Abstract
Introduction
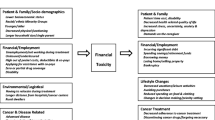
Financial toxicity is a developing research area to quantify the financial stress experienced by patients and caregivers, as well as the mechanisms by which they manage the costs associated with treatment and the very real harms that this stress can inflict upon cancer care. Patients with blood malignancies experience increased costs associated with their diagnosis due to possible inpatient admissions for treatment, frequent office visits, and even more frequent lab evaluations and testing.
Purpose of Review
Multiple studies have examined the causes and effects of financial toxicity on patient care and outcomes, and there have been several validated tools developed to identify patients experiencing or at risk for financial harm.
Discussion
However, few studies to date have focused on implementing successful interventions to assist in mitigating financial difficulties for patients diagnosed with hematologic malignancies and their families. In this review, we examine the current literature with an emphasis on levels of care, including providers, systems, and policies. Specifically, we discuss published interventions including physician education about treatment costs, financial navigation in cancer centers, and novel institutional multidisciplinary review of patients’ financial concerns. We also discuss the urgent need for societal and governmental interventions to lessen financial distress experienced by these highly vulnerable blood cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Purpose of Review
We are at a crossroads for many blood cancer patients. Survival in patients with hematologic malignancies has improved over the past 20 years, in large part due to novel therapeutics with less toxicity, improved supportive medications and interventions, and increased sensitivity of detection for residual disease [1, 2]. Yet, even as overall survival and management in patients improves, the financial burdens of blood cancer care, compounded by the eye-watering growth in prices for treatment, has had a toxic effect on the ability of patients and their families to seek and obtain this specialized care, and can have a lasting impact on their lives well beyond their diagnoses. In the oncology clinician community, there is growing interest in quantifying this harm and, more importantly, in addressing financial toxicity (FT) caused by cancer care
Research has linked FT with patients delaying or even foregoing all forms of medical care, ranging from office visits, prescriptions, seeking mental health treatment [3,4,5], and to worsened overall survival [6, 7]. There is also a compounding effect on the development of future treatments, as those at the highest risk of financial burdens remain the groups least likely to participate in clinical trials [8, 9]. Aside from clinical outcomes, FT impacts patient well-being. Patients undergoing cancer treatment are less likely to save or acquire assets [10], carry more debt [11], are more likely to declare bankruptcy [12], and see their long-term careers suffer from prolonged absences from work, unwanted job changes, and diminished productivity [13, 14]
The impact of FT on patients with hematologic malignancies is particularly acute, as they often require higher healthcare utilization than their solid tumor counterparts [2], including weeks-long inpatient hospitalizations for systemic treatment, frequent clinic visits and labs, costly novel therapeutic medications, and possible hematopoietic stem cell transplantation (HSCT) to improve their overall survival and quality of life [15,16,17,18,19]. Given the high morbidity and mortality associated with these diseases with inadequate delivery of treatment, there is a real need to identify and improve interventions to mitigate the effects of FT in this population. However, due to lack of dedicated research in the realm specific to hematologic malignancies—further testament to the need for further investigation in these patients—it should be noted that much of the conducted research discussed in this review included solid tumor oncology patients in addition to patient with blood cancers. In this review, we highlight specifically how these financial-related issues affect this highly vulnerable population of patients with hematologic malignancies in both these combined studies, and in studies looking specifically at the population of interest.
Defining Financial Toxicity and Its Impact on Patient Outcomes
At the outset, it is important to note that there is no standardized definition of FT. In 2007, the American Society of Clinical Oncology (ASCO) established the Cost of Care Task Force and issued guidance for oncologists to include the financial impacts of cancer care, including diagnostic and out-of-pocket expenses, into the shared decision-making process for treatment [20]. Since that time, the term “financial toxicity” was designated to measure the objective financial burden on patients and their families from cancer care [21]. Additionally, measures of FT typically attempt to quantify the psychological distress, coping behaviors, and lost productivity experienced by patients and their caregivers [14, 21, 22]. Many studies incorporate the financial stress experienced by patients in relation to their cancer care—including cost of treatment and supportive medications, testing, transportation, and time off work for patients and caregivers—into the definition [23]. Other variables included in FT encompass insurance or payor coverage, societal or cultural factors, and geography [23,24,25,26,27]. Several patient-reported outcomes in oncology have been associated with FT, including health-related quality of life (HR-QOL), burden of symptoms, medication noncompliance, and survival [27].
