Abstract
Hypertension is the leading factor in the global burden of disease. It is the predominant modifiable risk factor for stroke, heart disease, and kidney failure. Chronic kidney disease (CKD) is both a common cause and sequel of uncontrolled hypertension. The pathophysiology of CKD-associated hypertension is complex and multi-factorial. This paper reviews the key pathogenic mechanisms of CKD-associated hypertension, the importance of standardized blood pressure (BP) measurement in establishing the diagnosis and management plus the significance of ambulatory BP monitoring for assessment of diurnal BP variation commonly seen in CKD. The optimal BP target in CKD remains a matter of discussion despite recent clinical trials. Medical therapy can be difficult and challenging. In addition to lifestyle modification and dietary salt restriction, treatment may need to be individualized based on co-morbidities. Combination of antihypertensive drugs, including appropriate diuretic choice and dose, is of great significance in hypertension management in CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension, a global public health problem, is the major risk factor for adverse cardiovascular and cerebrovascular outcomes resulting in premature death and disability [1]. The prevalence of hypertension is rising despite increased awareness and improved treatment. Recent National Health and Nutrition Examination Survey shows 47% of adults aged over 20 with hypertension had uncontrolled blood pressure (BP) [2]. Chronic kidney disease (CKD), a recognized risk factor for cardiovascular disease (CVD), is another common and growing problem [3]. Hypertension prevalence, the most common CKD-associated comorbidity, increases with declining renal function [4]. The relationship between CKD and hypertension is complex and bidirectional. At some point, it becomes difficult to determine which disease process precedes the other, as both diseases share similar risk factors including age, obesity, minority descent, and comorbidities like diabetes or CVD. Additionally, the prevalence of resistant hypertension—defined as BP that remains above the goal despite the concurrent use of three antihypertensive agents of different classes, including a diuretic, prescribed at optimal dose without presence of a secondary cause of hypertension—increases with advanced CKD [5, 6]. Respectively, patients with CKD are at very high risk for CVD and mortality. Therefore, controlling elevated BP, is of great importance [7].
Blood Pressure Measurement in Clinical Practice
Accurate and standardized BP measurements are critical. Standardized measurements include using the appropriate cuff size; measurements should be repeated at intervals of 1 to 2 min and then averaged. BP should be measured in both arms to check for any between-arm discrepancies that could be commonly seen in CKD patients due to heavily calcified or arteriosclerotic arteries. Using the higher BP of the two arms is recommended for management purposes. These approaches require appropriate training of the clinical staff and are more time consuming than the single conventional BP measurement done routinely.
Home BP monitoring is superior to conventional auscultatory office BP readings, correlates more closely with ambulatory blood pressure monitoring (ABPM), and is more predictive of adverse cardiovascular outcomes [8,9,10]. In contrast, automated office BP readings are up to 20 mmHg lower than the standard conventional office BP measurements and are comparable to or even lower than daytime ambulatory BP readings [11]. However, 24-h ABPM provides critical information on diurnal BP variability and nocturnal BP [12]. Patients with CKD often lose the physiologic nocturnal 10–20% fall in systolic and diastolic BP levels. In advanced CKD, patients might even have a rise in nocturnal BP, a phenomena called riser. The loss of nocturnal dip and high BP variability are linked to increased risk of cardiovascular events and target organ damage including progression of CKD [13, 14]. Similarly, the loss of circadian rhythm and increased prevalence of the riser BP pattern is associated with highest risk of cardiovascular events among all possible BP patterns. It is 2.5-fold more prevalent in CKD and up to 5-fold more prevalent in end-stage renal disease (ESRD) [15]. In addition, ABPM is diagnostic for white-coat hypertension, a common cause of apparent resistant hypertension defined as persistently elevated clinic BP readings while out-of-office BP values measured by 24-h ABPM are normal [16]. Alternatively, masked hypertension, defined as normal office BP levels but out-of-office hypertension, appears to be remarkably prevalent in CKD [17]. There is a strong association between elevated nighttime BP and masked hypertension. Patients with masked hypertension are at increased risk for cardiovascular events and target organ damage including higher cumulative risk of ESRD [18].
Pathogenesis of Hypertension in CKD
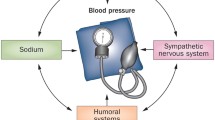
Multiple mechanisms of independent or interdependent pathways contribute to the pathogenesis of hypertension in CKD. The kidney is both the contributing and the target organ of the hypertensive processes.
