Abstract
Purpose of Review
Clinical trials represent a bedrock for measuring efficacy of interventions in biomedical research, but recruitment into clinical trials remains a challenge. Few data have focused on recruitment strategies from the perspective of clinical trial teams, especially in low- and middle-income countries (LMIC), where HIV is most prevalent.
Recent Findings
We summarized data from the literature and our experience with recruitment for the Renal Risk Reduction trial, aimed at reducing risk of kidney complications among people living with HIV in Nigeria. Using an implementation science framework, we identified strategies that contributed to successful clinical trial recruitment. For strategies that could not be categorized by this framework, we summarized key features according to selected action, actor, target, context, and time. We identified how these identified strategies could map to subsequent implementation outcomes at the patient and provider/health system level, as well as capacity-building efforts to meet needs identified by LMIC partners, which is a priority for success.
Summary
Our experience highlights the importance of considering implementation outcomes, and the strategies necessary to achieve those outcomes early, in the planning and execution of clinical trials. Clinical trial recruitment can be optimized via methodologies grounded in implementation science.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical trials are the most rigorous scientific approach for evaluating the efficacy and safety of medical, surgical, and behavioral interventions [1]. Without efficient recruitment of participants in clinical trials, efforts to develop more effective interventions to prevent, diagnose, or manage disease are hindered [2•]. Up to 86% of clinical trials do not reach recruitment goals within predefined timelines [2•]. Suboptimal recruitment is also the leading cause of early termination of clinical trials [3, 4]. In a 2015 analysis, approximately 19% of registered trials were closed or terminated due to insufficient participant accrual [2•]. Importantly, delays and barriers to recruitment may also significantly impact trial costs and workload, as well as dissemination of evidence-based interventions [5]. Further, challenges with recruitment driven by patient-level obstacles may inadvertently exclude individuals less likely to overcome those obstacles, thus resulting in findings that are less generalizable to important sub-populations [6, 7].
Recruitment challenges in clinical trials have been well documented in high-income settings [8]. Most research in this area has focused on patient-level barriers to recruitment, such as additional demands of the trial (visit intensity, blood draws, investigative procedures, etc.), patient preferences, concerns about uncertainty or randomization, and mistrust of the clinician or study team due to inability to understand information and consent [5]. While knowledge of these barriers is critical to proactively addressing recruitment concerns, additional considerations must be made for trials conducted in low- and middle-income countries (LMICs). Clinical trials in LMICs often present unique ethical, organizational, cultural, and infrastructure challenges [9]. These challenges are not only faced by those conducting the research but also by funding agencies, participants, their communities, and families [9]. These challenges may be even more pronounced when recruiting a highly stigmatized population, such as people living with HIV (PLWH) [10]. Research on clinical trial recruitment in LMICs is scarce and even more limited among PLWH in LMICs [9, 11,12,13]. Also, lessons learned from study teams during clinical trial recruitment may foreshadow important provider/health system or individual patient-level implementation obstacles when it is time to deploy successful biomedical interventions.
Other limited areas of research important to clinical trial recruitment are factors related to the functioning and capacity of the clinical trial team. Some factors that may hinder the success of clinical trial teams include issues with the protocol, poor communication, treatment preference, internal climate at the study site, poor recognition or staffing concerns, and lack of protected time [5, 8, 14,15,16]. Implementation science provides a helpful framework to consider which implementation strategies may optimize clinical trial recruitment from the perspective of the clinical trial team and to assess how implementation outcomes, resulting from these strategies, may inform subsequent intervention implementation—both from the perspective of the trial team/healthcare system and that of the target population. Indeed, the growing literature on hybrid implementation-effectiveness clinical trial designs, has demonstrated the value of studying implementation outcomes alongside effectiveness outcomes in order to facilitate the subsequent rollout of effective interventions [17]. Herein, we use an implementation science framework to [1] summarize our recruitment experience with the Renal Risk Reduction (R3) clinical trial, an National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-funded cooperative agreement supporting collaboration between US-based (Vanderbilt University Medical Center) and Nigeria-based (Aminu Kano Teaching Hospital; Kano, Nigeria) investigators; [2] identify implementation strategies impacting recruitment efforts from the perspective of the clinical trial team; and [3] make a case for adopting implementation strategies through hybrid study design to optimize recruitment efforts and inform later intervention implementation.