Recent Findings
The Landscape of Financial Toxicity in Hematologic Malignancies
Hematologic malignancies encompass a wide range of disease states and treatment modalities, which are often associated with both higher morbidity and mortality, and healthcare utilization. However, to date, the majority of FT-related work has been in solid tumor oncology, with few studies having assessed FT in patients with blood cancers [28,29,30]. One category of patients likely to experience severe FT are those diagnosed with acute leukemia, whether acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), as they are often diagnosed and treated in the inpatient setting initially with induction chemotherapy which is then followed by months of intensive treatment potentially including HSCT [31,32,33]. Myeloid disorders, such as chronic myeloid leukemia (CML), which are considered to be more chronic, can generally be managed in the outpatient setting. However, these conditions still cause patients to experience high rates of FT, with the price of treatments such as the oral tyrosine kinase inhibitors (TKIs) being prohibitive for many of these patients and can lead to noncompliance [34]. Similarly, patients with myelodysplastic syndrome (MDS) or myeloproliferative neoplasms (MPNs) also suffer from FT, although financial implications vary given the treatment can range from observation all the way to HSCT [35,36,37].
Financial-related concerns are also common in patients diagnosed with lymphoid disorders, with treatment varying from high-dose chemotherapy delivered in the inpatient setting to outpatient combination chemotherapy [38,39,40]. For example, in one study performed by Morrison et al., in newly diagnosed diffuse large B-cell lymphoma (DLBCL) patients and follicular lymphoma (FL) patients, the average per-patient, per-month (PPPM) costs during the first 12 months after diagnosis was $14,402 and $12,183, respectively [41]. Unfortunately, patients with plasma cell disorders fair no better financially. These patients have seen the development of several new and novel therapies that has increased survival over the past several decades [42], but these therapies do come at an increased cost, with one study demonstrating that multiple myeloma patients may use over one-third of their income on treatment-related costs within the first year of their diagnosis [43]. Compounding this financial distress is the fact that many patients receive multiple lines of therapy throughout their lifetime [44].
One up-and-coming therapy across a broad spectrum of hematologic malignancies is the use of chimeric antigen receptor T-cells (CAR T). However, this therapy comes with immense cost [45], with two of the CD-19 directed CAR-T products used in lymphoma—tisagenlecleucel and axi-cel—being priced at $475,000 and $373,000, respectively [46]. In addition to acquisition costs, many CAR-T products require hospitalization to monitor for adverse effects such as cytokine release syndrome (CRS) or neurotoxicity [47]. Also note that potential transportation, lodging, and follow-up evaluations at a CAR-T capable treatment center contribute to patient cost and FT related to their care.
Lastly, it is important that we recognize that the harmful effects of FT are not limited to patients alone. There is a growing recognition in the literature of the deleterious impact on patients’ families and caregivers, who often share in the financial hardships and challenges of a patient’s diagnosis. In one study involving 37 caregivers of patients 15–39 years old diagnosed with any cancer, 43.8% were suggested to be experiencing a high level of financial toxicity [48]. Specific to hematologic cancers, another study reported 85% of caregivers of patients with blood cancer indicating possible FT prior to the study intervention and 26.5% indicating issues with finances or insurance in the prior week [49]. However, this is an area in urgent need of additional research, as the available data remains limited to a handful of smaller-scale studies.
Assessing for Financial Toxicity in Blood Cancer Patients
One of the most fundamental interventions required to improve FT in cancer patients, generally, is identifying which patients are at risk for, or currently experiencing, the effects of FT [14, 30]. Current implementation of routine screening is variable [50] but improving, with one 2018 survey of 17 National Cancer Comprehensive Network (NCCN) member institutions reporting that 13 of these facilities routinely screened for financial distress. Similar rates were seen in a 2017 survey of community oncology practices, where 72% of respondents reported routine financial screening, albeit mainly on patient intake [51, 52]. The utility of recurrent screening and how often screening should occur throughout cancer care is expected to be a growing area of exploration [14]. To aid and standardize these assessments, several financial distress tools have been proposed and validated; however, it is extremely important to note the use and validation of many of these tools in the hematologic malignancy population which has been variable. In the hematologic malignancy population, the most used tools have been the COmprehensive Score for financial Toxicity (COST) measure, the Socioeconomic Wellbeing Scale (SWBS), and the European Organization for Research and Treatment of Cancer (EORTC). Additional tools include the InCharge Financial Distress/Financial Well-Being Scale (IFDFW) [53], the National Health Interview Survey (NHIS) [54], and the Medical Expenditure Survey (MEPS) [55]. A summary of these tools is provided in Table 1.
The most utilized and accepted assessment tool in the general FT research environment is the COST measure [28]. Lower values on the COST measure indicate increased FT, although there is no established threshold to categorize severe FT. The COST measure was subsequently validated in 2017; however, it should be noted that no patients with hematologic malignancies were included in the initial validation cohort [27]. The COST measure has been widely utilized in studies assessing FT in cancer patients and adopted by Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System as the standard for FT quantification [5, 56,57,58]. Given the length of the entire survey, some researchers have used pieces of this measure as a screening mechanism to attempt to identify patients at risk for FT prior to administration of the entire COST measure [30].