Loss of sodium regulation and impairment in excretion in the setting of CKD can result over time in volume-mediated hypertension and increases the prevalence of salt-sensitive hypertension [19]. There is a strong association of CKD and prevalence of resistant hypertension in part due to subclinical volume overload exacerbated by high dietary salt intake [20]. Increased dietary sodium intake can cause arterial vessel stiffness—commonly seen in elderly and CKD—resulting in increased risk of development of systolic hypertension, decreased nitric oxide release, and promotion of inflammatory processes [21, 22]. Excessive salt intake blunts the BP-lowering effect of most classes of antihypertensive drugs leading to development of resistant hypertension [23]. In contrast, low salt intake can have a synergistic effect on the actions of antihypertensive drugs that block the renin angiotensin aldosterone system (RAAS) [24]. These effects are more pronounced in salt-sensitive patients, including the elderly, African Americans, and CKD population [25].
Sympathetic nervous system (SNS) activity is increased in CKD [26]. The assessment of SNS activity and its contribution to BP regulation is imprecise, as circulating catecholamine levels provide only a rough estimate of the SNS activity. The renal artery is highly innervated with efferent renal nerves that originate from the central nervous system and afferent renal nerves that originate from the kidneys. Stimulation of efferent renal nerves via β-1 adrenoreceptors increases renin secretion from the juxtaglomerular apparatus that is also highly volume regulated [27]. Renin secretion activates the RAAS resulting in increased sodium reabsorption by both angiotensin II in the proximal tubule and aldosterone in the distal nephron in exchange for the secretion of potassium. RAAS activation also causes direct vasoconstriction via angiotensin II effect. Hypertension can cause and accelerate renal injury when impaired auto-regulation allows the transmission of high systemic pressures to the glomeruli, resulting in glomerulosclerosis [28].
The presence of proteinuria, commonly seen in CKD, may have an accentuating BP effect due to the filtered plasminogen and its conversion within the urinary space to plasmin by urokinase-type plasminogen activator that increases sodium retention by activating the epithelial sodium channel in the nephron collecting duct [29]. Similar results have been observed in patients with preeclampsia [30]. Similarly, CKD is more common in patients with obesity and metabolic syndrome. Although the relationship between obesity and hypertension is well described, the pathophysiology of obesity-induced hypertension is complex and not fully understood [31]. Angiotensin-II-independent release of aldosterone by adipocytes could be a reason for the increased occurrence of aldosterone-mediated-resistant hypertension [32].
Exogenous and commonly available over-the-counter drugs like non-steroidal anti-inflammatory drugs (NSAIDs) can add to this complexity [33, 34]. Inhibition of renal prostaglandin by NSAIDs can lead to sodium and fluid retention in patients with CKD [35]. Other agents that can add difficulty in BP management include decongestant and diet pills that contain sympathomimetics or amphetamine-like stimulants, oral contraceptives, and herbal preparations containing ephedra [36].
Evaluation of Hypertension in CKD
The first step in evaluation of difficult to treat hypertension in CKD is to confirm the diagnosis of true treatment resistance hypertension and exclude pseudo-resistance due to inaccurate blood pressure measurement technique and or treatment non-adherence [37]. Barriers to successful medication adherence include polypharmacy, drug costs, dosing inconvenience, and adverse effects of drugs. It is important to clarify the duration, course, and severity of the hypertension and if possible, the chronologic relation to the established diagnosis of CKD. Modifiable and contributing factors are to be identified and eliminated if possible (Table 1). Evaluation for secondary causes like renal artery stenosis should be considered in a young female, whose presentation may suggest the presence of fibromuscular dysplasia or in an older patient at increased risk of atherosclerotic disease, who has had a recent deterioration of renal function following therapy with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), or history of flash pulmonary edema [38]. Screening for target-organ damage including presence of proteinuria, left ventricular hypertrophy, or retinopathy and estimation of glomerular filtration rate (GFR) for the degree of CKD are essential to assess the risk of cardiovascular complications and further deterioration of renal function.
