Abstract
Purpose of Review
Obstructive sleep apnea (OSA) and hypertension are two phenomena deeply linked together and, although a causal relationship has been suggested, a recent meta-analysis showed only a very modest effect of OSA treatment on blood pressure (BP). However, a vast number of randomized controlled trials published so far share some limitations, mainly of methodological nature: neither OSA nor BP is always assessed in a standardized way. Moreover, compliance with OSA treatment is often sub-optimal making the results of these trials difficult to interpret.
Recent Findings
Recent studies have shown that antihypertensive drugs can reduce BP more than OSA treatment, showing a better compliance profile and very few side effects.
Summary
Considering the importance of reducing the overall cardiovascular risk of OSA patients, a more careful management of patient’s antihypertensive medication could allow a better BP control also in this condition. In addition, greater efforts should be made to improve patient’s acceptance of OSA treatment with the aim of improving their compliance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The relationship between obstructive sleep apnea (OSA) and hypertension (HT) has been extensively investigated in the past years and represents one of the main and strongest pathophysiological mechanisms that link cardiovascular diseases and sleep-disordered breathing (SDB), which includes a wide spectrum of breathing alterations during sleep ranging from respiratory-related arousals (RERAs) and hypopneas to the most severe forms of central or obstructive sleep apneas.
In fact, while the association between OSA and other cardiovascular risk factors such as diabetes and dyslipidemia seems weaker, albeit already supported by evidence [1], the association between OSA and HT appears to be more consistent and bi-directional in nature [2], having been confirmed in different populations [3]. It is still a matter of debate, however, whether OSA and HT are only associated with each other, an association possibly mediated by comorbidities such as obesity or hyperaldosteronism, or, on the contrary, OSA is one of the causes of blood pressure (BP) elevation.
Indeed, it is important to highlight that in order to demonstrate the occurrence of a causal link between OSA and HT, the treatment of OSA should not only reduce BP but also improve the HT-related consequences such as hypertension-mediated target organ damage and the frequency of cardiovascular complications such as stroke, heart failure, myocardial infarction, and death. A favorable impact of OSA treatment on cardiovascular outcomes has not been unequivocally demonstrated so far, and this is one of the reasons for the current uncertainty on whether OSA might be considered a causal factor for the development of hypertension over time.
To add even more complexity to the problem, identification of OSA requires an objective diagnostic assessment that involves polysomnographic examinations (full polysomnography or cardiorespiratory monitoring) which are variably and/or not frequently performed in the clinical management of hypertension, leading to a lack of diagnostic standardization. Furthermore, BP is a biological parameter that varies consistently within minutes, hours, and days. Thus, performing only office BP measurements, as done in many randomized controlled trials and in routine daily practice, without considering BP variability over time, does not allow to clearly define OSA-related BP phenotypes, which often include an increase in nighttime BP.
Lastly, BP response to OSA treatment might depend on the treatment choice, an issue which should be carefully addressed given the many different therapeutic options available such as positive airway pressure (PAP) devices, mandibular advancement devices (MAD) devices, weight loss, surgery, and electrical stimulation. With regard to PAP devices, which are believed to be the most effective treatment available, settings and type of ventilator can have an impact on BP response to this kind of treatment [4].
For all these reasons, despite a large number of studies published in the field, it is still a matter of debate whether treating OSA could help reduce the patients’ cardiovascular risk associated with HT. Also because of such an uncertainty, it is still unclear whether HT is actually caused by OSA, or whether the two conditions are only associated due to the presence of comorbidities, such as obesity. This uncertainty has not allowed so far to issue clear-cut recommendations for including OSA treatment in cardiovascular prevention.
In this review, we aim to discuss the most important aspects of the relationship between OSA and HT including the latest research in the field together with expert opinions and personal considerations.