The SWBS was also developed by Head and Faul [59] and was adapted for use in one study by the Mayo Clinic to assess financial burden in patients who received allogeneic hematopoietic cell transplant. In this study, 268 patients completed the survey with 73% of respondents stating their illness was detrimental to their finances, 35% reporting adverse health behaviors due to financial burden, and 3% of respondents declaring bankruptcy [60].
The EORTC has been used in some studies to assess HR-QOL in relation to FT [61] and has been validated in patients with hematologic malignancies [62]; however, the questionnaire itself only includes 1 question directly related to a patient’s finances [63].
Interventions
In general, few interventions have been explored to directly assist patients with the FT of cancer care, with even fewer in patients with hematologic malignancies. Since FT has been linked to increased symptomology, such as anxiety and depression, pain, and overall severity of symptoms, as well as to decreased compliance with therapy [64], alleviation of patient financial distress has the potential to greatly improve patient outcomes. We will examine the current literature with an emphasis on levels of care, including providers, systems, and policies.
At the center of cancer care is the clinician-patient relationship. Previous work has shown that provider discussion surrounding FT during treatment-planning and patient education regarding treatment costs and mitigation strategies can help alleviate some of the burden experienced by these patients and caregivers [22, 65]. This is in keeping with the 2009 ASCO statement addressing FT in oncology and the impact on patient care [20]. Recommendations—including a thorough discussion with patients about their goals for their therapy, anticipated cost of treatment, time away from work, and information about treatment scheduling and lower cost options—should be made in the treatment planning process for patients [20]. However, routine discussion of these issues has been found to be highly variable in published work, with conversations regarding the costs of cancer care primarily being initiated by patients [66, 67]. This may indirectly communicate to patients that their financial situation is insignificant within their overall cancer care and builds on patient concerns that they may receive “inferior treatment” if costs of treatment are discussed [68,69,70]. As hematologic oncology providers often serve as the primary provider for their patients, it is imperative that they feel comfortable and supported initiating these conversations about financial distress [14]. In order to accomplish this, there needs to be a far greater emphasis placed on financial-related issues from both a system and policy perspective, as oncologists have routinely identified both lack of knowledge and resources as barriers to initiating these discussions [71, 72]. Moreover, because support is likely to remain variable depending on system priorities, it is imperative that providers continue to leverage third-party assistance with programs from professional organizations including ASCO, the American Society of Hematology (ASH), and non-profits in the hematologic malignancy space, such as the Leukemia and Lymphoma Society. This can be beneficial in improving patients’ financial literacy and potentially provide direct assistance for anticipatory costs which may not have been initially considered, such as the cost of transportation to frequent office visits.
From both a provider and system-level perspective, the most studied and efficacious intervention identified in FT has been financial navigation, which has been shown to help decrease out-of-pocket expenses for patients and somewhat mitigate the impact of FT [14, 73]. By definition, financial navigation incorporates a comprehensive evaluation by a member of the oncology team, such as a nurse or social worker, of a patients’ financial situation and assistance with applications for grants and financial relief programs for out-of-pocket costs, such as healthcare co-payments, household expenses, and transportation [30, 73]. Financial navigation also traditionally includes patient insurance coverage assessment and optimization, coordination with clinic pharmacists to ensure that medication costs are minimized, and collaboration with supportive oncology resources, such as counseling, to ensure patients and their caregivers are receiving full psychosocial support through their cancer care and financial distress [14]. Early efforts in financial navigation have been encouraging, with Shankran et al. demonstrating the feasibility of implementing a comprehensive navigation program within the standard of care [74]. Wheeler et al. subsequently described patient-reported improvements in their financial distress via the COST measure after implementing a thorough financial navigation intervention for 50 patients experiencing FT. Patient satisfaction with the financial navigation intervention was also high, and internally, there was high-utilization of the financial resources offered within the protocol [75]. Financial navigation for cancer patients has also yielded institutional benefits, with Yezefski et al. demonstrating that financial navigators alleviated monetary losses for healthcare institutions by increasing payment for services rendered through improved patient access to insurance or patient assistance coverage [73], where previously these services would have gone unpaid.