Background, Study Setting, and Clinical Trials at AKTH
This study will examine the increasing prevalence of kidney diseases among HIV-positive adults in a West African population and the relationship between these diseases and apolipoprotein-1 (APOL1) high-risk genotype. By evaluating the addition of an angiotensin-converting enzyme inhibitor (ACEi) to the care of individuals with HIV infection who have microalbuminuria, our trial will provide definitive evidence to guide strategies for management and clinical care in this population, with the goal of reducing longer term HIV-related kidney complications [18]. The study setting for the R3 trial is a U.S. President’s Emergency Plan for AIDS Relief (PEPFAR)-funded HIV clinic at Aminu Kano Teaching Hospital (AKTH) in Kano, northern Nigeria. Kano is the most populous state in Nigeria and has an HIV prevalence of 1.3% [19]. AKTH is a large tertiary center that provides care for more than 10,000 HIV-positive adults. The first aim of the R3 Trial was to determine the prevalence of APOL1 risk variants and their association with markers of kidney disease (micro- and macroalbuminuria, reduced estimated glomerular filtration rate (eGFR), and serum creatinine) among HIV-positive adults on ART. This aim necessitated sampling urine and blood to assess kidney disease and APOL1 risk allele status, in addition to completion of baseline demographic and clinical forms.
Our study team, comprised of four US-based investigators and three project staff, and six Nigeria-based investigators and ten project staff, successfully recruited 2600 patients from the HIV clinic at AKTH over a 13-month period, achieving our recruitment goals 1 month earlier than anticipated [Fig. 1]. Among these individuals, only 1% (n=35) did not consent to study participation and 0.2% (n=5) withdrew participation after consent. An additional 60 individuals did not complete the study because of death (n=2), loss to follow-up (n=50), travel outside of the study area (n=30), or responsibility of caring for a sick relative (n=13). Among the remaining 2500, the median age was 40 years [IQR 34, 47], 70% were female, and 96% had a suppressed HIV viral load (HIV RNA <200/mL). In addition, 2% had self-reported diabetes mellitus, 15% had self-reported hypertension, 36% were overweight or obese, and 24% had sickle cell trait (hemoglobin SS or SA) [20, 21]. Importantly, our study population is representative of the clinic population with regard to age and sex demographics, along with HIV-specific clinical demographics and loss to follow-up. While the prevalence of non-communicable diseases has not been rigorously studied in this specific cohort, some findings were consistent with others from Nigeria (diabetes, overweight/obesity), while others like microalbuminuria, the focus of the R3 trial, were higher than anticipated and an important area for additional investigation for our team [20, 22,23,24]. As such, the success of our recruitment was lauded by both our study team and funders and motivated this review.
Approach and Implementation Science Framework
Implementation strategies are defined as the actions taken to enhance adoption, implementation, and sustainability of a clinical program or practice [25]. We were interested in clinical trial recruitment as the “clinical practice” and strategies to enhance successful recruitment. While the identification and utilization of implementation strategies was an integrated and iterative part of the study design, we later used two approaches to discern and codify implementation strategies that were important for optimal clinical trial recruitment in R3. First, we used a modified Delphi technique with the US-based study team to develop a consensus list of implementation strategies [26,27,28, 29•]. Second, we administered a 13-item survey containing multiple-choice and open-ended questions to our Nigeria-based study team to identify strategies contributing to outstanding clinical trial recruitment for the R3 study. The survey was administered via REDCap software and maintained respondent confidentiality [30]. We utilized the framework established by the Expert Recommendations for Implementing Change (ERIC) project to categorize implementation strategies identified by our team. As earlier literature on implementation strategies lacked consistency in definitions and adequate details for replication, Powell et al. developed (2013) and refined (2015) a set of 73 implementation strategies codified for use in isolation or in combination in implementation research and practice [31]. ERIC-defined implementation strategies are widely utilized in the field of implementation research. Finally, we used the Action, Actor, Context, Target, and Time (AACTT) framework to describe specific parameters of the implementation strategies identified by our team that were not described by the ERIC project [32].
