Abstract
Although Hodgkin lymphoma (HL) is largely curable with first-line therapy, approximately one-third of patients will not have a complete response to frontline treatment or will subsequently relapse. Only 50 % of these patients will be effectively salvaged with conventional therapies. The prognosis is particularly poor for those patients with chemotherapy refractory disease, who are unable to obtain even transient disease control, and for patients who relapse following high dose chemotherapy and autologous stem cell transplant. In this review, we summarize the most recent updates on the management of patients with relapsed HL, the role of novel therapies such as brentuximab vedotin, and an overview of promising new agents currently under investigation. We also discuss the role of consolidation strategies such as high-dose chemotherapy and autologous stem cell transplant, and reduced-intensity allogeneic hematopoietic stem cell transplant, and the need for new strategies in the elderly patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
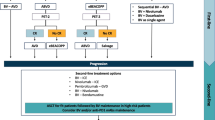
Hodgkin lymphoma (HL) is the most common lymphoma affecting the young population, with over 9,000 estimated new cases of HL in the US in 2014 [1]. The incidence of HL is bimodal with the highest incidence at age 15 to 34 years, and a second peak in those older than age 60 years. Despite the favorable outcome for most patients, approximately 25 % of patients will experience a relapse or be refractory to initial therapy. For these patients, only 50 % will be cured with standard salvage therapies [2]. Major challenges for the management of these relapsed patients include: improving risk stratification, the sequencing of standard therapy with promising new agents, and the role of consolidation strategies such as hematopoietic stem cell transplant for high-risk remission, both upfront and at the time of relapse.
Positron Emission Tomography and Other Predictive Models for Risk Stratification
While both established and novel risk stratification tools have been developed for newly diagnosed HL [3–8], predicting outcome for relapsed patients is less clearly defined. A remission duration of less than 1 year, advanced stage or extranodal disease at relapse, and B symptoms at relapse have all been reported as negative prognostic factors in this setting [9, 10]. More recently, for patients in first relapse undergoing autologous stem cell transplant (ASCT), a negative positron emission tomography (PET) scan suggestive of a complete remission (CR) has been identified as a significant predictor of progression-free survival (PFS) and overall survival (OS). Several retrospective studies reported a favorable PFS of 69 – 93 % at 3 – 5 years in patients with a normal PET prior to ASCT compared to PFS of between 10 – 41 % in patients with residual disease documented by PET pre-ASCT [11–15]. Similarly, the rate of OS in patients achieving a normal PET prior to ASCT was superior to OS in patients with disease showing abnormal PET, ranging between 87 – 97 % and 32 – 58 %, respectively [13–15].
The role of normal PET CR in predicting outcome after second and subsequent salvage regimens is less well defined. One phase II study of 97 patients suggested that the achievement of a CR after second-line salvage predicted a favorable outcome post ASCT, with an event-free survival (EFS) of 86 % versus 28.6 % in patients who had an abnormal PET [16••]. These data suggest that the elimination of minimal residual disease may be essential for ASCT-eligible patients to achieve long-term disease control in first and subsequent remissions. Early identification of patients with chemotherapy-sensitive disease, and selection of treatment regimens with the highest likelihood of disease control, is crucial for these patients.
For patients who relapse post-ASCT, cure is not possible with standard therapies. Some patients may obtain long-term disease control with experimental therapies or allogeneic stem cell transplant (allo-SCT). Relevant factors that have been reported by the European Group for Blood and Marrow Transplantation (EMBT) and the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) to be predictive of poor OS in the post-ASCT setting in a series of 511 patients include early relapse (<6 months from ASCT), stage IV, bulky disease, poor performance status, and age > =50 years at ASCT failure. In patients with no risk factors, the OS at 5 years was 62 % compared to 37 % and 12 % in those with 1 or 2 or more risk factors, respectively [17•].
The development and validation of predictive models for relapsed HL capable of identifying which patients are most likely to benefit from conventional salvage regimens and which patients should be considered for specific novel or intensive therapies, are increasingly necessary as treatment options for these patients continue to grow. Yet, risk-adapted therapeutic strategies for relapsed HL patients remain an unmet medical need.