Financial navigation has been less studied in patients with hematologic malignancies, but the practice is increasing. One recent study by Edward et al. incorporated 54 patients and 32 caregivers within the division of hematology and bone marrow transplant (BMT) who were screened for FT and subsequently placed into a financial navigation program. This intervention consisted of a financial navigator specific to the hematology and BMT division that would discuss FT and expected treatment-related costs with patients. They would also assist with applications for financial assistance and connect patients with other psychosocial resources as needed. Additionally, navigators would assist with financial aspects of discharge planning after hospitalization. Results showed that this intervention improved the psychological and COST scores of patients, as well as COST scores of caregivers. Notably, only 27% of eligible patients were enrolled in the study, but 100% of eligible caregivers participated [49]. Similarly, Knight et al. utilized a screening tool consisting of two questions from the COST measure to identify hematologic malignancy patients experiencing financial distress for focused intervention. The intervention group received financial navigation performed by oncology nurse navigators, as well as pharmacy, social work, and community pro bono financial counselors. Results showed improvements in both mental and physical quality of life for the intervention cohort. There was also a suggestion of improvement in mortality in the high-risk disease group who received the intervention [30]. Notably, this financial navigation related work has led to an ongoing study, S1912CD (NCT04960787), by the Southwest Oncology Group (SWOG) that includes patients with hematologic or lymphoid neoplasms and is analyzing a financial navigation intervention for patients and their spouses. Overall, these findings indicate that a multidisciplinary approach to reduce FT in these patients improves clinically significant outcomes and is necessary for continued improvement in the care of patients with blood cancer.
At the institutional level, published work has primarily focused on increased screening to leverage existing supportive resources. This is demonstrated in one recent study among pediatric oncology patients in which 9 poverty-exposed families of pediatric cancer patients were provided assistance with transportation or grocery delivery over a 3-month period to help alleviate hardships related to household needs with results showing high satisfaction with the program [76]. One novel idea that was published by Raghavan et al. was the establishment of a multidisciplinary monthly conference dubbed the “financial toxicity tumor board,” to address the FT experienced by patients being treated at its cancer institute [26]. Monthly meetings allow attendees to address specific patient cases, while also focusing on institutional and internal procedures to identify specific systemic improvements. Over the first 2 years of these interventions, they reported 3568 patients who have been helped through these efforts with $119,030,371 noted in savings [26]. As potential solutions for financial-related issues require the coordination of multiple disparate areas of a cancer center—including clinical, pharmaceutical, billing, and administration—who may not routinely interact, the establishment of a multidisciplinary discussion forum for financial concerns in cancer care can facilitate an exchange of ideas and raise awareness of solutions that may have previously been employed in similar dilemmas.
Finally, the necessity of governmental and policy interventions, both in terms of broad policies and focused initiatives, to mitigate the impact of FT on patients cannot be understated. Whereas many of the interventions described here rely on the hard work of providers and associated patient-focused staff to lessen the impact of FT on patients, only well-designed and carefully implemented governmental programs and policies will be able to achieve this effect on a nationwide scale. This is most clearly illustrated by the effects on outcomes following the expansion of Medicaid under the Affordable Care Act (ACA), which allows states to opt-in to Medicaid coverage—41 of which had done so by March 2023—for eligible adults with an income up to 138% of the poverty line [77]. Previous reports have documented that insurance coverage gaps have been associated with a decreased likelihood of initiating cancer treatment [78] and delays in treatment [79]. Conversely, a study of states that expanded Medicaid from 2000 to 2007 versus those that did not show significant reductions in all-cause mortality, with the greatest reductions among groups that frequently suffer from FT, including older adults and nonwhite patients [80]. Callison et al. demonstrated that Medicaid expansion in Louisiana was associated with decreased cancer deaths among the black population and decreased the racial mortality gap in cancer deaths by 57% for women and 49% for men [81]. Furthermore, Sommers found, in a follow-up to the 2000 to 2007 study, that Medicaid expansion reduced death rates among adults under 65 by 6% and that these gains were largely concentrated in treatable conditions that otherwise may have gone untreated. Sommers estimated that the improvements in death rates translated into one additional life being saved for every 239 to 316 extra individuals insured [82]. Extrapolating from this estimate, Mailankody et al. estimated that, based upon 20 million additional individuals gaining health insurance under the ACA, between 63,291 and 83,682 lives were saved in the first 7 years of the program [83]. Unfortunately, despite these efforts, there remains a significant proportion of patients without insurance, or with inadequate insurance, who will be at high risk for financial-related complications. Further policy reforms are urgently needed to both improve coverage issues and to address the structural imbalances that lead to financial hardship.
Summary
While FT is not a new problem in cancer care, awareness about FT and the effect that it has on patient quality of life and clinical outcomes still lags in needed research and changes to address its harms. Several studies have investigated causes of FT and the associated detriment to these patients, but relatively few have created effective strategies to intervene for patients at risk for or experiencing FT during their cancer care. More broadly, as the treatment landscape for hematologic malignancy management constantly evolves, so should the approaches we take to lessen the strain that cancer care places on patients and their families. With an ever-increasing number of cancer survivors, and patients receiving palliative therapies living longer, we have a responsibility to take action to reduce the prevalence and severity of FT, so that these patients and their families do not compound the tragedy of a cancer diagnosis with the tragic unraveling of their financial security as well.
