Abstract
Purpose of Review
Many of the risk factors for heart disease have recently been shown to develop during childhood such as left ventricular hypertrophy and fibrous plaque lesions. As risk for cardiovascular disease in children and adolescents has risen, sleep duration has decreased, and inadequate sleep in children and adolescents has been found to be associated with cardiovascular disease risk. The aims of this manuscript are to provide an updated systematic review of the literature assessing sleep, hypertension, and cardiovascular risk and evaluate the strength of the evidence based on the available research.
Recent Findings
A systematic review was conducted using six databases from January 1, 2015 through March 9, 2018. We sought studies which looked at the relationship between sleep duration, sleep timing, or sleep quality and outcome variables of hypertension, inflammation, obesity, glucose or insulin, and lipids in children and adolescents. We found 24 studies which met our criteria. Nine studies included hypertension as an outcome variable; fifteen included obesity; thirteen included glucose or insulin; eight included lipids; and three included measures of inflammation.
Summary
The existing literature on sleep and cardiovascular disease in children and adolescents is limited and relatively weak. Only one RCT was identified, and the overwhelming majority of studies had a high risk of bias. The strongest evidence of an association with sleep is with obesity, hypertension, and insulin sensitivity. Further research using more standardized methods and objective measures is needed to determine if a causal relationship truly exists between sleep and cardiovascular risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart disease is the primary killer of adult men and women in the USA [1], and risk factors for heart disease have been shown to develop during childhood. Specifically, research demonstrated the presence of fibrous plaque lesions at autopsy examination in the coronary arteries of 33% of 16- to 20-year-old adolescents who died of accidental causes [2]. Left ventricular hypertrophy, which is an independent risk factor for cardiovascular disease in adults, has also been shown to be present in childhood. Additionally, rates of hypertension and pre-hypertension have been increasing among children adolescents since around 1990 [3, 4].
One of the strongest predictors of hypertension in young adults is obesity in childhood and adolescence [5]. Over the last 30 years, the prevalence of obesity in children and adolescents has more than tripled [6]. The increased prevalence of obesity has also led to an increase in cardiovascular risk factors, including type 2 diabetes, hypertension, dyslipidemia, inflammation, as reflected in markers such as interlukin-6 and C-reactive protein, and atherosclerotic cardiovascular disease [7, 8]. One study found that 70% of obese children and adolescents had at least one risk factor for cardiovascular disease, including high blood pressure and cholesterol [7].
As risk for cardiovascular disease and obesity rates have risen, sleep duration has decreased [9]. Inadequate sleep in children and adolescents has been found to be associated with cardiovascular disease risk. Epidemiological studies have shown that short sleep duration in adolescents and adults is associated with excessive body fat [10]. Additionally, a recent meta-analysis reports an association between sleep and weight [11], with every added hour of sleep in adolescents associated with a 9.0% decrease in obesity risk [12].
The association between other cardiovascular disease risk factors and sleep has not received as much attention as obesity, but there is some evidence suggesting that they may also be related. Disturbed or short sleep in adults has recently been found to be associated with glucose intolerance and insulin resistance, and a reduction in acute insulin response to glucose was demonstrated [13]. In a healthy sample of adolescent males, insulin resistance was found to be 65% higher after three nights of sleep restriction, and obese adolescents with short sleep were found to have increased fasting insulin and insulin resistance [14]. Additionally, a recent review of research hypothesized that changes in sleep may also be associated cardiovascular disease through inflammatory mechanisms. They suggest that sleep loss leads to endothelial dysfunction, which activates pro-inflammatory cytokines associated with cardiovascular disease [15].
Because the presence of risk factors for cardiovascular disease has more clearly been identified in children and adolescents over the last 20 years, research assessing contributing factors is still emerging. Therefore, the relationship between sleep, hypertension, and cardiovascular risk is not yet clear. Matthews and colleagues conducted an enumerative review of the PubMed and PsychInfo databases for articles published between 2011 and 2014 that assessed the relationship between sleep and cardiovascular risk in children and adolescents. They found that strength of the evidence varied based on the risk factor and predominately found studies using cross-sectional study designs [16••]. Building on the previous enumerative review, the aims of this manuscript are to provide an updated systematic review of the literature assessing sleep, hypertension, and cardiovascular risk and evaluate the strength of the evidence based on the available research.