Target Blood Pressure in CKD
The optimal BP level in the treatment of hypertension in general and in CKD patients remains a matter of debate and controversial despite recent clinical guidelines and trial data [39, 40]. In CKD, the goal is to prevent cardiovascular events and delay the progression to ESRD requiring renal replacement therapy or renal transplant [41, 42]. Multiple trials in non-diabetics, including MDRD, AASK, and REIN-2 failed to show benefit from lower BP targets of <130/80 mmHg compared to <140/90 mmHg in slowing the progression of CKD to ESRD [43,44,45]. These trials had insufficient power to assess cardiovascular outcomes. The ACCORD trial that included a large number of diabetics with mild CKD (creatinine <1.5 mg/dl) detected a small but statistically insignificant reduction in cardiovascular events among diabetics treated to the intensive systolic BP goal of <120 mmHg compared to goal BP of <140 mmHg [46]. A follow-up report of ACCORD study suggests that intensive glycemic control might have been harmful and masked the cardiovascular benefits of intensive BP control [47]. In contrast, data from the SPRINT study which excluded diabetics but included 28% of participants with reduced estimated GFR of 20–60 ml/min/1.73m2 showed a 25% relative risk reduction in the cardiovascular events of myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes, and 27% reduction in all-cause mortality in individuals treated to the intensive systolic BP goal of <120 mmHg (mean systolic BP was 121.5 mmHg) [40]. Although there was no difference in rates of 50% reduction of estimated GFR or ESRD between the intensive BP-treated group compared to standard-BP group, more patients without incident CKD had >30% decline in estimated GFR with intensive treatment. Both the ACCORD and SPRINT trials showed an increase in the risk of serious adverse events (hypotension, electrolyte abnormalities, and acute kidney injury) with the more intensive BP lowering strategy. The benefit of BP target of <130/80 mmHg in patients with CKD and proteinuria is supported based on post hoc analyses [48].
Whether the results of SPRINT study can be implemented into future guidelines is a matter of discussion, as unattended office BP measurement technique used in the SPRINT study is not easily applicable in daily practice, cannot be directly compared to BP measurements in other trials, and provided readings comparable to lower systolic BP assessed by ABPM [49, 50]. Respectively, the current clinical practice guidelines for the management of hypertension in CKD without proteinuria recommending a goal BP of <140/90 mmHg remains valid (Table 2) [39, 51,52,53,54,55,56]. In the presence of proteinuria the recommendation, based on expert opinion, is a lower BP target of <130/80 mmHg. The Canadian Hypertension Education Program Guideline has an update based on SPRINT study results recommending intensive BP treatment to target systolic BP ≤120 mmHg in high-risk patients (Grade B) and automated office BP as the preferred method of obtaining in-office BP measurement (Grade D) [57].
Ultimately, it is important to balance the cardiovascular benefits of intensive targeted BP therapy in patients with higher cardiovascular risk, as adopted in the SPRINT trial, with possible increase in therapeutic costs, drug-related side effects, and serious adverse effects. Similarly, in the presence of commonly seen vascular pathology in CKD population, the risk of adverse effects including cerebral hypoperfusion, reduction in myocardial perfusion, or acute kidney injury may be increased if the BP is reduced below the threshold of autoregulation.
Treatment of Hypertension in CKD
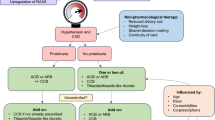
Achieving optimal BP control in CKD patients is frequently challenging even for nephrologists or hypertension specialists. A non-pharmacological approach with modification of lifestyle factors including dietary salt restriction, weight loss, regular exercise, and decreased alcohol ingestion are first steps in management of hypertension [58,59,60]. High salt diet blunts the effect of ACE inhibitors, and sodium reduction enhances the anti-proteinuric effect of ARBs [61, 62]. Accordingly, dietary sodium restriction to less than 100 mEq (2300 mg)/24 h is recommended. Additionally, ingestion of a diet rich in fruits and vegetables, with close monitoring of the potassium levels in setting of advanced CKD, reduces systolic and diastolic BP compared to a usual diet [63]. Identification and discontinuation of potentially interfering substances, like NSAIDs, is crucial.
Individualization of treatment should be considered based on the existing co-morbidities in addition to the CVD risk status, age, gender, and ethnicity of the patient. Avoidance of complex dosing regimens and high out-of-pocket costs improves patient’s compliance. Use of long-acting drugs with minimal adverse effects, chosen from different classes that increase the renal sodium excretion and inhibit both the RAAS and SNS activity can provide a synergistic pharmacological effect. There is strong evidence that combination regimens reduce cardiovascular events and are currently the standard of care recommended [39, 51, 64,65,66].