Implementation Strategies to Achieve Successful Trial Recruitment
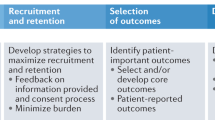
Our team identified several strategies focused on the clinical trial team, and others focused on the interaction with patients, which are consistent with strategies described in the ERIC framework. For the former, strategies included developing academic partnerships and organizing implementation team meetings, and for the latter, strategies included conducting educational meetings. However, several implementation strategies were identified by our team, which were not reflected in the ERIC project. These included fostering a positive clinical trial team dynamic, in addition to adopting a patient-centered approach and peer navigation for patient recruitment Table 1.
Clinical Trial Teams: Develop Academic Partnerships
Powell et al. defined this strategy as “partnering with a university or academic unit for the purposes of shared training and bringing research skills to an implementation project” [31]. Our study team has historically embodied such a partnership, and study team members highlighted the importance of the capacity building resulting from that partnership. Principal investigators of the R3 study are US-based faculty at Vanderbilt University Medical Center (Vanderbilt) who have been collaborating with Nigeria in general, and AKTH in particular, for more than 12 years. The relationship began with healthcare service delivery in 2008 when Vanderbilt received funding from the Centers for Disease Control and Prevention (CDC) through PEPFAR to implement a comprehensive HIV care and treatment program in northern Nigeria—a region served by AKTH. This program was led by Vanderbilt for 6 years, during which it was responsible for scale-up of care, treatment, and prevention services, eventually providing HIV testing services to 171,000 clients, prevention of mother-to-child HIV transmission (PMTCT) services to 80,300 women, and HIV care to 8577 Nigerians. This program ultimately transitioned to local leadership in 2013, and several program leaders, administrators, and staff with extensive experience in large-scale program implementation later joined AKTH in some capacity.
Subsequently, Vanderbilt and AKTH have collaborated on multiple research grants spanning primary and secondary prevention of stroke among children with sickle cell disease (R21NS080639, R01NS094041, Thrasher Foundation), task-shifted epilepsy screening and treatment (R21TW010899, R01NS113171, R01NS118483), and this HIV-associated kidney disease R3 Trial (U01DK112271). Throughout this partnership, Vanderbilt investigators have had a sustained commitment to building the capacity of AKTH investigators to conduct research. Two programs exemplify this commitment: the first, the Vanderbilt Institute for Research Development and Ethics (VIRDE) is a 1-month course on grant writing and research ethics facilitated by Vanderbilt Institute for Global Health (VIGH) and offered to international colleagues. VIRDE was launched in 2011 and has provided training for 12 Nigerian medical and public health professionals. The second, the Vanderbilt-Emory-Cornell-Duke (VECD) Fogarty Global Health Program for Fellows and Scholars (D43TW009337), is a training program whose purpose is to provide mentored global health research training opportunities in LMIC for pre- and post-doctoral candidates from the USA and LMICs. Through this NIH-sponsored program, Vanderbilt has supported seven Nigerian trainees to date. As a result of these relationships, Vanderbilt and AKTH have developed a successful and productive partnership resulting in more than 50 co-authored manuscripts by Vanderbilt and AKTH faculty over the past 12 years, primarily in the HIV/AIDS field.
This collaborative relationship with ongoing training for individual investigators in research methods has led to additional NIH-funded capacity-building grants focused on clinical trials research for non-communicable diseases associated with HIV (D43TW011544) and capacity building for personnel and other infrastructure to enhance grant submissions and management (1G11TW011819). Establishing such collaborative relationships is critical for the feasibility of successful trial and study recruitment, but also crucial for the later deployment of effective interventions.
Clinical Trial Teams: Implementation Team Meetings
The ERIC project defined this strategy as “developing and supporting teams of clinicians who are implementing the innovation and give them protected time to reflect on the implementation effort, share lessons learned, and support one another’s learning.” The R3 team has fully embraced this strategy, and has held standing weekly meetings since the study’s inception. [1] US- and Nigeria-based investigators and study staff meet weekly to share progress and challenges to study recruitment and retention, sample collection and processing, and data collection and analysis; the team also holds a journal club to apprise members of important literature related to the study aims. [2] US- and Nigeria-based project staff meet weekly to discuss the progress of the ongoing research and troubleshoot any issues that arise. [3] The US-based team meet weekly to (a) discuss pertinent research topics, any changes in the direction of the research projects, or new project ideas, (b) review current studies relevant to the current project via a journal club, (c) address budgeting concerns, and (d) distribute resources and workload required for the optimal execution of the project. [4] The Nigeria-based team meet weekly and impromptu to discuss and troubleshoot local problems and other logistic issues related to the study as they arise. These meetings are in addition to regular, ad hoc communication between team members in both countries by phone, email, and other forms of electronic communication. Efficient and effective communication in these meetings is especially critical, as investigators in Nigeria and other LMICs, though grant-supported, are typically not provided with the same protected time to which US-based investigators are accustomed. As such, time dedicated to the R3 study is in addition to any clinical, administrative, or other research commitments held by other co-investigators. Since these meetings integrate teaching about the rationale and premise of the study, in addition to the study protocol, it is likely to have an impact on both feasibility of clinical trial recruitment and later intervention acceptability (at the level of the clinic and trial teams) in addition to intervention penetration and sustainability (for the clinical teams). This may be especially true if the intervention is novel and changes the standard of care to which the clinical teams are accustomed. For the R3 study, the next phase of investigation does involve the randomization of lisinopril for PLWH and evidence of CKD, but this practice is not the standard of care in this setting, and thus requires both provider and patient buy-in.