Methods
Systematic Review of the Literature
The following databases were searched for available articles from January 1, 2015 through March 9, 2018: PubMed, EMBASE, PsychINFO, Scopus, CINAHL, and Cochrane. This review was registered with the PROSPERO registry: CRD42018090760 and can be viewed at http://www.crd.york.ac.uk/PROSPERO.
Inclusion Criteria
-
1.
Humans
-
2.
English language articles
-
3.
Studies had to measure sleep duration, sleep quality, timing of sleep, sleep architecture, or daytime sleepiness
-
4.
Outcomes had to include one of the following: blood pressure, pulse pressure, mean arterial pressure, BMI, BMI z-score, BMI percentile, waist circumference, abdominal circumference, percent body fat, fat mass, glucose, insulin, lipids, C-reactive protein, or interlukin-6
-
5.
Study population was limited to participants 21 years or younger
-
6.
Published between January 1, 2015 and the search date of March 9, 2018.
Exclusion Criteria
Studies which evaluated broad lifestyle interventions were excluded as the unique effect of sleep could not be evaluated separately. We also excluded studies of participants with sleep apnea or other sleep disorders and studies of participants with comorbid diseases (e.g., asthma, cystic fibrosis, epilepsy).
Study Search Terms
An example of the search syntax used in PubMed is as follows: Child [mh] OR adolescent [mh] OR child [tiab] OR children [tiab] OR adolescen* [tiab] OR teenager* [tiab] OR girls [tiab] OR boys [tiab] OR paediatric* [tiab] OR “Pediatrics” [Mesh] OR “Adolescent Medicine” [Mesh] OR “Adolescent Health” [Mesh] OR “Child Health” [Mesh] OR “Adolescent Behavior” [Mesh] OR “Child Behavior” [Mesh] AND Sleep [mh] OR sleep* [tiab] OR “Sleep Wake Disorders” [Mesh] OR insomnia* [tiab] OR hypersomn* [tiab] OR somnolence [tiab] OR narcolepsy [tiab] OR syssomnia* [tiab] AND Cardiovascular Disease [mh] OR cardiovascular [tiab] OR hypertens* [tiab] OR prehypertens* [tiab] OR “Blood Pressure” [Mesh] OR blood-pressure* [tiab] OR metabolic diseases [mh] OR cardiometabolic [tiab] OR metabolic [tiab].
Filters applied were: English language.
Study Selection
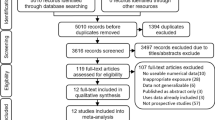
See the PRISMA diagram in Fig. 1 for the search and study selection process. Studies were screened by removing duplicates and removing any non-English language articles that were not screened out by filters applied to the original search. All three authors evaluated articles by title and abstract to determine if they met inclusion/exclusion criteria before full articles were retrieved. Full text articles were reviewed by all three authors.
Risk of Bias Assessment
A risk of bias assessment was completed for all included studies. Two authors (ADF, LE) independently reviewed each paper and evaluated areas of potential risk of bias. Given that the overwhelming majority of studies included in this review were observational studies, risk of bias was assessed using the RTI Item Bank. The RTI Item Bank was developed as a tool for identifying sources of bias and confounding in observational studies. The RTI Item Bank assesses selection, performance, detection and attrition biases, confounding, selective outcome reporting, and overall quality of a study. This approach aims to offer an assessment of how confident (low, medium, or high) the reviewer is that the observed effect in a study is close to the true effect. Non-randomized/observational studies are assumed to have low confidence (i.e., high risk of bias), but may be upgraded if they exhibit features which merit an increase in reviewers’ confidence as outlined in the instructions in the RTI Item Bank [17]. Discrepancies in ratings were discussed between the two authors until consensus was reached.