The Role of Consolidation: Autologous Stem Cell Transplant (ASCT)
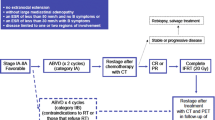
For over two decades, the standard management of fit patients with refractory or relapsed disease who obtain adequate disease control (CR or minimal residual disease) from salvage chemotherapy is ASCT which can induce durable responses in approximately 50 % of these patients [18, 19].
Commonly used salvage regimens include ifosfamide, carboplatin, and etoposide (ICE), which has been primarily investigated by the Memorial Sloan Kettering Cancer Center group with an overall response rate (ORR) of 84 %, including a CR rate of 26 % [20, 21], and the combination of dexamethasone, high-dose cytarabine, and cisplatin (DHAP), which shows similar response rates, (ORR 89 % including 21 % of CRs) [22]. Other gemcitabine-based combination regimens, including gemcitabine, dexamethasone, and cisplatin (GDP) [23]; gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVP) [24]; and gemcitabine, cisplatin, and prednisone (GEM-P) [25], have been developed with reported ORRs around 70 %, including approximately 20 % CR. In a recent, single-institution study reported by Hawkes, GEM-P produced an 80 % ORR with 37 % CR in a cohort of 47 relapsed HL patients with the added benefits of outpatient delivery, and non-cross toxicity with first-line therapy [26•].
More intensive alkylator based salvage regimens such as BCNU, etoposide, cytarabine, and melphalan (Mini-BEAM) and the German Hodgkin Study Group modification with dexamethasone (Dexa-BEAM) are rarely used as first-line salvage regimens in ASCT-eligible patients due to high toxicity and potential impairment of hematopoietic stem cell collection [27, 28].
Strategies Post-ASCT and/or Refractory Disease
Approximately 50 % of the patients who relapse after first-line therapy, do not achieve disease control with high-dose chemotherapy and ASCT. In this setting, HL is particularly challenging and requires innovative approaches. The FDA-approved antibody drug conjugate brentuximab vedotin, and several other novel agents, described in detail below, offer long-term disease control for subsets of these patients, but to date, HL relapsed after ASCT or refractory to chemotherapy remains largely incurable and is an area of unmet medical need.
Brentuximab Vedotin
Brentuximab vedotin is an anti-CD30 antibody conjugated to monomethyl auristatin E, an anti-microtubule. A member of the tumor necrosis factor (TNF) receptor superfamily, CD30 is a trans-membrane glycoprotein receptor expressed on the surface of Hodgkin Reed Steinberg cells (HRS) and anaplastic large-cell lymphoma (ALCL) cells. It is involved in regulating lymphocyte proliferation, differentiation, and apoptosis, and is not expressed on resting T and B cells, or cells outside the immune system [29]. Early trials, which targeted CD30 with the naked antibody (SGN-30), did not demonstrate significant activity in HL [30, 31], as outlined in Table 1, which summarizes the results of all major clinical trials conducted with agents targeting CD30.
Early phase I studies of relapsed HL and ALCL treated with brentuximab demonstrated that it has significant activity with a favorable safety profile [32•, 33]. Subsequently, a pivotal open-label phase II study treated 102 patients with relapsed or refractory HL with brentuximab until disease progression or unacceptable toxicity with a goal of a maximum of 16 cycles. The ORR was 75 % including 34 % of CRs. The median PFS for all patients was 5.6 months, and the median duration of response (DOR) for those achieving a CR was 20.5 months. Grade 3 or higher adverse events were recorded in 55 % of the patients, with neutropenia (20 %), thrombocytopenia (8 %), peripheral sensory neuropathy (8 %), and anemia (6 %) being the most common [34••]. The German Hodgkin Study Group (GHSG) reported ORR of 60 % and 22 % CRs in a series of 45 heavily pretreated HL patients, including 64 % refractory patients. Median DOR was 8 months [35]. This high ORR in heavily pretreated HL patients with a tolerable safety profile led to the accelerated FDA approval of brentuximab in 2011 for the treatment of HL relapsed either after ASCT or after two lines of combination chemotherapy in patients not eligible for ASCT. Based on the rationale that patients refractory to salvage chemotherapy are not eligible for ASCT, the National Comprehensive Cancer Network guidelines includes brentuximab as a therapeutic option for patients with HL relapsed after ASCT or at least two prior therapies regardless of their eligibility for transplant.