Modification of a regimen by adding a diuretic, increasing the dose of the diuretic, or changing the class of prescribed diuretic based on GFR can significantly improve BP control [67, 68]. Lack or underuse of diuretics in patients with CKD is a common cause of treatment-resistant hypertension. Long-acting thiazide-like diuretic, chlorthalidone, is the most potent thiazide diuretic [69]. Although KDIGO guideline recommends a switch from thiazide-type diuretic to loop diuretic at CKD Stage 4 (GFR <30 mL/min/1.73 m2), this recommendation has been challenged recently, based on few small studies that reported efficacy of thiazide-type diuretics even at low GFR [70]. Loop diuretics are more potent natriuretic agents, and individuals with advanced CKD with or without proteinuria may require even higher doses to achieve natriuresis and BP reduction. Use of long acting diuretics avoids counter-regulatory rebound sodium reabsorption and volume retention in patients with CKD [71]. And finally, sequential blockade of sodium channels along the nephron with both a thiazide and loop diuretic is very effective, but the combination requires frequent monitoring of serum creatinine and electrolytes [72].
RAAS blockers are recommended in many guidelines for management of hypertension in CKD patients with or without proteinuria. The reno-protective effect of ACE inhibitors or ARBs is exerted by reducing the intra-glomerular pressure and thereby decreasing proteinuria [73]. Respectively, an associated GFR loss of up to 30% is physiologic and is associated with a better renal outcome [74]. There is no indication for cessation of the RAAS blocker, unless there is the complication of persistent hyperkalemia refractory to treatment. Although a combination of RAAS blockers could improve BP control in resistant hypertension, it is associated with significant adverse effects including risk of severe hyperkalemia and acute renal failure and should be avoided [75, 76].
Dihydropyridine calcium channel blockers (CCBs) in contrast to the non-dihydropyridine CCBs, which have more an anti-proteinuric effect are very effective antihypertensive drugs [77]. Patients may experience lower extremity edema due to the higher pre-capillary arterial dilatation effect of the drug class. This condition is refractory to diuretics but improves or resolves with the use of an ACE inhibitor or ARB. The combination of a CCB with an ACE inhibitor might be more effective in slowing the progression of CKD, particularly in black patients [78, 79].
Aldosterone antagonist is the drug of choice for patients uncontrolled on multidrug regimens and low-renin status as the result of volume expansion or possible aldosterone escape phenomenon [80, 81]. The effect of the drug is independent of a patient’s baseline plasma aldosterone or 24-h urinary aldosterone level, plasma renin activity, or plasma aldosterone/renin ratio [82]. Although an aldosterone antagonist use in combination with an ACE inhibitor or ARB is not contraindicated in CKD, they should be restricted to patients with GFR >30 mL/min/1.73 m2 and plasma potassium concentrations of <4.5 mmol/L due to high risk of hyperkalemia, particularly seen in patients taking NSAIDs or have co-morbidities such as diabetes.
Beta-blockers, as the fifth drug of choice, are more often used when there is a coexisting cardiac disease such as ischemic heart disease or heart failure [83]. Combined alpha and beta antagonists are the more effective agents. Alpha-blockers despite their possible favorable effect on BP and vascular remodeling have limited role due to common side effect of dizziness. Clonidine, a centrally acting agent, can be very effective but requires frequent dosing, has a significant side effect profile, and is associated with rebound hypertension if the dose is missed when using a daily dose over 0.6 mg. Potent vasodilators such as hydralazine or minoxidil have a higher incidence of adverse effects including lower extremity edema or tachycardia and respectively are less preferred drugs.
A very important factor in management of hypertension in CKD is the concept of chronotherapy. Bedtime administration of at least one of the drugs is associated with a better 24-h mean BP control and could induce the desired nocturnal dip in non-dippers, thereby reducing cardiovascular event risk [84, 85]. Finally, frequent home BP monitoring by the patients can enhance medication adherence and improvement of hypertension [86].