Results
Results of Literature Search
Our initial aim was to only include randomized controlled trial studies in the review in an effort to better understand the causal relationship between sleep and cardiovascular risk in children and adolescents. However, since only one randomized controlled trial was found [18••], we expanded our criteria to include any study assessing the relationship between sleep and cardiovascular risk in children and adolescents. Twenty-four unique studies from over 10 countries were found that met all search criteria, and populations included both representative population samples and samples of overweight and obese children and adolescents. Several of the studies reported results for more than one of our outcome variables. We found nine studies that assessed the relationship between sleep and hypertension [19,20,21, 22•, 23,24,25,26,27,28]. Fifteen studies were found that assessed the relationship between sleep and obesity including variables of BMI, BMI z-score, BMI percentile, percent body fat, and waist and abdominal circumference [19,20,21, 22•, 23,24,25, 27,28,29,30,31,32,33,34,35]. Thirteen studies evaluated the relationship between sleep and glucose or insulin [18••, 20, 21, 22•, 29, 33,34,35,36,37,38,39,40], eight evaluated sleep and lipids [20, 21, 22•, 23, 32,33,34,35, 39], and three assessed sleep and inflammation [20, 41, 42].
Results of Sleep and Hypertension
Nine studies examined blood pressure or risk for hypertension (Tables 1). Five cross-sectional studies found a significant negative association between sleep duration and blood pressure [20, 21, 23, 25, 27]. However, Anjuo et al. only found a significant association between sleep and blood pressure in African Surinamese and not for other ethnicities [19]. Navarro and colleagues also found a significant association between short sleep and pulse pressure and mean arterial pressure in ages 7–16 [25]. Peach and colleagues reported a negative association between sleep duration on school nights and risk for hypertension. In Derks and colleagues’ longitudinal study, they found that sleep duration at 2 months of age was significantly associated with systolic blood pressure, but this relationship was no longer present at 6 years [22•]. Another longitudinal study reported a significant gender interaction in which longer sleep duration was associated with greater systolic blood pressure in females and lower levels of both systolic and diastolic blood pressure in males [26]. Only one study found no significant association between sleep duration and blood pressure [24].
Results of Sleep and Obesity
Of the fifteen studies that assessed sleep and obesity, thirteen found a significant relationship (Table 2). Eight cross-sectional studies reported a significant negative association between sleep duration and BMI, BMI z-score, or BMI percentile [19,20,21, 25, 27, 32, 34, 35] while one cross-sectional study found that greater BMI z-score was associated with later weekend bedtime and greater shift in bedtime between weeknights and weekends [31]. Deacon and colleagues conducted a multi-staged cluster survey and found a negative association between sleep duration and BMI z-score [30]. Additionally, Derks and colleagues reported that sleep duration at age 2 months significantly predicted BMI at age 6 years [22•]. Four studies reported a negative relationship between sleep duration and waist circumference [20, 21, 25, 32], and a negative relationship was found between sleep quality and abdominal circumference in Gonzaga and colleagues’ cross-sectional study [23]. Three studies also found a significant relationship between sleep duration and fat percentage [25, 32, 34].
While the majority of the studies found a significant association among sleep and obesity, two studies found no evidence of a relationship. A cross-sectional study by Morita et al. found no significant association between sleep duration and BMI, and a cohort study found no significant associations between sleep duration and BMI or waist circumference [24, 33].
Results of Sleep and Glucose and Insulin
Results of the studies assessing sleep and glucose and insulin are mixed (Table 3). Zhu and colleagues found a negative association between glucose and sleep duration, sleep efficiency, and percent of total sleep time in stage 3 [40]. However, three other studies found no relationship between sleep duration and glucose [18••, 33, 39]. However, one study demonstrated that higher salivary glucose levels were a positive partial mediator in the relationship between waist circumference and bedtime [29].
Of the four studies that evaluated sleep and insulin, three found no significant association [18••, 20, 22•]. Sluggett and colleagues found that short sleepers were more likely to have hyperinsulinemia, but the association was no longer significant after controlling for age, sex, chronic disease, education, and income [35].
