia disorder in adults. Ann Intern Med. 2016;165(2):125–33. https://doi.org/10.7326/M15-2175.
Li Y, Yang Y, Li Q, Yang X, Wang Y, Ku WL, et al. The impact of the improvement of insomnia on blood pressure in hypertensive patients. J Sleep Res. 2017;26(1):105–14. https://doi.org/10.1111/jsr.12411.
McGrath ER, Espie CA, Power A, Murphy AW, Newell J, Kelly C, et al. Sleep to lower elevated blood pressure: a randomized controlled trial (SLEPT). Am J Hypertens. 2017;30(3):319–27. https://doi.org/10.1093/ajh/hpw132.
Bathgate CJ, Edinger JD, Krystal AD. Insomnia patients with objective short sleep duration have a blunted response to cognitive behavioral therapy for insomnia. Sleep 2017;40(1). https://doi.org/10.1093/sleep/zsw012.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Fernandez-Mendoza reports grants from American Heart Association. Dr. Bathgate reports grants from Merck, grants from Phillips/Respironics, and personal fees from myStrength Digital Health, outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Sleep and Hypertension
Rights and permissions
About this article
Cite this article
Bathgate, C.J., Fernandez-Mendoza, J. Insomnia, Short Sleep Duration, and High Blood Pressure: Recent Evidence and Future Directions for the Prevention and Management of Hypertension. Curr Hypertens Rep 20, 52 (2018). https://doi.org/10.1007/s11906-018-0850-6
Published:
DOI: https://doi.org/10.1007/s11906-018-0850-6