Conclusion
Hypertension is the major modifiable risk factor for cardiovascular disease and renal failure. The interaction between hypertension and CKD is complex and increases the risk of adverse cardiovascular outcomes. This risk is particularly significant in the setting of resistant hypertension commonly seen in CKD. Sodium dysregulation, increased SNS activity, and alterations in RAAS are some of the key pathogenic mechanisms of CKD-associated hypertension and do play an important role in determining the choice of antihypertensive medication. Out-of-office BP measurements including ABPM provide a better assessment of diurnal BP variation commonly seen in CKD. The optimal BP target in CKD population remains a matter of debate despite recent clinical trial results. Medical therapy can be difficult, challenging, and even frustrating in the setting of commonly seen resistant hypertension. Combination of different drug classes including appropriate diuretic choice in addition to the non-pharmacological approaches, most of important of which is dietary salt restriction, is the optimal management practice.
References
World Health Organization. A global brief on hypertension: silent killer, global public health crisis. World health day 2013. Geneva: World Health Organization; 2013. p. 1–39.
Yoon SS, Fryar CD, Carroll MD. Hypertension prevalence and control among adults: United States, 2011–2014. NCHS data brief, no 220. Hyattsville, MD: National Center for Health Statistics. 2015.
Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–70.
Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, et al. Hypertension awareness, treatment, and control in adults with CKD: results from the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis. 2010;55:441–51.
Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association professional education committee of the Council for High Blood Pressure research. Hypertension. 2008;51:1403–19.
De Nicola L, Gabbai FB, Agarwal R, Chiodini P, Borrelli S, Bellizzi V, et al. Prevalence and prognostic role of resistant hypertension in chronic kidney disease patients. J Am Coll Cardiol. 2013;61:2461–7.
Townsend RR, Taler SJ. Management of hypertension in chronic kidney disease. Nat Rev Nephrol. 2015;11:555–63.
Drawz PE, Abdalla M, Rahman M. Blood pressure measurement: clinic, home, ambulatory, and beyond. Am J Kidney Dis. 2012;60:449–62.
Cohen DL, Huan Y, Townsend RR. Home blood pressure monitoring in CKD. Am J Kidney Dis. 2014;63:835–42.
Niiranen TJ, Hänninen MR, Johansson J, Reunanen A, Jula AM. Home measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn-home study. Hypertension. 2010;55:1346–51.
Myers MG, Godwin M, Daves M, et al. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. Hypertension. 2010;55:195–200.
Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69:1175–80.
Kanno A, Kikuya M, Asayama K, Satoh M, Inoue R, Hosaka M, et al. Night-time blood pressure is associated with the development of chronic kidney disease in a general population: the Ohasama study. J Hypertens. 2013;31:2410–7.
Ciobanu AO, Gherghinescu CL, Dulgheru R, Magda S, Dragoi Galrinho R, Florescu M, et al. The impact of blood pressure variability on subclinical ventricular, renal and vascular dysfunction, in patients with hypertension and diabetes. Maedica (Buchar). 2013;8:129–36.
Mojón A, Ayala DE, Piñeiro L, Otero A, Crespo JJ, Moyá A, et al. Comparison of ambulatory blood pressure parameters of hypertensive patients with and without chronic kidney disease. Chronobiol Int. 2013;30:145–58.
Agarwal R. Hypertension diagnosis and prognosis in chronic kidney disease with out-of-office blood pressure monitoring. Curr Opin Nephrol Hypertens. 2006;15:309–13.
Agarwal R, Pappas MK, Sinha AD. Masked uncontrolled hypertension in CKD. J Am Soc Nephrol. 2016;27:924–32.
Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193–8.
Koomans HA, Roos JC, Boer P, Geyskes GG, Mees EJ. Salt sensitivity of blood pressure in chronic renal failure. Evidence for renal control of body fluid distribution in man. Hypertension. 1982;4:190–7.
Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell'Italia LJ, et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009;54:475–81.
Briet M, Boutouyrie P, Laurent S, London GM. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012;82:388–400.
Hovater MB, Sanders PW. Effect of dietary salt on regulation of TGF-beta in the kidney. Semin Nephrol. 2012;32:269–76.
Luft FC, Weinberger MH. Review of salt restriction and the response to antihypertensive drugs: satellite symposium on calcium antagonists. Hypertension. 1988;11:I-229–32.
Kwakernaak AJ, Krikken JA, Binnenmars SH, Visser FW, Hemmelder MH, Woittiez AJ, et al. Effects of sodium restriction and hydrochlorothiazide on RAAS blockade efficacy in diabetic nephropathy: a randomised clinical trial. Lancet Diabetes Endocrinol. 2014;2:385–95.