OSA and HT: a Complex Relationship
The occurrence of fluctuations in BP during episodes of intermittent partial or complete airway obstructions at night has been reported since the first studies by Coccagna [5] and colleagues and later confirmed by Guilleminault and colleagues at Stanford University [6].
While these authors focused initially on right heart hemodynamics, it appeared clearer at a later stage that OSA has a more relevant impact on systemic arterial hypertension rather than on pulmonary hypertension.
In fact, a series of longitudinal studies performed in different populations confirmed the association between OSA and hypertension and suggested such an association to be characterized by a proportional dose-response-like link, with an increasing apnea-hypopnea index (AHI), which is the number of apneas plus hypopneas per hour of sleep, corresponding to an increased odds ratio for hypertension [7]. However, other trials such as the Sleep Heart Health Study [8] and the study by Cano-Pumarega et al. [9] were not able to confirm a causal role of OSA in hypertension development, after accounting for confounders.
The potential pathophysiological mechanisms behind this association are possibly intertwined together but the most relevant ones seem to be intermittent hypoxia and sympathetic activation [10, 11], with the accompanying reduction in arterial baroreflex sensitivity [12, 13].
Intermittent hypoxia, caused by repetitive airway obstructions, on one side promotes the peripheral chemoreceptor stimulation which causes an enhanced sympathetic activation, and on the other side, it contributes to the production of reactive oxygen species (ROS), activates systemic inflammation, and ultimately impairs endothelial function [14].
The activation of the sympathetic branch of the autonomic nervous system is the consequence not only of intermittent hypoxia but also of arousals from sleep and of increased pleural pressure swings which stimulate pulmonary stretch receptors. As a confirmation of this hypothesis, several studies have shown that patients with OSA have a greater catecholamine production [15, 16] and exhibit an increased sympathetic neural traffic measured by means of microneurography [17].
The main consequences of the increased sympathetic discharge are on one side the intermittent release of dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline) which have positive inotropic, chronotropic, and dromotropic effects. On the other side, the sympathetic activation stimulates the renin-angiotensin-aldosterone system and in particular an increased production of angiotensin II promoting vasoconstriction in venous and arterial walls smooth muscles, and an enhanced aldosterone production [10].
Although all these processes explain nocturnal hypertension in patients with sleep apnea, it is still unclear why there might be also a carry-over effect of hypertension during the daytime. Indeed, daytime hypertension is common in OSA but as these patients share often many common risk factors for hypertension such as obesity, disentangling the effect of OSA on nighttime versus daytime BP is very difficult.
Effects of OSA Treatments on BP
One of the challenges of the interaction between OSA and BP is that the efficacy of treatments currently available for sleep-disordered breathing is very much dependent on patients’ compliance and adherence to the prescribed interventions. In fact, compared with other chronic diseases where the main treatment is administered orally in the form of capsules or pills, in order to treat OSA effectively, patients must wear either a mask or an oral appliance.
Continuous Positive Airway Pressure
Since its discovery in the 1980s, continuous positive airway pressure (CPAP) is by far the most effective treatment for OSA [18].
CPAP is made of a mask, either with nasal or full-face application, connected by a plastic tube to a ventilator. It works by delivering positive air pressure in order to keep the airway patent at night with a pneumatic splint. Treatment with CPAP restores normal breathing and improves nighttime and daytime symptoms such as snoring, choking at night, and daytime sleepiness.
One of the first randomized controlled studies by Engelmann and colleagues [19] highlighted the potentials and also the possible problems of the CPAP-induced BP-lowering effect. In fact, in a small sample of hypertensive patients treated with CPAP for 3 weeks with good compliance, the authors showed that in the whole group, BP was not reduced at follow-up when compared with placebo. Interestingly, a post hoc analysis showed that persons with non-dipping BP had a significant reduction in mean ambulatory BP when compared with their counterparts with dipping BP. This suggests that CPAP can be effective only in a selected subgroup of patients.