Treatment with brentuximab has been explored also as a bridge to ASCT for patients refractory to conventional chemotherapy. In a retrospective study, 14 patients with primary refractory or relapsed HL, who had not received prior high-dose chemotherapy and ASCT secondary to refractory disease (n = 9), comorbidity (n = 4), or unknown reasons (n = 1), were treated with brentuximab with an ORR of 71 % (10/14) with 34 % CRs. Of the nine patients with refractory disease, five obtained a response (two PR and three CR) becoming eligible for consolidation with ASCT [36]. In another series, 18 patients with relapsed or refractory HL were treated with brentuximab followed by reduced-intensity conditioning (RIC) allogeneic (allo)-SCT. Of these, six achieved a CR and eight a PR. Eleven required unrelated or mismatched donors. All patients engrafted; the incidence of acute GVHD was 27.8 % and chronic GVHD was 56.3 %. The 2-year OS and PFS were 79 % and 57.5 %, respectively [37]. In another series, 24 patients with relapsed or refractory HL underwent four cycles of therapy with brentuximab, 15 achieved adequate disease control (ORR 66 %, including 45.8 % of CR) and proceeded to consolidation with allo-SCT (9/15), ASCT (3/15), and tandem ASCT (3/15). With a median follow up of 20 months no deaths or relapses observed. No data on the incidence of GVHD provided [38•].
Two ongoing studies are examining the role of brentuximab as first-line salvage therapy prior to ASCT. Chen reported the interim results of brentuximab as first-line salvage therapy at the 2012 meeting of the American Society of Hematology, reporting an ORR of 87.5 % and a CR rate of 50 % [39]. MSKCC presented their interim results of sequential brentuximab-ICE chemotherapy at the 2013 International Conference on Malignant Lymphoma, reporting a CR rate of 33 % with single agent brentuximab and a CR rate of 92 % with the sequential strategy [40].
A randomized, double-blind, placebo-controlled, multicenter phase III study to evaluate the potential of brentuximab to prevent relapse in patients at high risk of residual HL post-ASCT is currently ongoing, and expected to be completed in 2016 (AETHERA trial NCT01100502). No data have been reported to date.
Brentuximab also appears to be effective as a retreatment strategy in selected patients. In 21 patients with HL who were retreated with brentuximab, the ORR was 60 % with 30 % CR and an estimated median DOR of 9.5 months. Adverse events leading to treatment discontinuation occurred in 31 % of the patients, and were generally similar to those observed with upfront therapy with the exception of peripheral neuropathy, which was more severe in this population. At pretreatment baseline, 48 % of the patients entering the study had preexisting neuropathy, and 69 % of the patients experienced newly occurring or worsening of baseline neuropathy [41•].
In patients relapsed after allo-SCT, brentuximab is effective, but appears to have significant toxicity. In a series of 25 brentuximab naïve relapsed HL patients who were heavily pre-treated (median of nine lines of therapy), the ORR was 50 % including 38 % CRs, with a median PFS of 7.8 months. More than a third of the patients in this study (36 %) had to suspend the treatment because of adverse effects, however. This increase in adverse effects, 36 % compared to 20 % reported in the pivotal trial [34••], is likely a consequence of the cumulative toxicity of brentuximab in the context of comorbidities from prior lines of intensive therapy [42•].
Novel Agents Under Investigation
Several novel agents are under active investigation for patients with relapsed HL. These are discussed below, and summarized in Table 2.