The results of studies assessing sleep and insulin sensitivity are heterogeneous. Two studies reported the greatest HOMA-IR levels among children and adolescents who did not receive adequate sleep, one in a normative sample and one in a sample with obesity [38, 39]. Rudnicka and colleagues found a negative association between HOMA-IR and sleep duration [34]. However, Dorenbos and colleagues found a negative association between HOMA-IR and sleep duration and efficiency among a sample of adolescent girls with overweight and obesity but not boys, and they accounted for 59 and 58%, respectively, of the variance in HOMA-IR [36]. Another study reported a negative association between HOMA-IR and time spent in stage 3 sleep suggesting that the relationship may vary by sleep architecture [37]. Conversely, two studies reported a positive relationship between sleep and insulin sensitivity. One study found that sleep deprivation was associated with lower HOMA-IR [21], and another found that lower sleep duration, sleep efficiency, and time spent in stage 3 sleep were positively associated with insulin sensitivity and insulin secretion–sensitivity index-2 [40].
Results of Sleep and Lipids
Results on the association between sleep and cholesterol are heterogeneous (Table 4). Sleep duration was found to be negatively associated with HDL in later childhood by Plumptre and colleages [33]. Another study found an interaction with age in which HDL was highest among those that slept between 9 and 10 h in 10–13-year olds, but in 14–15-year olds, it was highest among those that slept less than 8 h on average [39]. Derks and colleagues reported that shorter sleep at 6 months was associated with higher HDL, but this association was not present at 6 years after adjusting for BMI [22•]. Conversely, normal weight adolescents who were sleep deprived have also been found to have significantly lower HDL, but there was no effect for adolescents who were overweight or obese [21]. A positive association between sleep and HDL was also reported by Li et al. [32]. Three cross-sectional studies found no association between sleep duration and HDL [20, 34, 35]. None of the five studies which assessed the relationship between sleep and triglycerides found a significant effect [20, 21, 33, 34, 39].
Results of Sleep and Inflammation
Only three studies measured the relationship between sleep and inflammation (Table 5). One study found that shorter weekday sleep duration on school nights was associated with a greater likelihood of being in the C-reactive protein high risk group, and those who obtained greater than 2 h of catch up sleep on weekend nights were twice as likely to be in the C-reactive protein high-risk group [18••]. Jakubowski and colleagues demonstrated that a greater proportion of days napped was associated with greater interlukin-6, but they found no relationship between napping and C-reactive protein [42]. Carson and colleagues also found no relationship between C-reactive protein and sleep duration [20].
Results of Risk of Bias
The overwhelming majority of studies included in this review presented with high risk of bias. Of the 24 studies included in the review, only one (Shaw [18••]) merited low risk of bias, and only 3 were found to have moderate risk of bias (Alqaderi [29], Dorenbos [36], Pacheco [37]). Much of the existing bias within these studies is due to the nature of the design of the study, with the majority of them being cross-sectional or longitudinal studies without a control or comparison group.
Discussion/Observation
This systematic review of the association between sleep, hypertension, and cardiovascular risk in children and adolescents demonstrated several findings. First, the strongest evidence of a relationship with sleep is with obesity, hypertension, and insulin resistance. Of the 15 studies that measured obesity, 13 found a significant relationship with sleep regardless of measure of sleep (bedtime, sleep duration, etc.) or obesity (BMI z-score, waist circumference, etc.). It is noteworthy that although the relationship between sleep and obesity has been the most commonly assessed over the last decade [16••] and despite calls for increased randomized trials [11], there were no randomized controlled trials assessing the causal mechanisms in the last 3 years, and all of the studies relied on self- or parent-report of sleep as opposed to objective measures.
There was also consistent evidence of a relationship between sleep and hypertension, regardless of age. One study demonstrated that sleep at 2 months can have at least a short-term effect on blood pressure [22•]. Specifically, both systolic blood pressure [20, 22•, 25, 26] and diastolic blood pressure [23, 26] seem to be related to sleep duration and quality. Only one study found no relationship between sleep and blood pressure [24].