Since the 1990s, several randomized controlled studies have been published on the same topic and the results have consistently shown that CPAP use, when compared with a passive control group, determines a significant although small drop in BP (systolic 2.6 ± 0.6 mmHg, diastolic 2.0 ± 0.4 mmHg, p < 0.001) which is even more pronounced when nocturnal BP is considered [20].
Although a net BP change of a few millimeters of mercury does not seem much from a clinical perspective, it nevertheless corresponds to a considerable reduction in stroke mortality [21].
Furthermore, withdrawal studies by the group of Kohler and collaborators [22] confirmed that when CPAP treatment is discontinued, relapse of symptoms is accompanied by a clinically relevant increase in BP.
Oral Appliances
Similarly to CPAP, oral appliances (OA) can reduce sleep-disordered breathing by moving the jaw and the tongue forward, thereby preventing pharyngeal collapse. The first randomized controlled studies investigating the effect of OA on BP were published in 2004: Gotsopoulos and colleagues [23] showed that in 61 patients diagnosed with OSA (AHI > 10 events/h) on polysomnography, the use of mandibular advancement splint reduced 24-h diastolic, but not systolic, BP (by 1.8 ± 0.5 mmHg) compared with the control group using a sham device. Interestingly, no changes in nocturnal BP were present after interventions.
Afterwards, several studies confirmed these findings until the very recent randomized controlled trial published by Gagnadoux et al. [24]. They randomized 150 patients with severe OSA and no overt cardiovascular disease to either effective mandibular advancement device or to a sham device for 2 months. Surprisingly, they did not find any difference between groups in terms of ambulatory BP and reactive hyperemia index, a validated measurement of endothelial function. It is important to note that baseline BP values were within the normal range, again posing the question as to whether the degree of BP elevation at baseline matters more that OSA severity in studies aimed at assessing the BP effects of OSA treatment.
Pitfalls and Limitations of the Main Trials
Since the 1990s, more than 220 randomized controlled studies have been published on the effect of OSA treatment. Only in half of them BP was assessed and analyzed, however, with most of the studies measuring office BP and only less than 10 studies assessing ambulatory BP.
This methodological aspect is crucial given that OSA patients exhibit increased BP variability when compared with non-OSA subjects, making office BP less reliable and possibly not representative of the real patient’s BP profile [25].
In addition to this, it is well known that ambulatory BP better predicts cardiovascular outcomes in hypertensive patients as compared with office BP; its assessment thus represents a preferable approach particularly in patients with sleep-disordered breathing [26].
From a methodological standpoint, other confounding factors must be considered, including the methodology of OSA diagnosis. Although most studies assessed patients with full polysomnography, which represents the gold standard for OSA diagnosis, in other studies, patients were investigated through the use of portable devices or nocturnal oxymeters only. Although these differences are perhaps less critical, it should be noted that portable devices or oxymeters could have missed arousals which are known to determine sympathetic activations and BP surges during apneic events.
On the contrary, recent studies have stressed the importance of the OSA-related hypoxic burden on the development of cardiovascular disease suggesting that not only the frequency but also the depth and duration of sleep-related upper airway obstructions are important disease-characterizing features [27].
In this context, a more thorough assessment of the relationship between hypoxia and blood pressure surges, for instance via a triggered nocturnal BP monitoring method based on an oxygen-trigger function that initiates a BP measurement when the patient’s oxygen saturation falls below a variable threshold, could help better defining the impact of OSA-related hypertension on cardiovascular consequences [28].
Another methodological aspect which deserves to be discussed involves the control groups, given that a huge heterogeneity exists among the studies on OSA treatment published so far. In fact, many studies have included in the placebo arm patients on sham CPAP, represented by either a CPAP machine with low or no pressures or by CPAP machines with drilled holes on the mask used, to allow for an adequate air exchange with the environment. Other studies used tablets as a placebo or compared the effects of specific OSA treatment with those of standard usual care. All these methodological differences are responsible for a significant heterogeneity among trials, making it difficult to compare their results in meta-analyses and systematic reviews. Lastly, in conducting trials on OSA, as in all studies comparing a medical device with placebo, it is barely impossible to blind both patients and investigators to the treatment allocation. As suggested by Djavadkhani and colleagues [29], although patient blinding may be possible with the lack of full disclosure, investigator blinding is unlikely to be achieved, again posing the question as to whether the assessment of the effects of CPAP treatment might be biased.