Cytotoxic Agents
Bendamustine
Bendamustine is a chemotherapy agent that combines the alkylating activity of a mustard group with the antimetabolite properties of a purine analog. Bendamustine was initially synthesized in Germany in the 1960 s [43] and has been used since that time against both HL and NHL. Bendamustine has activity in the upfront treatment of indolent NHL [44, 45], and also appears to have significant activity in relapsed HL [46]. In 2013, three reports were published on the activity of bendamustine in the treatment of heavily pretreated HL patients [47••, 48•, 49•]. The efficacy of bendamustine was tested in a phase II study of 36 relapsed and refractory HL patients, with a median age of 34 years (range 21 – 75) and a median of four prior treatments, including ASCT (75 %) and allo-SCT (6 %). Patients were treated with bendamustine at a dose of 120 mg/m [2] on days 1 and 2 of a 28-day cycle, for six planned cycles. The ORR was 53 %, including 33 % CRs, and the median PFS was 5.2 months. No responses were seen in patients who relapsed within 3 months of ASCT. Interestingly, only 20 % of the patients eligible for allo-SCT ultimately underwent transplant. Authors attributed the disappointing progression to allo-SCT to the fact that many patients progressed on treatment, after obtaining an initial response. Toxicity was mild, with infrequent grade ≥ 3 adverse events, most commonly thrombocytopenia (20 %), anemia (14 %), and infection (14 %) [47••].
Two retrospective studies, one multicenter Italian study, and one single-institution French study, showed consistent results confirming the high ORR [48•, 49•]. The patient population was similar and included heavily pretreated, relapsed or refractory HL, with a median age of 32 – 33 years, and an average of four to five prior therapies, including ASCT in the majority of the cases. The dosing and schedule of bendamustine varied between the studies, and the patients included in each study (dose range 90–120 mg/ m2 every 21 or 28 days). Despite the differences in treatments the ORR was 58 % and 50 % respectively with a CR rate of approximately 33 %, and a median PFS of 9 and 5 months, respectively. Data for the first response evaluation, provided in the Italian study, showed ORR of 78 % after two courses of chemotherapy, which fell to 58 % at the final assessment. Of note, patients attaining a PR at early evaluation did not convert to CR upon treatment prolongation [49•].
These studies suggest that bendamustine has potential utility in the management of relapsed HL; however, the short response duration suggests that its impact is more likely as a bridge to transplant, or in combination with other agents, then as a stand-alone therapy. Combinations of bendamustine with other novel agents are currently being evaluated in clinical trials, including the combination of bendamustine plus gemcitabine (NCT01535924), bendamustine plus lenalidomide (NCT01412307), and bendamustine plus brentuximab for patients with HL after first relapse (NCT01657331).
Cell Signaling and Epigenetic Approaches
Everolimus
Everolimus is an antineoplastic agent, which inhibits the mammalian target of rapamycin (mTOR), a key regulatory kinase involved in cell growth, proliferation and survival, which has been suggested in vitro to have a role in HL cell survival [50]. The clinical activity of everolimus has been evaluated in a phase II study in 19 heavily pretreated patients with RR HL. The ORR was 47 % with eight patients achieving a PR and one patient a CR. The median time to progression (TTP) was 7.2 months, with four responders remaining progression-free at 12 months. The therapy was well tolerated with reversible myelosuppression the main toxicity [51].
Synergistic activity between everolimus and the histone deacetylase inhibitor (HDACI) panobinostat has been suggested by in vitro studies. That prompted a phase I trial of this novel combination; the ORR for panobinostat and everolimus was 43 % in 14 patients with RR HL; however, grade 3 or higher thrombocytopenia was reported in 64 % of the patients limiting the future development of this combination [52•].
Histone Deacetylase Inhibitors (HDACI)
The HDACI vorinostat may have a direct antiproliferative effect on HRS cells, and possibly, by an immune-mediated effect by altering the balance of cytokines and chemokines in the tumor microenvironment [53]. Vorinostat, and other HDACIs including mocetinostat and panobinostat, have been investigated as mono-therapies with panobinostat demonstrating the more promising single agent data [54•, 55, 56]. An ORR of 27 %, including 23 % PRs and 4 % CRs, was reported in a cohort of 129 patients with relapse post-ASCT, or refractory HL treated with panobinostat. The median PFS was 6.1 months with an estimated 1-year OS of 78 %. Primary toxicity was severe (grade 3/4) myelosuppression. Responses were associated with early reductions in serum thymus and activation-regulated chemokine (TARC) levels [54•, 55–57].