Data Availability
Data sharing not applicable – no new data generated.
References
Fitch K, Ferro C, Pittinger S. The cost burden of blood cancer care in Medicare A longitudinal analysis of Medicare Advantage and Fee for Service patients diagnosed with blood cancer. Published online 2019.
Dieguez G, Ferro C, Rotter D. The cost burden of blood cancer care: a longitudinal analysis of commercially insured patients diagnosed with blood cancer. 2018.
Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119(20):3710–7. https://doi.org/10.1002/CNCR.28262.
Knight TG, Deal AM, Dusetzina SB, et al. Financial toxicity in adults with cancer: adverse outcomes and noncompliance. J Oncol Pract. 2018;14(11) https://doi.org/10.1200/JOP.18.00120.
Huntington SF, Weiss BM, Vogl DT, et al. Financial toxicity in insured patients with multiple myeloma: a cross-sectional pilot study. Lancet Haematol. 2015;2(10):e408–16. https://doi.org/10.1016/S2352-3026(15)00151-9.
Ramsey SD, Bansal A, Fedorenko CR, et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;34(9):980–6. https://doi.org/10.1200/JCO.2015.64.6620.
Ma SJ, Iovoli AJ, Attwood K, et al. Association of significant financial burden with survival for head and neck cancer patients treated with radiation therapy. Oral Oncol. 2021:115. https://doi.org/10.1016/J.ORALONCOLOGY.2021.105196.
Chino F, Zafar SY. Financial toxicity and equitable access to clinical trials. Am Soc Clin Oncol Educ Book. 2019;39(39):11–8. https://doi.org/10.1200/EDBK_100019.
Nipp RD, Lee H, Gorton E, et al. Addressing the financial burden of cancer clinical trial participation: longitudinal effects of an equity intervention. Oncologist. 2019;24(8):1048–55. https://doi.org/10.1634/THEONCOLOGIST.2019-0146.
Pak TY, Kim H, Kim KT. The long-term effects of cancer survivorship on household assets. Health. Econ Rev. 2020;10(1) https://doi.org/10.1186/S13561-019-0253-7.
Doroudi M, Coughlan D, Banegas MP, Han X, Robin YK. Is cancer history associated with assets, debt, and net worth in the United States? JNCI Cancer Spectr. 2018;2(2) https://doi.org/10.1093/JNCICS/PKY004.
Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood). 2013;32(6):1143–52. https://doi.org/10.1377/HLTHAFF.2012.1263.
Mehnert A, De Boer A, Feuerstein M. Employment challenges for cancer survivors. Cancer. 2013;119(SUPPL11):2151–9. https://doi.org/10.1002/CNCR.28067.
Smith GL, Banegas MP, Acquati C, et al. Navigating financial toxicity in patients with cancer: a multidisciplinary management approach. CA Cancer J Clin. 2022;72(5):437–53. https://doi.org/10.3322/caac.21730.
Preussler JM, Meyer CL, Mau LW, et al. Healthcare costs and utilization for patients age 50 to 64 years with acute myeloid leukemia treated with chemotherapy or with chemotherapy and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2017;23(6):1021–8. https://doi.org/10.1016/J.BBMT.2017.02.017.
Zhu F, Wei G, Zhang M, et al. Cell Transplant. 2020:29. https://doi.org/10.1177/0963689720919434.
Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. 2003;348(19):1875–83. https://doi.org/10.1056/NEJMOA022340.
Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349–61. https://doi.org/10.1001/JAMA.2009.813.
Rashidi A, Ebadi M, Cashen AF. Allogeneic hematopoietic stem cell transplantation in Hodgkin lymphoma: a systematic review and meta-analysis. Bone Marrow Transplant. 2016;51(4):521–8. https://doi.org/10.1038/BMT.2015.332.
Meropol NJ, Schrag D, Smith TJ, et al. American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol. 2009;27(23):3868–74. https://doi.org/10.1200/JCO.2009.23.1183.
Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18(4):381–90. https://doi.org/10.1634/THEONCOLOGIST.2012-0279.
Lentz R, Benson AB, Kircher S. Financial toxicity in cancer care: prevalence, causes, consequences, and reduction strategies. J Surg Oncol. 2019;120(1):85–92. https://doi.org/10.1002/JSO.25374.
Ouchveridze E, Banerjee R, Desai A, et al. Financial toxicity in hematological malignancies: a systematic review. Blood Cancer J. 2022;12(4). https://doi.org/10.1038/S41408-022-00671-Z.