Lastly, it is worth noting that many trials in the field of OSA and hypertension did not consider the role of important confounders such as the non-pharmacological treatment for high blood pressure. In fact, weight loss, but most of all low sodium intake diet, has not always been accounted as confounders. In particular, a change in sodium intake can have profound effects on BP even in the short term.
Potential Predictors of OSA Treatment Effects on BP
Studies on CPAP withdrawal [22] and a recent meta-analysis [20] pointed out that severity of OSA was associated with a higher BP rise in response to CPAP withdrawal and with a greater net BP decrease in patients treated with CPAP, respectively. However, studies focusing on the determinants of CPAP effect on BP are lacking.
Among CPAP-related factors, patients’ compliance and adherence remain the main predictors of its BP-lowering effects, as confirmed in large randomized controlled trials [30]. The type of PAP device does not seem to matter with regard to the effect on BP [31]. However, a recent report from the European Sleep Apnea Database showed that fixed CPAP use was associated with a lower decline of kidney function when compared with auto-adjusting CPAP [32].
Other CPAP-related issues can represent not only the cause of low compliance, such as mask discomfort or leaks, but also a cause of increased BP, such as excessive CPAP pressures. Xiao and colleagues demonstrated that higher CPAP pressures are associated with breathlessness and activation of expiratory muscles in patients on CPAP [33] and the same group showed that this was associated with increases in both BP and BP variability [34]. It is therefore of crucial importance ensuring a careful CPAP titration in obese subjects in particular. Among patient-related factors, some studies found that both BMI at baseline and improvement in excessive daytime sleepiness were predictors of BP fall following CPAP therapy [35]. Interestingly, the same group found that there was also a correlation between the fall in 24-h BP and the fall in pulse rate (r = 0.44, p < 0.0001) as confirmed in another cohort of patients [36]. These data suggest somehow that the degree of sympathetic activation at the time of CPAP initiation could predict the improvement in BP control at follow-up [37].
It is worth mentioning that the higher BP values at baseline, the more prominent the BP change after CPAP treatment could be, suggesting perhaps a “regression to the mean” confounding phenomenon. In patients with resistant hypertension and OSA, the BP fall observed on treatment was greater in terms of absolute values when compared with the change seen in non-resistant hypertensive patients: in patients with resistant hypertension, 24-h diastolic BP dropped by 3.2 mmHg (95% CI, 1.0 to 5.4; p = .005) while no differences were seen for systolic 24-h BP [30]. Recent studies have emphasized the importance of assessing patients with OSA also by means of 24-h ambulatory blood pressure monitoring (ABPM), as this might be helpful to predict those who benefit more of CPAP treatment in terms of BP reduction. Castro-Grattoni showed in a small sample of patients that nocturnal hypertension, circadian BP pattern, and nighttime heart rate could be clinical predictors of BP response to CPAP, further supporting the usefulness of 24-h ABPM for OSA patients before treatment initiation [38•].
What if BP Medications Work better?
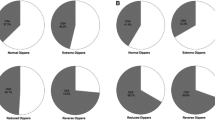
All the issues related to OSA treatment, such as low compliance and potential hypertensive effects due to device intolerance and associated sleep disruption, raise the question of why we would need to seek the BP-lowering effects of such devices when we could obtain the same result by simply optimizing the dose or the number of BP medications (Fig. 1).