Hematological toxicity has been a challenging factor to date in the development of combination strategies of HDACI with other agents, such as everolimus as described above and chemotherapy agents.
Immune-Modulating Therapies
Lenalidomide
Lenalidomide is an immunomodulatory and antiangiogenetic agent that has been investigated in a multicenter phase 2 study in relapsed and refractory HL patients. Thirty-six patients, 86 % with prior ASCT, were treated with lenalidomide 25 mg daily on days 1 – 21 of a 28-day cycle until disease progression or an unacceptable adverse event. The ORR was 19 %. The treatment was well tolerated, with moderate hematological toxicity [58•]. This study along with other smaller ones [59, 60] suggest that lenalidomide has modest single agent in relapsed HL, and its activity may be enhanced by combinations with chemotherapy or other novel agents. Trials of the combinations of lenalidomide and temsirolimus, lenalidomide and bendamustine, and lenalidomide and romidepsin are currently being evaluated in phase I/II studies in relapsed HL (NCT01076543; NCT01412307; NCT01742793).
Rituximab
CD20 is rarely expressed by HRS, but it is usually expressed by reactive B cells present in the tumor microenvironment, which have been hypothesized as playing a role in supporting HRS via survival signals, and suppressing host immune response. A pilot study conducted in 2003 showed an ORR of 22 % in 22 patients with HL recurrent after a minimum of two therapies. The median DOR was 7.8 months. Interestingly, remissions were observed irrespectively of CD20 expression by the primary HRS tumor cells [61]. Based on these results, a prospective clinical trial using rituximab in combination with gemcitabine was conducted in RR HL. The ORR was 48 %, and appeared again to be independent of HRS cell CD20 expression, but the DOR was short (2.7 months) [62].
Iplimumab and Nivolumab
Another promising strategy currently under investigation for relapsed and refractory HL are immune-activating drugs, both as single agents and in combination with HRS-targeting drugs. The rationale is to enhance the tumor-directed immune activation of the tumor microenvironment, and in the case of combination therapy, simultaneously targeting tumor bulk and providing antigen release with a targeted therapy. A phase I study exploring the combination of the anti-CTLA-4 antibody ipilimumab with brentuximab is currently ongoing and enrolling patients (NCT01896999). The anti-PD-1 checkpoint inhibitor nivolumab has recently received breakthrough therapy designation for the treatment of patients with HL after the failure of ASCT and brentuximab. Trials of the anti-PD-1 checkpoint inhibitors nivolumab and MK-3475 currently include HL patients in their design (NCT01592370; NCT01953692).
Allogeneic Stem Cell Transplant
Over time, a significant improvement in survival for patients with hematologic diseases after allo-SCT has been reported [63]. Reasons postulated for these advances are the introduction of RIC regimens, improvements in high resolution HLA typing, better supportive care, more careful patient selection, and earlier referral for SCT. However, despite these advances in relapsed HL, the long-term disease control with allo-SCT remains suboptimal with a PFS ranging from 25 – 30 % and an OS of 35 – 60 % at 2 years. Relapse remains the major cause of treatment failure, followed by treatment related mortality [64, 65].
A recent, large prospective Phase II study conducted by the Grupo Español de Linfomas/Trasplante de Médula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation enrolled 78 relapsed and refractory HL patients who underwent a RIC allo-SCT with the conditioning regimen of fludarabine (150 mg/m2 iv) and melphalan (140 mg/m2 iv). Fourteen patients (18 %) were unable to be transplanted due to inadequate disease control and died from progressive HL after a median time from trial entry of 10 months; they were excluded from the analysis. For the allografted population, PFS was 48 % at 1 year and 24 % at 4 years. Patients allografted in complete remission had a significantly better outcome, with PFS at 1 and 4 years of 70 and 50 %, respectively. Post-allo-SCT outcomes were similar for related and unrelated donor transplants [66••]. Another recent study confirmed that disease burden at the time of transplant is an important predictor for clinical outcome; minimal residual disease (<2 cm) versus gross residual disease (>2 cm), was an independent predictor of outcome with 4 year OS of 71 % vs. 8 %, respectively [67].