Clinical Trial Teams: Team Dynamics
Nearly all of our study team members commented on the importance of facets of the study team dynamic in achieving our recruitment goals. Study team members described the importance of prior working relationships, a central focus on teamwork, and coordinated efforts and effective time management as key elements of a successful team dynamic. More than 90% of our AKTH team members have previously worked with others on the study team in some capacity. These prior relationships facilitated understanding of interpersonal dynamics, along with key competencies and strengths of other team members, and were leveraged for the current project. One respondent wrote:
[Prior work with other members of the R3 team] has helped in providing seamless access and interaction with the relevant team members and provided a common ground for all of us to contribute effectively to the study.
Teamwork was consistently described by study team members as a critical component of the team’s success in clinical trial recruitment. For some, this stood in stark contrast to their experiences working with other clinical trial teams. One respondent wrote:
R3 Study is entirely different from other studies I've worked on. In my previous studies, time management wasn't enforced as in this study. Although we worked as a team, there was no effective coordination of activities as found in R3 study. Meetings were infrequent and, in most cases, there was no room to identify and seize opportunities. Team members of my previous studies were committed to the success of the study but were not as enthusiastic as the R3 study team. The team members did not take ‘ownership’ of the study.
While not directly represented as an implementation strategy in the ERIC project, team dynamics and teamwork is a basic tenet of team effectiveness in the field of organizational behavior [33]. Researchers in this field have identified key enabling conditions for effective teams, which include a compelling direction (articulated via clear, challenging goals), a strong structure (including members with a diversity of relevant skills), a supportive context (maintaining appropriate support and rewards), and a shared mindset. Overlapping literature originating from the perspective of healthcare delivery teams has defined six key characteristics of practice teams: shared goals, clearly defined roles, shared knowledge and skills, effective and timely communication, mutual respect, and an optimistic attitude [33, 34]. We propose that optimizing dynamics among study team members will impact the feasibility of meeting enrollment targets and later sustainability. Certainly, intervention sustainability will require, among other elements, champions within the clinical setting; and our trial team members represent an important part of this population. Our team members have described many of these factors in the context of effective team dynamics. As one R3 team member shared:
Team members supported one another and were able to carry out individual and group tasks effectively. In addition, team members were dedicated and committed to the success of the study to the extent that they took ‘ownership’ of the study.
Patient Interaction: Conduct Educational Meetings
ERIC investigators described this strategy as “holding meetings targeted toward different stakeholder groups (e.g., providers, administrators, other organizational stakeholders, and community, patient/consumer, and family stakeholders) to teach them about the clinical innovation.” The R3 team leveraged existing infrastructure within the AKTH clinic to easily deliver information to patients and identified this as an important recruitment strategy. At the AKTH HIV clinic, and in most high-volume outpatient health facilities in this setting, appointments are often not scheduled. Consequently, there is often a large, captive audience of patients who are waiting to be seen by a clinician. Many clinics use this opportunity to disseminate health information and other important announcements with patients, helping the time pass before they are called to be seen by a provider. One of the AKTH-based team members shared that:
Morning health talk has been an effective way of passing information to clients. It has worked well for us in previous research. It has also helped in establishing trust with clients.
Another R3 team member wrote:
Frequent targeted health talks: This has helped increase awareness about the study to the extent that participants referred many other participants to us.