Su CT. Financial toxicity interventions in hematologic malignancies are timely and necessary. JCO Oncol Pract. 2022;18(9):607–9. https://doi.org/10.1200/op.22.00357.
Su CT, Shankaran V. Defining the role of the modern oncology provider in mitigating financial toxicity. J Am Coll Radiol. 2022. https://doi.org/10.1016/j.jacr.2022.10.011.
Raghavan D, Keith NA, Warden HR, et al. Levine Cancer Institute Financial Toxicity Tumor Board: a potential solution to an emerging problem. JCO Oncol Pract. 2021;17(10):e1433–9. https://doi.org/10.1200/op.21.00124.
de Souza JA, Yap BJ, Wroblewski K, et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: the validation of the COmprehensive Score for financial Toxicity (COST). Cancer. 2017;123(3):476–84. https://doi.org/10.1002/CNCR.30369.
De Souza JA, Yap BJ, Hlubocky FJ, et al. The development of a financial toxicity patient-reported outcome in cancer: the COST measure. Cancer. 2014;120(20):3245–53. https://doi.org/10.1002/CNCR.28814.
Chino F, Peppercorn JM, Rushing C, et al. Out-of-pocket costs, financial distress, and underinsurance in cancer care. JAMA Oncol. 2017;3(11):1582–4. https://doi.org/10.1001/JAMAONCOL.2017.2148.
Knight TG, Aguiar M, Robinson M, et al. Financial toxicity intervention improves outcomes in patients with hematologic malignancy. JCO Oncol Pract. 2022;18(9):e1494–504. https://doi.org/10.1200/op.22.00056.
Pelcovits A, Niroula R. Acute myeloid leukemia: a review. R I Med J (2013). 2020;103(3):38-40. http://www.ncbi.nlm.nih.gov/pubmed/32236160 . Accessed 3/19/2023
Litzow MR, Ferrando AA. How I treat T-cell acute lymphoblastic leukemia in adults. Blood. 2015;126(7):833–41. https://doi.org/10.1182/blood-2014-10-551895.
Hoelzer D, Bassan R, Dombret H, Fielding A, Ribera JM, Buske C. Acute lymphoblastic leukaemia in adult patients: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(February):v69–82. https://doi.org/10.1093/annonc/mdw025.
Dusetzina SB, Winn AN, Abel GA, Huskamp HA, Keating NL. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32(4):306–11. https://doi.org/10.1200/JCO.2013.52.9123.
Jain AG, Elmariah H. BMT for myelodysplastic syndrome: when and where and how. Front Oncol. 2022;11(January):1–13. https://doi.org/10.3389/fonc.2021.771614.
Fenaux P, Platzbecker U, Ades L. How we manage adults with myelodysplastic syndrome. Br J Haematol. 2020;189(6):1016–27. https://doi.org/10.1111/bjh.16206.
Knight TG, Robinson M, Ai J, et al. Patient reported financial toxicity in myeloproliferative neoplasms. Blood. 2019;134(Supplement_1):2099. https://doi.org/10.1182/BLOOD-2019-128858.
Sehn LH, Salles G. Diffuse large B-cell lymphoma. Longo DL, ed. N Engl J Med. 2021;384(9):842-858. doi:https://doi.org/10.1056/NEJMRA2027612.
Freedman A, Jacobsen E. Follicular lymphoma: 2020 update on diagnosis and management. Am J Hematol. 2020;95(3):316–27. https://doi.org/10.1002/AJH.25696.
Ansell SM. Hodgkin lymphoma: a 2020 update on diagnosis, risk-stratification, and management. Am J Hematol. 2020;95(8):978–89. https://doi.org/10.1002/AJH.25856.
Morrison VA, Bell JA, Hamilton L, et al. Economic burden of patients with diffuse large B-cell and follicular lymphoma treated in the USA. Future Oncol. 2018;14(25):2627–42. https://doi.org/10.2217/FON-2018-0267.
Rajkumar SV, Kumar S. Multiple myeloma current treatment algorithms. Blood Cancer J. 2020;10(9) https://doi.org/10.1038/s41408-020-00359-2.
Goodwin JA, Coleman EA, Sullivan E, et al. Personal financial effects of multiple myeloma and its treatment. Cancer Nurs. 2013;36(4):301–8. https://doi.org/10.1097/NCC.0b013e3182693522.
Fiala MA, Silberstein AE, Schroeder MA, Stockerl-Goldstein KE, Vij R. The dynamics of financial toxicity in multiple myeloma. Clin Lymphoma Myeloma Leuk. 2023:1–7. https://doi.org/10.1016/j.clml.2023.01.008.