Strategies to improve disease control prior to allo-SCT and increase the potential durable benefit from this high-risk therapy are an area of active investigation [68].
HL in Elderly Patients
HL in the elderly has a worse prognosis, and age is a risk factor in the International Prognostic Score (IPS) for this disease [5]. Several unfavorable factors associated with inferior outcomes appear with a higher incidence in the elderly patient population, including mixed cellularity histology, EBV-related disease, and advanced stage [69, 70]. A recent report described that decreased forkhead box P3 (FOXP3) Tregs and increased Granzyme–B-positive cells in tumor samples, predictive of poor prognosis, are associated with older age [71]. Patients older than 60 years are also more prone to have increased toxicities of chemotherapy and radiation therapy, due to underlying comorbidities, frequently resulting in insufficient dose-intensity [72]. Additionally, these patients are often excluded from randomized trials, so an evidence-based standard of care is lacking.
A recent, retrospective analysis on 105 patients investigated the second-line treatment and survival in older patients (median age 66 years) with relapsed or refractory HL after first-line therapy according to German Hodgkin Study Group during 1993 – 2007. Second-line treatment strategies included intensified salvage regimens (22 %), with only 20 % of the patients receiving ASCT (due to HL progression or intolerable toxicity), conventional poly-chemotherapy and/or salvage-radiotherapy with curative intent (42 %), and palliative approaches (31 %). Median OS for the entire cohort was 12 months. Patients were risk-stratified into three categories, based on early relapse (<12 months from initial therapy), advanced stage at relapse (III – IV), and anemia. In low-risk patients, the impact of therapy on survival was significant in favor of the conventional poly-chemotherapy/salvage radiotherapy approach. In high-risk patients, OS was low overall and did not differ significantly among treatment strategies [73•]. Toxicity leading to fatal events ranged between 7 and 13 % in the different treatment groups. The authors conclude that primarily low-risk older patients appear to benefit from conventional poly-chemotherapy/salvage radiotherapy approach. In frail or high-risk elderly patients, intensified therapies are toxic, and not efficacious enough to warrant their use.
In contrast, a small, retrospective study on 15 patients with relapsed and refractory HL aged 60 years or older, who completed high-dose chemotherapy and ASCT, showed OS and PFS not statistically different from those observed in a matched cohort of 157 younger patients treated in a similar manner. At a median follow-up of 2.5 years, OS were 88 % and 84 % and PFS were 73 % and 56 %, respectively (p = 0.80). This study suggested that in selected fit elderly patients with good risk relapsed HL, who are able to tolerate intensive chemotherapy, ASCT consolidation gives similar results to younger patients in terms of survival and PFS, without increased toxicity [74].
A recently published, retrospective study investigated the safety and efficacy of brentuximab in patients older than 60 years. An ORR was observed in 56 % of these patients with refractory/relapsed HL, establishing a role for this drug in a monitored setting. However, toxicity was significant in comparison to a younger patient cohort, with higher rates anemia (30 % vs. 10 %), peripheral sensory neuropathy (60 % vs. 46 %), and fatigue (58 % vs. 43 %). Other grade 3 – 4 adverse events (neutropenia, anemia, and thrombocytopenia), however, appeared to be similar in the groups (20 % vs. 16 %) [75•].
Conclusion
While multiply relapsed HL remains incurable, currently there are more treatment options than ever before available. Brentuximab is currently being investigated in frontline and first relapse, and in combination with both conventional and novel agents. Other exciting therapeutic strategies in development include immune modulating therapies which may further alter the landscape of relapsed HL if they are able to provide long term disease control. Improvements in risk stratification will help us better tailor appropriate therapies and therapeutic intensity to HL patients in relapse. For elderly and frail patients, dedicated clinical trails are needed to characterize better the fine balance between intensity of therapy, comorbidities, and disease risk.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of outstanding importance
Available at: http://seer.cancer.gov/statfacts/html/hodg.html. Accessed May 11th, 2014.
Evens AM, Hutchings M, Diehl V. Treatment of Hodgkin lymphoma: the past, present, and future. Nat Clin Pract Oncol. 2008;5:543–56.