A vast amount of literature has focused on how to share recruitment information with patients. In a 2018 Cochrane review on strategies to improve recruitment into clinical trials, the authors reviewed 68 trials with more than 74,000 studies and interestingly found that bespoke, user-tested approaches to developing informational leaflets to facilitate recruitment made little to no difference in recruitment outcomes (absolute improvement 1%, 95% confidence interval, CI; −1 to 3%) [35]. In contrast with such tailored approaches, the health talks are a mechanism widely used in this setting to share information and which our team members identified as an important approach for R3.
Patient Interaction: Patient-Centered Approach
While not an implementation strategy identified by the ERIC investigators, our study team described the importance of a patient-centered approach to recruitment. Proctor et al. distinguished between distinct but related implementation and service outcomes [36]. The latter are derived from quality improvement aims indexed by the Institute of Medicine in their landmark report “Crossing the Quality Chasm” [37]. Service outcomes define many factors that patients would likely prioritize in their healthcare experience, including efficiency, safety, effectiveness, equity, patient-centeredness, and timeliness. Clinical facilities that systematically espoused patient-centeredness by developing patient-centered medical homes may deliver better quality care at a lower cost [38]. Following the lead of programs in high-income countries, many HIV treatment centers in LMIC adopted this multidisciplinary treatment approach in the early phases of the scale-up of our global AIDS response [38,39,40]. As such, it is not surprising that our team described the effectiveness of patient-centered approaches in their trial recruitment efforts. These approaches exemplified our team’s commitment to respecting the time and competing priorities of study participants. To accomplish this, flow through the clinic was streamlined, other clinical needs were facilitated (such as referrals to other specialists), transportation costs were provided, and ultimately time away from competing priorities (work, school, etc.) was minimized. Reflecting on such patient-centered efforts, one survey respondent from AKTH shared:
Most of the participants were interested in the study but couldn't have completed all their visits if not for their transportation that was compensated. Therefore, transport compensation has contributed a lot. We try as much as we can to ensure that we don't take much time of the patients.
Another team member offered:
We streamlined the recruitment process which helped in the seamless movement of participants through the various contact points of the study… The team has provided support to the participants, including in areas not directly linked to the study, e.g. clinic appointments and consultations, and that helped build cordial relationships between them…. Also, this has prevented the study from unnecessarily increasing participants' stay in the clinic, and in some cases, participants were able to complete their clinic visits long before the usual time.
While transportation reimbursement might be considered an expected component of financial remuneration for study participants, R3 study team members thought of potential barriers to study participation holistically, considering competing priorities of patients while valuing their time and contribution. As such, the study team went beyond simply providing funds for transportation, but also optimized flow through the clinic to reduce wait times and facilitated the scheduling of other referrals and appointments.
Patient Interaction: Peer Navigation
Our study team identified peer navigation as a key strategy for successful clinical trial recruitment for R3. One study team member is a peer navigator who has been employed by the AKTH HIV clinic for 14 years. In that capacity, this team member was charged with outreach to patients who missed clinic visits to encourage them to return to care. Given this longstanding relationship in the clinic, the peer navigator had nourished trusting relationships over time with many clinic attendees, thus helping to facilitate recruitment into our clinical trial. Healthcare navigation has been recommended as an intervention to improve linkage to and engagement in care for people living with HIV. It is a process by which an individual guides a patient through and around the barriers of a complex care system, to help ensure timely diagnosis and treatment [41,42,43]. Peer navigators can help traverse both structural and social barriers to promote healthcare engagement based on trust and shared experiences [41, 44]. As such, it is not surprising that peer navigation services would be successful in settings such as clinical trial participation, particularly in a population of PLWH, where complex enrollment criteria and study protocols may be challenging for patients to navigate on their own.
While not identified by ERIC as an implementation strategy, this may have been one of the most important strategies identified by our study team as reflected in the responses below. One team member shared:
All team roles were essential in the R3 study. However, participant navigators were most essential in recruiting the participants while other team members facilitated participant retention.
Another offered:
Participants’ navigation by the research navigator was behind the recruitment success and collective staff teamwork was responsible for the success of retention.
We propose that all of the strategies related to patient interaction will impact the feasibility of meeting recruitment targets in addition to the feasibility, acceptability, and appropriateness of the subsequent intervention among patients. Specifically, educational meetings and interactions with the peer navigator will provide important information on the intervention rationale and delivery by (potentially trusted) messengers within the clinic setting—both of which may subsequently influence how patients view its acceptability and appropriateness.