Comparison of the net negative changes in blood pressure (OSA treatment vs no OSA treatment) resulting from the 4 latest published meta-analysis (lower part of the figure) with the negative changes in blood pressure observed in 3 randomized trials comparing CPAP vs antihypertensive treatment (upper part of the figure). SBP, systolic blood pressure; DBP, diastolic blood pressure. Asterisk indicates the difference in office BP change (bosentan-CPAP), p value NS for both SBP and DBP. # indicates the difference in 24-h BP change (valsartan-CPAP), p values < 0.001 for SBP and 0.002 for DBP. $ indicates the difference in office BP change (acetazolamide-CPAP), p values n/a
Several studies tried to address this issue by using different drugs in order to counteract the known molecular and pathophysiologic mechanisms linking OSA with hypertension. Joyeux-Faure and colleagues [39•] for example tested bosentan, an endothelin receptor antagonist, given that patients with OSA exhibit high levels of endothelin-1 (ET1), one of the most potent vasoconstrictors in nature [40]. They showed that bosentan did not modify 24-h BP but only reduced office BP, concluding that ET1 blockade does not play a major role in the treatment of OSA-related hypertension. In this context, it should also be noted that measuring ET1 levels is rather challenging given its short half-life. Indeed, other authors focused on the precursors of ET1 showing more convincing results on their role in OSA-related hypertension [41].
Pepin and collaborators [42] focused on angiotensin receptor blockers. This was done because, in OSA, the renin-angiotensin-aldosterone system is hyperactivated through angiotensin II type 1 receptors [43]. They randomized 23 hypertensive patients with OSA to receive valsartan 160 mg or CPAP treatment for 8 weeks according to a crossover study design. These investigators found that valsartan induced a fourfold higher decrease in mean 24-h BP than CPAP in untreated hypertensive patients with OSA. Although a BP change was seen also in patients treated with CPAP, there was a difference of − 7.0 mmHg (95% confidence interval, − 10.9 to − 3.1 mmHg; p < 0.001) in favor of drug therapy, which again poses the question of whether patients with OSA and hypertension deserve a more careful titration of their antihypertensive drugs. An even better BP reduction was obtained by combining valsartan and CPAP treatment, suggesting that improved BP control in OSA patients might benefit from this kind of combination treatment.
Additionally, there are data suggesting that antagonism of mineralcorticoid receptors by spironolactone reduces AHI, in particular in patients with resistant hypertension [1].
Eskandari and colleagues [44••] approached the problem from a different perspective testing acetazolamide (AZT) in patients with OSA. Acetazolamide is a carbonic anhydrase inhibitor which modulates BP in conditions of hypoxia. Indeed, our group demonstrated that the hemodynamic changes and central and peripheral BP rise induced by the acute exposure to high altitude hypoxia can be effectively counteracted by treatment with acetazolamide [45].
Eskandari and colleagues explored the same biological effects of AZT in patients with OSA characterized by cyclic intermittent hypoxia. They randomized 13 male patients with hypertension and moderate-to-severe OSA to receive either AZT, CPAP, or AZT plus CPAP for 2-week periods. They showed that AZT alone and AZT plus CPAP, but not CPAP alone, reduced not only office mean arterial pressure but also aortic systolic BP and augmentation index, assessed by radial artery tonometry.
Although this study suffers from some limitations such as a small sample size, it clearly points out that carbonic anhydrase inhibition might constitute a potential target for drug therapy in patients with sleep apnea and comorbid hypertension.
Conclusions and Perspectives
In the era of precision medicine, more studies are needed to better understand the metabolic and hemodynamic profiles of patients with OSA and hypertension before starting treatment. In the attempt of better understanding the predictors of BP response to CPAP, the Spanish Sleep Network group managed to find a group of differential micro-ribonucleic acids (miRNA) that were associated with a favorable BP response to CPAP [46].