Boudville N, Ward S, Benaroia M, House AA. Increased sodium intake correlates with greater use of antihypertensive agents by subjects with chronic kidney disease. Am J Hypertens. 2005;18:1300–5.
Klein IH, Ligtenberg G, Neumann J, Oey PL, Koomans HA, Blankestijn PJ. Sympathetic nerve activity is inappropriately increased in chronic renal disease. J Am Soc Nephrol. 2003;14:3239–44.
Davis JO, Freeman RH. Mechanisms regulating renin release. Physiol Rev. 1976;56:1–56.
Bidani AK, Polichnowski AJ, Loutzenhiser R, Griffin KA. Renal microvascular dysfunction, hypertension and CKD progression. Curr Opin Nephrol Hypertens. 2013;22:1–9.
Svenningsen P, Friis UG, Versland JB, Buhl KB, Møller Frederiksen B, Andersen H, et al. Mechanisms of renal NaCl retention in proteinuric disease. Acta Physiol (Oxf). 2013;207:536–45.
Buhl KB, Friis UG, Svenningsen P, Gulaveerasingam A, Ovesen P, Frederiksen-Møller B, et al. Urinary plasmin activates collecting duct ENaC current in preeclampsia. Hypertension. 2012;60:1346–51.
Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–33.
Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, et al. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci U S A. 2003;100:14211–6.
LeLorier J, Bombardier C, Burgess E, Moist L, Wright N, Cartier P, et al. Practical considerations for the use of nonsteroidal anti-inflammatory drugs and cyclo‑oxygenase‑2 inhibitors in hypertension and kidney disease. Can J Cardiol. 2002;18:1301–8.
Conlin PR, Moore TJ, Swartz SL, Barr E, Gazdick L, Fletcher C, et al. Effect of indomethacin on blood pressure lowering by captopril and losartan in hypertensive patients. Hypertension. 2000;36:461–5.
Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A Meta-Analysis Ann Intern Med. 1994;121:289–300.
Mansoor GA. Herbs and alternative therapies in the hypertension clinic. Am J Hypertens. 2001;14:971–5.
Burnier M, Wuerzner G, Struijker-Boudier H, Urquhart J. Measuring, analyzing, and managing drug adherence in resistant hypertension. Hypertension. 2013;62:218–25.
Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014;35:1245–54.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–20.
SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–16.
McCullough PA, Steigerwalt S, Tolia K, Chen SC, Li S, Norris KC, et al. Cardiovascular disease in chronic kidney disease: data from the kidney early evaluation Program (KEEP). Curr Diab Rep. 2011;11:47–55.
Whaley-Connell AT, Sowers JR, Stevens LA, McFarlane SI, Shlipak MG, Norris KC, et al. CKD in the United States: kidney early evaluation program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999-2004. Am J Kidney Dis. 2008;51:S13–20.
Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease study group. N Engl J Med. 1994;330:877–84.
Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–31.
Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN‑2): multicentre, randomised controlled trial. Lancet. 2005;365:939–46.
Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85.
Margolis KL, O’Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care. 2014;37(6):1721–8.
Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med. 2011;154:541–8.
Drawz PE, Pajewski NM, Bates JT, et al. Effect of intensive versus standard clinic-based hypertension management on ambulatory blood pressure. Results from the SPRINT (systolic blood pressure intervention trial) ambulatory blood pressure study. Hypertension. 2017;69.
Kjeldsen SE, Lund-Johansen P, Nilsson PM, Mancia G. Unattended blood pressure measurements in the systolic blood pressure intervention trial: implications for entry and achieved blood pressure values compared with other trials. Hypertension. 2016;67:808–12.
Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337–414.
National Institute for Health and Care Excellence. Chronic kidney disease http://www.nice.org.uk/guidance/cg182/evidence/cg182-chronic-kidney-disease.
Dasgupta K, Quinn RR, Zarnke KB, Rabi DM, Ravani P, Daskalopoulou SS, et al. The 2014 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2014;30:485–501.
Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–357.
Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. 2014;32:3–15.
American Diabetes Association. Standards of medical care in diabetes. Chapter 8 ‘cardiovascular disease and risk management’. Diabetes Care. 2016;39(Suppl. 1):S60–71.
Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32(5):569–88.
Aucott L, Poobalan A, Smith WC, Avenell A, Jung R, Broom J. Effects of weight loss in overweight/obese individuals and long-term hypertension outcomes: a systematic review. Hypertension. 2005;45:1035–41.
Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503.