Areas for Improvement
The single most important factor identified by the R3 study team that needed improvement and was a barrier to study activities was the delay in the transfer of funds to execute study activities. This delay represents the antithesis of a supportive team environment, one of the four features of effective teams described by Haas and Mortenson [33]. Delays in the transfer of study funds also created a physical obstacle to continuing to work on the study and to optimizing study logistics (such as lab consumables, storage, processing of samples, etc.). One of the R3 team members wrote:
Some activities need funds to work; when there is a delay in sending funds for such activities, it tends to affect the flow of the program.
This feedback from the R3 study teams is an important reminder that assumptions cannot be made about the financial reserves of our partner institutions in LMICs and that study activities may not be able to proceed on the mere “promise” of funds from a subcontract.
In addition, despite the efforts of the US-based Vanderbilt team to support capacity building at AKTH, several R3 team members at AKTH shared that the team activities could be enhanced with additional capacity-building initiatives. This sentiment underscores the importance of developing a shared understanding of training needs from both collaborative partners and for additional training grants that could help build capacity in specific areas of need.
Description of Newly Identified Implementation Strategies Using the AACTT Framework
Three implementation strategies—namely [1] optimizing team dynamics, [2] utilizing a patient-centered approach, and [3] employing peer navigation—were identified as important implementation strategies by our study team, but were not described by the ERIC project. We used the AACTT framework to specify the important actions in these strategies that were utilized by our study team [Table 2] [32]. Our study team described the execution of regular team meetings as an important feature of optimizing the team dynamic. It was important to the team that these meetings were consistent, timely, and reinforced teamwork. While this may seem obvious on the surface, several team members described being a part of studies that rarely or infrequently met at all. A patient-centered approach to recruitment was also described as a potential implementation strategy. Our study team members went to great lengths to schedule study visits at the same time as routine clinical visits and labs and to facilitate clinical referrals in order to minimize the time that patients spent in the clinic. As a result of their efforts, many patients described spending less time in clinic than they would have for a routine (non-study) follow-up visit. Finally, our use of a peer navigator to facilitate recruitment was identified as an important implementation strategy. One particular responsibility of the peer navigator was to call participants to invite them to learn more about the study and to schedule study visits. As a follow-up, the navigator also reminded participants about appointments via phone call the day prior. These touchpoints provided information and reminders from a trusted person in the clinic to whom patients were accustomed.
Conclusions/Lessons Learned
The R3 study will be the first to evaluate therapy to treat microalbuminuria among HIV-positive, ART-treated adults with the goal of preventing the progression of kidney disease. Our study team, at a large teaching hospital in northern Nigeria, exceeded our trial recruitment goals and enrolled 2600 patients within 13 months. Considering clinical trial recruitment as an important implementation outcome for a clinical trial, and that implementation strategies utilized to optimize recruitment could also provide important knowledge about facilitators and barriers of implementation outcomes for the later intervention, we [1] used a framework codified by the ERIC project investigators to assess implementation strategies to facilitate successful recruitment from the study team perspective and [2] used the AACTT framework to describe features of strategies identified by our team that were not included in the ERIC project. Our study team identified six implementation strategies that were important for clinical trial recruitment. While the ERIC framework was helpful, it was not exhaustive, as identified by our study team three of the six implementation strategies were not identified by this framework (optimizing team dynamics, adopting a patient-centered approach, and utilizing peer navigation) but were also critical for recruitment success. Our experience highlights the importance of considering implementation outcomes and the strategies necessary to achieve those outcomes early in the planning and execution of clinical trials.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
NIH. What are clinical trials and studies? 2020.
• Huang GD, Bull J, Johnston McKee K, Mahon E, Harper B, Roberts JN, et al. Clinical trials recruitment planning: A proposed framework from the Clinical Trials Transformation Initiative. Contemp Clin Trials. 2018;66:74–9 This study provides a comprehensive guide to approaching clinical trial recruitment through design and protocol development, trial feasibility, site selection and communication, and also provides tools to facilitate adoption of the recommended guidelines.
Blümle A, Schandelmaier S, Oeller P, Kasenda B, Briel M, von Elm E, et al. Premature Discontinuation of Prospective Clinical Studies Approved by a Research Ethics Committee - A Comparison of Randomised and Non-Randomised Studies. PLoS One. 2016;11(10):e0165605.