Cusatis R, Tan I, Piehowski C, et al. Worsening financial toxicity among patients receiving chimeric antigen receptor t-cell (CAR-T) therapy: a mixed methods longitudinal study. Blood. 2021;138(Supplement 1):567. https://doi.org/10.1182/BLOOD-2021-146032.
Hay AE, Cheung MC. CAR T-cells: costs, comparisons, and commentary. J Med Econ. 2019;22(7):613–5. https://doi.org/10.1080/13696998.2019.1582059.
Schubert ML, Schmitt M, Wang L, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32(1):34–48. https://doi.org/10.1016/j.annonc.2020.10.478.
Baum LV, Koyama T, Schremp EA, et al. Posttraumatic stress symptoms and financial toxicity among adolescent and young adult oncology patients and their caregivers at cancer diagnosis. Cancer. 2022;128(10):2005–14. https://doi.org/10.1002/cncr.34146.
Edward JS, Mclouth LE, Rayens MK, Eisele LP, Davis TS. Coverage and cost-of-care links: addressing financial toxicity among patients with hematologic cancer and their caregivers coverage and cost-of-care links: addressing financial toxicity among patients with hematologic cancer and their caregivers. J Clin Oncol. 2023. https://doi.org/10.1200/OP.22.00665.
Voleti SS, Warsame R, Mead-Harvey C, et al. Assessing patient-reported financial hardship in patients with cancer in routine clinical care. JCO Oncol Pract. 2022;18(11):e1839–53. https://doi.org/10.1200/OP.22.00276.
Khera N, Sugalski J, Krause D, et al. Current practices for screening and management of financial distress at NCCN member institutions. JNCCN J Natl Compr Canc Netw. 2020;18(7):825–31. https://doi.org/10.6004/jnccn.2020.7538.
McLouth LE, Nightingale CL, Dressler EV, et al. Current practices for screening and addressing financial hardship within the NCI Community Oncology Research Program. Cancer Epidemiol Biomarkers Prev. 2021;30(4):669–75. https://doi.org/10.1158/1055-9965.EPI-20-1157.
Prawitz A, Garman ET, Sorhaindo B, O’Neill B, Kim J, Drentea P. Incharge financial distress/financial well-being scale: development, administration, and score interpretation. J Financial Couns Plan. 2006;17(1):34–50.
Statistics NC for H. About the National Health Interview Survey. Published 2022. https://www.cdc.gov/nchs/nhis/about_nhis.htm. Accessed 5/31/2023
Quality A for HR and Medical Expenditure Panel Survey: survey background. Published 2019. https://meps.ahrq.gov/mepsweb/about_meps/survey_back.jsp . Accessed 5/31/2023
Bouberhan S, Shea M, Kennedy A, et al. Financial toxicity in gynecologic oncology. Gynecol Oncol. 2019;154(1):8–12. https://doi.org/10.1016/J.YGYNO.2019.04.003.
Honda K, Gyawali B, Ando M, et al. Prospective survey of financial toxicity measured by the comprehensive score for financial toxicity in Japanese patients with cancer. J Glob Oncol. 2019;2019(5):1–8. https://doi.org/10.1200/JGO.19.00003.
FACIT Group. COST: a FACIT measure of financial toxicity. Accessed April 3, 2023. https://www.facit.org/measures/FACIT-COST.
Head B, Faul AC. Development and validation of a scale to measure socioeconomic well-being in persons with cancer. J Support Oncol. 2008. https://doi.org/10.1037/E548132012-061.
Khera N, Chang Y, Hui HS, et al. Financial burden in recipients of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20(9):1375–81. https://doi.org/10.1016/J.BBMT.2014.05.011.
Rogers SN, Harvey-Woodworth CN, Hare J, Leong P, Lowe D. Patients’ perception of the financial impact of head and neck cancer and the relationship to health related quality of life. Br J Oral Maxillofac Surg. 2012;50(5):410–6. https://doi.org/10.1016/j.bjoms.2011.07.026.
Efficace F, Cottone F, Sommer K, et al. Validation of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 Summary Score in Patients With Hematologic Malignancies. Value Health. 2019;22(11):1303–10. https://doi.org/10.1016/j.jval.2019.06.004.
Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L23073154 . Accessed 3/21/2023
Chan RJ, Gordon LG, Tan CJ, et al. Relationships between financial toxicity and symptom burden in cancer survivors: a systematic review. J Pain Symptom Manage. 2019;57(3):646–660.e1. https://doi.org/10.1016/j.jpainsymman.2018.12.003.
Khera N, Kumbamu A, Langer SL, et al. Developing an educational intervention to address financial hardship in cancer patients. Mayo Clin Proc Innov Qual Outcomes. 2020;4(4):424. https://doi.org/10.1016/J.MAYOCPIQO.2020.04.004.