This could be the direction where to drive the future efforts of the scientific community to understand which patients are more likely to benefit from OSA treatment to reduce their BP values. While waiting for further progress in this direction, from a practical standpoint, the current approach to explore how to assess the cardiovascular effects of OSA treatment should focus on the results of studies evaluating the predictive power of some of the promising markers in this field, such as nocturnal heart rate at baseline, according to a longitudinal prospective design, in order to provide clinicians with simple tools for their daily practice. Moreover, based on the available evidence, a more accurate evaluation of the patient’s BP profile by means of 24-h ABPM and an appropriate titration of antihypertensive drugs might represent effective approaches to help further reduce the cardiovascular risk profile of OSA patients.
Last but not least, clinical and experimental observations in patients with hypertension strongly suggest that better results can be obtained in hypertension control and cardiovascular risk reduction through an early start of antihypertensive treatment, before organ damage develops and before hypertension might become resistant [47]. This is also likely to apply to the treatment of OSA patients, in whom CPAP effects on BP might be more evident when OSA is diagnosed early in the clinical history of this disease and when treatment is started promptly.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Newman AB, Nieto FJ, Guidry U, Lind BK, Redline S, Pickering TG, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;154(1):50–9.
Friedman O, Bradley TD, Chan CT, Parkes R, Logan AG. Relationship between overnight rostral fluid shift and obstructive sleep apnea in drug-resistant hypertension. Hypertension. 2010;56(6):1077–82.
Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–8.
Parati G, Lombardi C, Hedner J, Bonsignore MR, Grote L, Tkacova R, et al. Position paper on the management of patients with obstructive sleep apnea and hypertension: Joint recommendations by the European Society of Hypertension, by the European Respiratory Society and by the members of European COST (Cooperation in Scientific and Technological research) ACTION B26 on Obstructive Sleep Apnea. J Hypertens. 2012;30(4):633–46.
Coccagna G, Mantovani M, Brignani F, Parchi C, Lugaresi E. Continuous recording of the pulmonary and systemic arterial pressure during sleep in syndromes of hypersomnia with periodic breathing. Bull Physiopathol Respir. 1972;8(5):1159–72.
Guilleminault C, Tilkian A, Dement WC. The sleep apnea syndromes. Annu Rev Med. 1976;27:465–84.
Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307(20):2169–76.
O'Connor GT, Caffo B, Newman AB, Quan SF, Rapoport DM, Redline S, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009;179(12):1159–64.
Cano-Pumarega I, Duran-Cantolla J, Aizpuru F, Miranda-Serrano E, Rubio R, Martinez-Null C, et al. Obstructive sleep apnea and systemic hypertension: longitudinal study in the general population: the Vitoria Sleep Cohort. Am J Respir Crit Care Med. 2011;184(11):1299–304.
Bisogni V, Pengo MF, Maiolino G, Rossi GP. The sympathetic nervous system and catecholamines metabolism in obstructive sleep apnoea. J Thorac Dis. 2016;8(2):243–54.
Parati G, Lombardi C, Narkiewicz K. Sleep apnea: epidemiology, pathophysiology, and relation to cardiovascular risk. Am J Physiol Regul Integr Comp Physiol. 2007;293(4):R1671–83.
Bonsignore MR, Parati G, Insalaco G, Marrone O, Castiglioni P, Romano S, et al. Continuous positive airway pressure treatment improves baroreflex control of heart rate during sleep in severe obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2002;166(3):279–86.
Parati G, Di Rienzo M, Bonsignore MR, Insalaco G, Marrone O, Castiglioni P, et al. Autonomic cardiac regulation in obstructive sleep apnea syndrome: evidence from spontaneous baroreflex analysis during sleep. J Hypertens. 1997;15(12 Pt 2):1621–6.
Iturriaga R, Oyarce MP, Dias ACR. Role of carotid body in intermittent hypoxia-related hypertension. Curr Hypertens Rep. 2017;19(5):38.
Fletcher EC, Miller J, Schaaf JW, Fletcher JG. Urinary catecholamines before and after tracheostomy in patients with obstructive sleep apnea and hypertension. Sleep. 1987;10(1):35–44.