Williams RJ, Tse T, DiPiazza K, Zarin DA. Terminated Trials in the ClinicalTrials.gov Results Database: Evaluation of Availability of Primary Outcome Data and Reasons for Termination. PLoS One. 2015;(5):10, e0127242.
Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52(12):1143–56.
Heumann C, Cohn SE, Krishnan S, Castillo-Mancilla JR, Cespedes M, Floris-Moore M, et al. Regional variation in HIV clinical trials participation in the United States. South Med J. 2015;108(2):107–16.
Castillo-Mancilla JR, Cohn SE, Krishnan S, Cespedes M, Floris-Moore M, Schulte G, et al. Minorities remain underrepresented in HIV/AIDS research despite access to clinical trials. HIV clinical trials. 2014;15(1):14–26.
Institute of Medicine (US). Recruitment Challenges in Clinical Trials for Different Diseases and Conditions. Washington DC: National Academies Press (US); 2012.
Mbuagbaw L, Thabane L, Ongolo-Zogo P, Lester RT, Mills E, Volmink J, et al. The Cameroon mobile phone SMS (CAMPS) trial: a protocol for a randomized controlled trial of mobile phone text messaging versus usual care for improving adherence to highly active anti-retroviral therapy. Trials. 2011;12:5.
Mills E, Nixon S, Singh S, Dolma S, Nayyar A, Kapoor S. Enrolling women into HIV preventive vaccine trials: an ethical imperative but a logistical challenge. PLoS Med. 2006;3(3):e94.
Ranjani H, Weber MB, Anjana RM, Lakshmi N, Narayan KMV, Mohan V. Recruitment challenges in a diabetes prevention trial in a low- and middle-income setting. Diabetes Res Clin Pract. 2015;110(1):51–9.
Pitisuttithum P, Choopanya K, Rerk-Ngnam S. HIV-vaccine research and development in Thailand: evolution and challenges. Vaccine. 2010;28(Suppl 2):B45–9.
Eholié SP, Aoussi FE, Ouattara IS, Bissagnéné E, Anglaret X. HIV treatment and care in resource-constrained environments: challenges for the next decade. J Int AIDS Soc. 2012;15(2):17334.
Abraham NS, Young JM, Solomon MJ. A systematic review of reasons for nonentry of eligible patients into surgical randomized controlled trials. Surgery. 2006;139(4):469–83.
Strong S, Paramasivan S, Mills N, Wilson C, Donovan JL, Blazeby JM. 'The trial is owned by the team, not by an individual': a qualitative study exploring the role of teamwork in recruitment to randomised controlled trials in surgical oncology. Trials. 2016;17(1):212.
Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al. Recruitment to randomised trials: strategies for trial enrollment and participation study. The STEPS study. Health Technol Assess. 2007;11(48) iii:ix–105.
Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–26.
Aliyu MH, Wudil UJ, Ingles DJ, Shepherd BE, Gong W, Musa BM, et al. Optimal management of HIV- positive adults at risk for kidney disease in Nigeria (Renal Risk Reduction "R3" Trial): protocol and study design. Trials. 2019;20(1):341.
Government of Nigeria. Nigeria AIDS indicator and impact survey, national summary sheet: Preliminary findings. In: Aids FMoH, National Agency for the Control o, editors. 2019.
Wudil UJ, Aliyu MH, Ingles DJ, Ahonkhai AA, Musa BM, Muhammad H, et al. Apoliproprotein-1 (APOL1) risk variants and associated kidney phenotypes in an HIV cohort in Nigeria. Kidney international. 2021(In press)
Musa BM, Coker M, Bussell S, Aliyu M, Babashani M, Muhammad H, et al. Long-term outcomes of antiretroviral therapy in an adult HIV program: a 10-year retrospective cohort study in Kano, Nigeria. Annals of Saudi Medicine. 2015;35(4):303–11.
Uloko AE, Musa BM, Ramalan MA, Gezawa ID, Puepet FH, Uloko AT, et al. Prevalence and Risk Factors for Diabetes Mellitus in Nigeria: A Systematic Review and Meta-Analysis. Diabetes Ther. 2018;9(3):1307–16.