There is not strong evidence of a relationship between glucose or insulin and sleep. However, there appears to be a link between insulin resistance and sleep. Of the seven studies that assessed insulin sensitivity, all of them found an association with sleep, but the direction of the relationship was inconsistent. This did not appear to be affected by weight status or method of measuring insulin sensitivity.
There is no evidence of a relationship between sleep and triglycerides, and evidence on the relationship between sleep and HDL is inconsistent. Several studies found no relationship between sleep and HDL, but this could be due to differences in age or weight status, which was suggested by several of the studies [21, 22•, 39]. Finally, there are few studies assessing the association between sleep and inflammation.
Despite the significant impact of cardiovascular disease and the large amount of correlational data supporting the relationship between sleep and cardiovascular risk factors, to our knowledge, only one randomized controlled trial addressing the casual relationship among these variables was published in the last 3 years. This is surprising, especially given that the association between sleep and obesity has been well-established for a number of years and previous meta-analyses have called for more randomized trials [11]. Even among the longitudinal and cross-sectional studies, very few use objective measures of sleep and instead rely on self-report, and the majority of studies we reviewed were found to have high risk of bias. Therefore, based on current research, we are unable to make conclusions about the direction of the relationship among sleep and cardiovascular variables. It is unclear if intervening on children and adolescents’ sleep duration would improve obesity, hypertension, or insulin sensitivity or if it might enhance current treatments. Future research should utilize more rigorous research methods including conducting randomized controlled trials and objective measures of sleep such as polysomnography or actigraphy.
Although a few of the studies assessed the effects of later bedtime, most studies did not assess the effect of obtaining too much sleep or the circadian timing of sleep. Most adolescents go to bed late and are then unable to obtain adequate sleep due to school. However, studies in adults suggest that circadian misalignment could have a negative effect on cardiovascular risk [43].
This review has many strengths. First, we provided a systematic review of the literature over the last 3 years to provide an updated overview of our current knowledge on the relationship between sleep and cardiovascular risk in children and adolescents. To our knowledge, this is the first systematic review that assessed all cardiovascular risk factors, including obesity, hypertension, insulin, glucose, insulin sensitivity, and inflammation. We also provided a clear picture of the populations and methods of the recent research on this topic. Weaknesses include the lack of randomized controlled trials to assess for causality and few longitudinal studies, use of self-report of sleep, inconsistency in measures used (e.g., HOMA-IR vs. insulin secretion–sensitivity index-2), few comparisons of outcomes based on SES or race, and lack of assessment for circadian misalignment or obtaining greater sleep than recommended. These issues should be addressed in future research.
In conclusion, this systematic review highlights cross-sectional and some longitudinal evidence from the last 3 years suggesting that sleep duration or quality is associated with obesity, hypertension, and insulin resistance. The results of this review call for additional research using more standardized methods and objective measures in order to determine if a causal relationship truly exists between sleep and cardiovascular risk.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–603.
Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med. 1998;338(23):1650–6.
Din-Dzietham R, Liu Y, Bielo M-V, Shamsa F. High blood pressure trends in children and adolescents in National Surveys, 1963 to 2002. Circulation. 2007;116(13):1488–96.
Ostchega Y, Carroll M, Prineas RJ, McDowell MA, et al. Trends of elevated blood pressure among children and adolescents: data from the National Health and nutrition examination survey 1988–2006. Am J Hypertens. 2009;22(1):59–67.
Sinaiko AR, Donahue RP, Jacobs DR, Prineas RJ. Relation of weight and rate of increase in weight during childhood and adolescence to body size, blood pressure, fasting insulin, and lipids in young adults. Circulation. 1999;99(11):1471–6.
Statistics NCfH. Health, United States. With special features on socioeconomic status and health. Hyattsville: U.S. Department of Health and Human Services; 2011. p. 2012.
Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150(1):12–7.e2.
Kaufman FR. Type 2 diabetes in children and youth. Endocrinol Metab Clin N Am. 2005;34(3):659–76. ix-x
Matricciani LA, Olds TS, Blunden S, Rigney G, Williams MT. Never enough sleep: a brief history of sleep recommendations for children. Pediatrics. 2012;129(3):548–56.