Gilardini L, Lombardi C, Redaelli G, Mattaliano P, Fanari P, Cornacchia M, et al. Effect of continuous positive airway pressure in hypertensive patients with obstructive sleep apnea and high urinary metanephrines. J Hypertens. 2018;36(1):199–204.
Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98(8):772–6.
Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1(8225):862–5.
Engleman HM, Gough K, Martin SE, Kingshott RN, Padfield PL, Douglas NJ. Ambulatory blood pressure on and off continuous positive airway pressure therapy for the sleep apnea/hypopnea syndrome: effects in “non-dippers”. Sleep. 1996;19(5):378–81.
Fava C, Dorigoni S, Vedove FD, Danese E, Montagnana M, Guidi GC, et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysis. Chest. 2014;145(4):762–71.
Palmer AJ, Bulpitt CJ, Fletcher AE, Beevers DG, Coles EC, Ledingham JG, et al. Relation between blood pressure and stroke mortality. Hypertension. 1992;20(5):601–5.
Schwarz EI, Schlatzer C, Rossi VA, Stradling JR, Kohler M. Effect of CPAP withdrawal on BP in OSA: data from three randomized controlled trials. Chest. 2016;150(6):1202–10.
Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial. Sleep. 2004;27(5):934–41.
Gagnadoux F, Pepin JL, Vielle B, Bironneau V, Chouet-Girard F, Launois S, et al. Impact of mandibular advancement therapy on endothelial function in severe obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine Conference: American Thoracic Society International Conference, ATS. 2017;195(no pagination).
Marrone O, Bonsignore MR. Blood-pressure variability in patients with obstructive sleep apnea: current perspectives. Nat Sci Sleep. 2018;10:229–42.
O'Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31(9):1731–68.
Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J. 2018. https://doi.org/10.1093/eurheartj/ehy624.
Shirasaki O, Kuwabara M, Saito M, Tagami K, Washiya S, Kario K. Development and clinical application of a new technique for detecting ‘sleep blood pressure surges’ in sleep apnea patients based on a variable desaturation threshold. Hypertens Res. 2011;34(8):922–8.
Djavadkhani Y, Marshall NS, D'Rozario AL, Crawford MR, Yee BJ, Grunstein RR, et al. Ethics, consent and blinding: lessons from a placebo/sham controlled CPAP crossover trial. Thorax. 2015;70:265–9.
Martinez-Garcia MA, Capote F, Campos-Rodriguez F, Lloberes P, Diaz de Atauri MJ, Somoza M, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310(22):2407–15.
Marrone O, Salvaggio A, Bue AL, Bonanno A, Riccobono L, Insalaco G, et al. Blood pressure changes after automatic and fixed CPAP in obstructive sleep apnea: relationship with nocturnal sympathetic activity. Clin Exp Hypertens. 2011;33(6):373–80.
Marrone O, Cibella F, Pepin JL, Grote L, Verbraecken J, Saaresranta T, et al. Fixed but not autoadjusting positive airway pressure attenuates the time-dependent decline in glomerular filtration rate in patients with OSA. Chest. 2018;154(2):326–34.
Xiao S, Bastianpillai J, Ratneswaran C, Pengo MF, Luo Y, Jolley CJ, et al. Continuous positive airway pressure and breathlessness in obese patients with obstructive sleep apnea: a pilot study. Sleep. 2016;39(6):1201–10.
Ratneswaran C, Pengo MF, Xiao S, Luo Y, Rossi GP, Polkey MI, et al. The acute effect of continuous positive airway pressure titration on blood pressure in awake overweight/obese patients with obstructive sleep apnoea. Blood Press. 2018:1–9.
Robinson GV, Langford BA, Smith DM, Stradling JR. Predictors of blood pressure fall with continuous positive airway pressure (CPAP) treatment of obstructive sleep apnoea (OSA). Thorax. 2008;63(10):855–9.