Adeloye D, Ige-Elegbede JO, Ezejimofor M, Owolabi EO, Ezeigwe N, Omoyele C, et al. Estimating the prevalence of overweight and obesity in Nigeria in 2020: a systematic review and meta-analysis. Ann Med. 2021;53(1):495–507.
Dakum P, Avong YK, Okuma J, Sorungbe T, Jatau B, Nedmbi N, et al. Prevalence and risk factors for obesity among elderly patients living with HIV/AIDS in a low-resource setting. Medicine. 2021;100(15):e25399.
University of Washington. What is implementation strategy? 2020.
Dalkey N, Helmer O. An experimental application of the Delphi method to the use of experts. Manag Sci. 1963;9:458–67.
Dalkey N. The Delphi Method: An experimental study of group opinion. Santa Monica: Rand Corp Public RM-58888-PR; 1969.
Wood L, Black P, Heng D, Kollmannsberger C, Moore R, Soulieres D, et al. Using the Dephi technique to improve clinical outcomes through the development of quality indicators in renal cell carcinoma. J Oncology Practice. 2013:262–7.
• Murphy M, Black N, Lamping D, McKee C, Sanderson C, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess. 1998:i–88 This review examines the effects of strategies for improving recruitment of participants to randomised trials.
Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, Conde J. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.
Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21.
Presseau J, McCleary N, Lorencatto F, Patey AM, Grimshaw JM, Francis JJ. Action, actor, context, target, time (AACTT): a framework for specifying behaviour. Implement Sci. 2019;14(1):102.
Hass M, Mortenson M. The Secrets of Great Teamwork: Harvard Business Review; 2016.
Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511–44.
Treweek S, Pitkethly M, Cook J, Fraser C, Mitchell E, Sullivan F, et al. Strategies to improve recruitment to randomised trials. Cochrane Database Syst Rev. 2018;2(2):Mr000013.
Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Admin Pol Ment Health. 2011;38(2):65–76.
Committee on Quality of Health in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
Ahonkhai AA, Onwuatuelo I, Regan S, Adegoke A, Losina E, Banigbe B, et al. The patient-centered medical home: a reality for HIV care in Nigeria. Int J Qual Health Care. 2017;29(5):654–61.
Jackson GL, Powers BJ, Chatterjee R, Bettger JP, Kemper AR, Hasselblad V, et al. Improving patient care. The patient centered medical home. A Systematic Review. Ann Intern Med. 2013;158(3):169–78.
Pappas G, Yujiang J, Seiler N, Malcarney MB, Horton K, Shaikh I, et al. Perspectives on the role of patient-centered medical homes in HIV Care. Am J Public Health. 2014;104(7):e49–53.
Koester KA, Morewitz M, Pearson C, Weeks J, Packard R, Estes M, et al. Patient navigation facilitates medical and social services engagement among HIV-infected individuals leaving jail and returning to the community. AIDS Patient Care STDs. 2014;28(2):82–90.
International Advisory Panel on HIVCCO. IAPAC Guidelines for Optimizing the HIV Care Continuum for Adults and Adolescents. J Int Assoc Provid AIDS Care. 2015;14(Suppl 1):S3–S34.
CDC. Effective interventions: HIV Prevention that Works [Available from: https://effectiveinterventions.cdc.gov/en/care-medication-adherence/group-1/artas/core-elements-and-fact-sheet%2D%2D-artas.
Bauman LJ, Braunstein S, Calderon Y, Chhabra R, Cutler B, Leider J, et al. Barriers and facilitators of linkage to HIV primary care in New York City. J Acquir Immune Defic Syndr (1999). 2013;64(Suppl 1):S20–6.
Acknowledgments
The authors wish to acknowledge the contributions of all our research staff and participants at Aminu Kano Teaching Hospital (AKTH) in Kano, Nigeria. This work is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH), U01DK112271. The findings and conclusions are those of the authors and do not necessarily represent the official position of the NIDDK, the Department of Health and Human Services, or the government of the USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Implementation Science
Rights and permissions
About this article
Cite this article
Ahonkhai, A.A., Wudil, U.J., Dankishiya, F.S. et al. Strategies for Successful Clinical Trial Recruitment of People Living with HIV in Low- and Middle-Income Countries: Lessons Learned and Implementation Implications from the Nigeria Renal Risk Reduction (R3) Trial. Curr HIV/AIDS Rep 18, 289–298 (2021). https://doi.org/10.1007/s11904-021-00566-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-021-00566-x