Taheri S. The link between short sleep duration and obesity: we should recommend more sleep to prevent obesity. Arch Dis Child. 2006;91(11):881–4.
Capers PL, Fobian AD, Kaiser KA, Borah R, Allison B, Systemic Review DA. Meta-analysis of randomized controlled trials of the impact of sleep duration on adiposity and components of energy balance. Obes Rev. 2015;16(9):771–82.
Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity. 2008;16(2):265–74.
Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5(5):253–61.
Flint J, Kothare SV, Zihlif M, Suarez E, Adams R, Legido A, et al. Association between inadequate sleep and insulin resistance in obese children. J Pediatr. 2007;150(4):364–9.
Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol. 2007;5(2):93–102.
•• Matthews KA, Pantesco EJ. Sleep characteristics and cardiovascular risk in children and adolescents: an enumerative review. Sleep Med. 2016;18:36–49. This review examines the relationship between sleep and multiple factors of cardiovascular risk, and highlights the need for stronger research in this area.
Viswanathan M, Berkman ND, Dryden DM, et al. Assessing Risk of Bias and Confounding in Observational Studies of Interventions or Exposures: Further Development of the RTI Item Bank [Internet]. Rockville: Agency for Healthcare Research and Quality (US); 2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK154461/
•• Shaw ND, McHill AW, Schiavon M, Kangarloo T, et al. Effect of Slow Wave Sleep Disruption on Metabolic Parameters in Adolescents. Sleep. 2016;39(8):1591–9. This is the only RCT found in the last 3 years, and one of only a handful in the last several years; the results found no relationship between slow wave sleep disruption and glucose, insulin or C-peptide levels.
Anujuo KO, Vrijkotte TG, Stronks K, Jean-Louis G, Agyemang CO. Ethnic differences in sleep duration at 5 years, and its relationship with overweight and blood pressure. Eur J Pub Health. 2016;26(6):1001–6.
Carson V, Tremblay M, Chaput J, Chastin S. Associations between sleep duration, sedentary time, physical activity, and health indicators among Canadian children and youth using compositional analyses. Appl Physiol Nutr Metab. 2016;41:S294–302.
De Bernardi Rodrigues AM, da Silva Cde C, Vasques AC, Camilo DF, Barreiro F, Cassani RS, et al. Association of Sleep Deprivation with Reduction in insulin sensitivity as assessed by the hyperglycemic clamp technique in adolescents. JAMA Pediatr. 2016;170(5):487–94.
• Derks IPM, Kocevska D, Jaddoe VWV, Franco OH, Wake M, Tiemeier H, et al. Longitudinal Associations of Sleep Duration in Infancy and Early Childhood with Body Composition and Cardiometabolic Health at the Age of 6 Years: The Generation R Study. Child Obes. 2017;13(5):400–8. This is one of a few recent longitudinal studies examining sleep and cardiometabolic health; the results found no clear evidence for a relationship between sleep duration in early life and childhood cardiometabolic health.
Gonzaga NC, Sena ASS, Coura AS, Dantas FG, Oliveira RC, Medeiros CCM. Sleep quality and metabolic syndrome in overweight or obese children and adolescents. Rev Nutr. 2016;29(3):377–89.
Morita N, Kambayashi I, Okuda T, Oda S, Takada S, Nakajima T, et al. Inverse relationship between sleep duration and cardio-ankle vascular index in children. J Atheroscler Thromb. 2017;24(8):819–26.
Navarro-Solera M, Carrasco-Luna J, Pin-Arboledas G, Gonzalez-Carrascosa R, Soriano JM, Codoner-Franch P. Short sleep duration is related to emerging cardiovascular risk factors in obese children. J Pediatr Gastroenterol Nutr. 2015;61(5):571–6.
Paciencia I, Araujo J, Ramos E. Sleep duration and blood pressure: a longitudinal analysis from early to late adolescence. J Sleep Res. 2016;25(6):702–8.
Peach H, Gaultney JF, Reeve CL. Sleep characteristics, body mass index, and risk for hypertension in young adolescents. J Youth Adolesc. 2015;44(2):271–84.