Pengo MF, Drakatos P, Kosky C, Williams A, Hart N, Rossi GP, et al. Nocturnal pulse rate and symptomatic response in patients with obstructive sleep apnoea treated with continuous positive airway pressure for one year. J Thorac Dis. 2014;6(6):598–605.
Lombardi C, Parati G, Cortelli P, Provini F, Vetrugno R, Plazzi G, et al. Daytime sleepiness and neural cardiac modulation in sleep-related breathing disorders. J Sleep Res. 2008;17(3):263–70.
• Castro-Grattoni AL, Torres G, Martinez-Alonso M, Barbe F, Turino C, Sanchez-de-la-Torre A, et al. Blood pressure response to CPAP treatment in subjects with obstructive sleep apnoea: the predictive value of 24-h ambulatory blood pressure monitoring. Eur Respir J. 2017;50(4). Finding predictors of BP response to CPAP is of crucial importance. In this paper, the authors showed that nocturnal hypertension, circadian BP pattern, and nighttime heart rate could be clinical predictors of BP response to CPAP.
• Joyeux-Faure M, Jullian-Desayes I, Pepin JL, Cracowski JL, Baguet JP, Tamisier R, et al. Comparison of continuous positive airway pressure and bosentan effect in mildly hypertensive patients with obstructive sleep apnoea: A randomized controlled pilot study. Respirology. 2016;21(3):546–52. In this randomized controlled trial, authors tested the effect of bosentan on blood pressure in patients with OSA. They showed no significant changes in blood pressure suggesting that endothelin-1 blockade does not play a major role in treatment of OSA-related hypertension.
Phillips BG, Narkiewicz K, Pesek CA, Haynes WG, Dyken ME, Somers VK. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens. 1999;17(1):61–6.
Jordan W, Reinbacher A, Cohrs S, Grunewald RW, Mayer G, Ruther E, et al. Obstructive sleep apnea: plasma endothelin-1 precursor but not endothelin-1 levels are elevated and decline with nasal continuous positive airway pressure. Peptides. 2005;26(9):1654–60.
Pepin JL, Tamisier R, Barone-Rochette G, Launois SH, Levy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;182(7):954–60.
Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension. 1999;34(2):309–14.
•• Eskandari D, Zou D, Grote L, Hoff E, Hedner J. Acetazolamide reduces blood pressure and sleep-disordered breathing in patients with hypertension and obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2018;14(3):309–17. First study assessing the effect of acetazolamide in OSA patients in a randomized controlled fashion showing that inhibition of carbonic anhydrase can not only ameliorate BP profile but also improve vascular stiffness and sleep-disordered breathing.
Parati G, Revera M, Giuliano A, Faini A, Bilo G, Gregorini F, et al. Effects of acetazolamide on central blood pressure, peripheral blood pressure, and arterial distensibility at acute high altitude exposure. Eur Heart J. 2013;34(10):759–66.
Sanchez-de-la-Torre M, Khalyfa A, Sanchez-de-la-Torre A, Martinez-Alonso M, Martinez-Garcia MA, Barcelo A, et al. Precision medicine in patients with resistant hypertension and obstructive sleep apnea: blood pressure response to continuous positive airway pressure treatment. J Am Coll Cardiol. 2015;66(9):1023–32.
Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 3. Effects in patients at different levels of cardiovascular risk--overview and meta-analyses of randomized trials. J Hypertens. 2014;32(12):2305–14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Sleep and Hypertension
Rights and permissions
About this article
Cite this article
Parati, G., Pengo, M.F. & Lombardi, C. Obstructive Sleep Apnea and Hypertension: Why Treatment Does Not Consistently Improve Blood Pressure. Curr Hypertens Rep 21, 30 (2019). https://doi.org/10.1007/s11906-019-0935-x
Published:
DOI: https://doi.org/10.1007/s11906-019-0935-x