Warsame R, Kennedy CC, Kumbamu A, Branda M, Fernandez C, Kimball B. Conversations about financial issues in Routine Oncology Practices: a multicenter study conversations about financial issues in Routine Oncology Practices: a multicenter study. J Oncol Pract. 2019;15(8):e690–703.
Hamel LM, Penner LA, Eggly S, et al. Do patients and oncologists discuss the cost of cancer treatment? An observational study of clinical interactions between African American patients and their oncologists. J Oncol Pract. 2017;13(3):e249–58. https://doi.org/10.1200/JOP.2016.015859.
Irwin B, Kimmick G, Altomare I, et al. Patient experience and attitudes toward addressing the cost of breast cancer care. Oncologist. 2014;19(11):1135–40. https://doi.org/10.1634/THEONCOLOGIST.2014-0117.
Bullock AJ, Hofstatter EW, Yushak ML, Buss MK. Understanding patients’ attitudes toward communication about the cost of cancer care. J Oncol Pract. 2012;8(4) https://doi.org/10.1200/JOP.2011.000418.
Meisenberg BR, Varner A, Ellis E, et al. Patient attitudes regarding the cost of illness in cancer care. Oncologist. 2015;20(10):1199–204. https://doi.org/10.1634/theoncologist.2015-0168.
Shih YCT, Chien CR. A review of cost communication in oncology: patient attitude, provider acceptance, and outcome assessment. Cancer. 2017;123(6):928–39. https://doi.org/10.1002/CNCR.30423.
Altomare I, Irwin B, Zafar SY, et al. Physician experience and attitudes toward addressing the cost of cancer care. J Oncol Pract. 2016;12(3):e281–8. https://doi.org/10.1200/JOP.2015.007401.
Yezefski T, Steelquist J, Watabayashi K, Sherman D, Shankaran V. Impact of trained oncology financial navigators on patient out-of-pocket spending. Am J Manag Care. 2018;24(5):S74–9.
Shankaran V, Leahy T, Steelquist J, et al. Pilot feasibility study of an oncology financial navigation program. J Oncol Pract. 2018;14(2):e122–9. https://doi.org/10.1200/JOP.2017.024927.
Wheeler SB, Rodriguez-O’Donnell J, Rogers C, Fulcher J, Deal A, Manning ML, Gellin M, Padilla NR. Reducing cancer-related financial toxicity through financial navigation: results from a pilot intervention. Cancer Epidemiol Biomark Prevent. 2020;29(3):694. https://doi.org/10.1158/1055-9965.EPI-20-0067.
Umaretiya PJ, Revette A, Seo A, et al. PediCARE: Development of a poverty-targeted intervention for pediatric cancer. Pediatr Blood Cancer. 2021;68(10). https://doi.org/10.1002/PBC.29195.
Foundation KF. Status of State Medicaid Expansion Decisions: Interactive Map.; 2023. https://www.kff.org/medicaid/issue-brief/status-of-state-medicaid-expansion-decisions-interactive-map/. Accessed 4/2/2023
Dawes AJ, Louie R, Nguyen DK, et al. The impact of continuous Medicaid enrollment on diagnosis, treatment , and survival in six surgical cancers. Health Serv Res. 2014;49(6):1787–811. https://doi.org/10.1111/1475-6773.12237.
Tsui J, DeLia D, Stroup AM, Nova J, Kulkarni A, Ferrante JM, Cantor JC. Association of Medicaid enrollee characteristics and primary care utilization with cancer outcomes for the period spanning Medicaid expansion in New Jersey. Cancer. 2019;125(8):1330–40. https://doi.org/10.1002/cncr.31824.
Sommers BD, Baicker K, Epstein AM. Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012;367(11):1025–34. https://doi.org/10.1056/NEJMSA1202099.
Callison K, Segal L, Zacharia G. Medicaid expansion and cancer mortality by race and sex in Louisiana. Am J Prevent Med. 2022;62(4):242–7.
Sommers BD. State medicaid expansions and mortality, revisited: a cost-benefit analysis. Am J Health Econ. 2017;3(3):392–421. https://doi.org/10.1162/ajhe_a_00080.
Mailankody S. Affordable Care Act, Health Insurance Coverage, and Cancer Outcomes. J Clin Oncol. 2023;35(35):3893–5. https://doi.org/10.1200/JCO.2017.75.4259.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sears-Smith, M., Knight, T.G. Financial Toxicity in Patients with Hematologic Malignancies: a Review and Need for Interventions. Curr Hematol Malig Rep 18, 158–166 (2023). https://doi.org/10.1007/s11899-023-00707-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-023-00707-6