Tavasoli A, Saeidi M, Hooman N. Correlation between sleep quality and blood pressure changes in Iranian children. J Compr Ped. 2015;6(1):e24805.
Alqaderi H, Redline S, Tavares M, Goodson JM. Effect of late bedtime on salivary glucose and abdominal obesity in children. Sleep Biol Rhythms. 2017;15(3):227–33.
Deacon-Crouch M, Skinner I, Tucci J, Skinner T. Association between short sleep duration and body mass index in Australian indigenous children. J Paediatr Child Health. 2017;54(1):49-54.
Hayes JF, Balantekin KN, Altman M, Wilfley DE, Taylor CB, Williams J. Sleep patterns and quality are associated with severity of obesity and weight-related behaviors in adolescents with overweight and obesity. Child Obes. 2017;14(1):11-17.
Li L, Fu J, Yu XT, Li G, Xu L, Yin J, et al. Sleep duration and Cardiometabolic risk among Chinese school-aged children: do Adipokines play a mediating role? Sleep. 2017;40(5):zsx042
Plumptre L, Anderson LN, Chen Y, Carsley S, Narang I, Hamilton J, et al. Longitudinal analysis of sleep duration and Cardiometabolic risk in young children. Child Obes. 2017;13(4):291–9.
Rudnicka AR, Nightingale CM, Donin AS, Sattar N, Cook DG, Whincup PH, et al. Sleep duration and risk of type 2 diabetes. Pediatrics. 2017;140(3):1–10.
Sluggett L, Wagner SL, Hardy C, Harris RL. Associations between sleep duration and indicators of Cardiometabolic disease in Canadian children and adolescents: analyses of the 2007-2009 Canadian health measures survey. Child Obes. 2016;12(5):325–33.
Dorenbos E, Rijks JM, Adam TC, Westerterp-Plantenga MS, Vreugdenhil AC. Sleep efficiency as a determinant of insulin sensitivity in overweight and obese adolescents. Diabetes Obes Metab. 2015;17(Suppl 1):90–8.
Pacheco SR, Miranda AM, Coelho R, Monteiro AC, Braganca G, Loureiro HC. Overweight in youth and sleep quality: is there a link? Arch Endocrinol Metab. 2017;61(4):367–73.
Peplies J, Börnhorst C, Günther K, Fraterman A, Russo P, Veidebaum T, et al. Longitudinal associations of lifestyle factors and weight status with insulin resistance (HOMA-IR) in preadolescent children: the large prospective cohort study IDEFICS. Int J Behav Nutr Phys Act. 2016;13:1–12.
Sayin FK, Buyukinan M. Sleep duration and media time have a major impact on insulin resistance and metabolic risk factors in obese children and adolescents. Child Obes. 2016;12(4):272–8.
Zhu Y, Li AM, Au CT, Kong AP, Zhang J, Wong CK, et al. Association between sleep architecture and glucose tolerance in children and adolescents. J Diabetes. 2015;7(1):10–5.
Hall MH, Lee L, Matthews KA. Sleep duration during the school week is associated with C-reactive protein risk groups in healthy adolescents. Sleep Med. 2015;16(1):73–8.
Jakubowski KP, Hall MH, Marsland AL, Matthews KA. Is daytime napping associated with inflammation in adolescents? Health Psychol. 2016;35(12):1298–306.
Morris CJ, Yang JN, Scheer FAJL. The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res. 2012;199:337–58.
Acknowledgements
We would like to thank Carolyn Holmes and Geeta Malik with the Lister Hill Library at the University of Alabama at Birmingham for their assistance in developing the search strategy for this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Sleep and Hypertension
Rights and permissions
About this article
Cite this article
Fobian, A.D., Elliott, L. & Louie, T. A Systematic Review of Sleep, Hypertension, and Cardiovascular Risk in Children and Adolescents. Curr Hypertens Rep 20, 42 (2018). https://doi.org/10.1007/s11906-018-0841-7
Published:
DOI: https://doi.org/10.1007/s11906-018-0841-7