Aguilera MT, de la Sierra A, Coca A, Estruch R, Fernandez-Sola J, Urbano-Marquez A. Effect of alcohol abstinence on blood pressure: assessment by 24-hour ambulatory blood pressure monitoring. Hypertension. 1999;33:653–7.
Singer DR, Markandu ND, Sugden AL, Miller MA, MacGregor GA. Sodium restriction in hypertensive patients treated with a converting enzyme inhibitor and a thiazide. Hypertension. 1991;17:798–803.
Kwakernaak AJ, Krikken JA, Binnenmars SH, Visser FW, Hemmelder MH, Woittiez AJ, et al. Effects of sodium restriction and hydrochlorothiazide on RAAS blockade efficacy in diabetic nephropathy: a randomised clinical trial. Lancet Diabetes Endocrinol. 2014;2:385–95.
Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH collaborative research group. N Engl J Med. 1997;336:1117–24.
Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomized controlled trial. Lancet. 2005;366:895–906.
Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomized controlled trial. Lancet. 2007;370:829–40.
Chalmers J, Arima H, Woodward M, Mancia G, Poulter N, Hirakawa Y, et al. Effects of combination of perindopril, indapamide, and calcium channel blockers in patients with type 2 diabetes mellitus: results from the action in diabetes and vascular disease: Preterax and Diamicron controlled evaluation (ADVANCE) trial. Hypertension. 2014;63:259–64.
Tamargo J, Segura J, Ruilope LM. Diuretics in the treatment of hypertension. Part 1: thiazide and thiazide-like diuretics. Expert Opin Pharmacother. 2014;15:527–47.
Tamargo J, Segura J, Ruilope LM. Diuretics in the treatment of hypertension. Part 2: loop diuretics and potassium-sparing agents. Expert Opin Pharmacother. 2014;15:605–21.
Ernst ME, Carter BL, Goerdt CJ, Steffensmeier JJ, Phillips BB, Zimmerman MB, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47:352–8.
Cirillo M, Marcarelli F, Mele AA, Romano M, Lombardi C, Bilancio G. Parallel-group 8-week study on chlorthalidone effects in hypertensives with low kidney function. Hypertension. 2014;63:692–7.
Shankar SS, Brater DC. Loop diuretics: from the Na-K-2Cl transporter to clinical use. Am J Physiol Renal Physiol. 2003;284:F11–21.
Izzo JL. Value of combined thiazide-loop diuretic therapy in chronic kidney disease: heart failure and reninangiotensin-aldosterone blockade. J Clin Hypertens. 2012;14:344.
Anderson S, Brenner BM. Therapeutic benefit of converting-enzyme inhibition in progressive renal disease. Am J Hypertens. 1988;1:380S–3S.
Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80:282–7.
ONTARGET Investigators, Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–59.
Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892–903.
Bakris GL, Weir MR, Secic M, Campbell B, Weis-McNulty A. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int. 2004;65:1991–2002.
Bakris GL, Sarafidis PA, Weir MR, Dahlöf B, Pitt B, Jamerson K, et al. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet. 2010;375:1173–81.
Weir MR, Bakris GL, Weber MA, Dahlof B, Devereux RB, Kjeldsen SE, et al. Renal outcomes in hypertensive black patients at high cardiovascular risk. Kidney Int. 2012;81:568–76.
Eide IK, Torjesen PA, Drolsum A, Babovic A, Lilledahl NP. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J Hypertens. 2004;22:2217–26.
Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–68.
Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925–30.
Rosendorff C, Lackland DT, Allison M, Aronow WS, Black HR, Blumenthal RS, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Hypertension. 2015;65:1372–407.
Hermida RC, Diana E, Ayala DE, Mojón A, Fernández JR. Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol. 2011;22:2313–21.
Hermida RC, Ayala DE, Mojon A, Fernandez JR. Decreasing sleep-time blood pressure determined by ambulatory monitoring reduces cardiovascular risk. J Am Coll Cardiol. 2011;58:1165–73.
Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens. 2006;8:174–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Hamrahian declares no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pediatric Hypertension
Rights and permissions
About this article
Cite this article
Hamrahian, S.M. Management of Hypertension in Patients with Chronic Kidney Disease. Curr Hypertens Rep 19, 43 (2017). https://doi.org/10.1007/s11906-017-0739-9
Published:
DOI: https://doi.org/10.1007/s11906-017-0739-9