Steidl C, Diepstra A, Lee T, et al. Gene expression profiling of microdissected Hodgkin Reed-Sternberg cells correlates with treatment outcome in classical Hodgkin lymphoma. Blood. 2012;120:3530–40.
Scott DW, Chan FC, Hong F, et al. Gene expression-based model using formalin-fixed paraffin-embedded biopsies predicts overall survival in advanced-stage classical Hodgkin lymphoma. J Clin Oncol. 2013;31:692–700.
Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med. 1998;339:1506–14.
Gallamini A, Rigacci L, Merli F, et al. The predictive value of positron emission tomography scanning performed after two courses of standard therapy on treatment outcome in advanced stage Hodgkin's disease. Haematologica. 2006;91:475–81.
Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18 F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin's lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–52.
Diefenbach CS LH, Hong F. Evaluation Of a Novel 3 Factor Prognostic Score (PS-3) For Patients With Advanced Hodgkin Lymphoma (HL) Treated On US Intergroup E2496. Blood. 2013;122(21):4277.
Viviani S, Santoro A, Negretti E, et al. Salvage chemotherapy in Hodgkin's disease. Results in patients relapsing more than twelve months after first complete remission. Ann Oncol. 1990;1:123–7.
Brice P, Bastion Y, Divine M, et al. Analysis of prognostic factors after the first relapse of Hodgkin's disease in 187 patients. Cancer. 1996;78:1293–9.
Smeltzer JP, Cashen AF, Zhang Q, et al. Prognostic significance of FDG-PET in relapsed or refractory classical Hodgkin lymphoma treated with standard salvage chemotherapy and autologous stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1646–52.
Moskowitz AJ, Yahalom J, Kewalramani T, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. 2010;116:4934–7.
Jabbour E, Hosing C, Ayers G, et al. Pretransplant positive positron emission tomography/gallium scans predict poor outcome in patients with recurrent/refractory Hodgkin lymphoma. Cancer. 2007;109:2481–9.
Devillier R, Coso D, Castagna L, et al. Positron emission tomography response at the time of autologous stem cell transplantation predicts outcome of patients with relapsed and/or refractory Hodgkin's lymphoma responding to prior salvage therapy. Haematologica. 2012;97:1073–9.
Castagna L, Bramanti S, Balzarotti M, et al. Predictive value of early 18 F-fluorodeoxyglucose positron emission tomography (FDG-PET) during salvage chemotherapy in relapsing/refractory Hodgkin lymphoma (HL) treated with high-dose chemotherapy. Br J Haematol. 2009;145:369–72.
Moskowitz CH, Matasar MJ, Zelenetz AD, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119:1665–70. The prospective study that provides evidence that the goal of salvage chemotherapy in patients with HL should be a negative FDG-PET scan before ASCT.
Martinez C, Canals C, Sarina B, et al. Identification of prognostic factors predicting outcome in Hodgkin's lymphoma patients relapsing after autologous stem cell transplantation. Ann Oncol. 2013;24:2430–4. Largest study so far conducted to assess the predictors of outcome of patients with Hodgkin lymphoma relapsing after autologous stem cell transplant.
Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–4.
Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359:2065–71.
Moskowitz C. Risk-adapted therapy for relapsed and refractory lymphoma using ICE chemotherapy. Cancer Chemother Pharmacol. 2002;49(Supp 1):S9–12.
Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97:616–23.
Josting A, Rudolph C, Reiser M, et al. Time-intensified dexamethasone/cisplatin/cytarabine: an effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin's disease. Ann Oncol. 2002;13:1628–35.
Baetz T, Belch A, Couban S, et al. Gemcitabine, dexamethasone and cisplatin is an active and non-toxic chemotherapy regimen in relapsed or refractory Hodgkin's disease: a phase II study by the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol. 2003;14:1762–7.
Bartlett NL, Niedzwiecki D, Johnson JL, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol. 2007;18:1071–9.
Ng M, Waters J, Cunningham D, et al. Gemcitabine, cisplatin and methylprednisolone (GEM-P) is an effective salvage regimen in patients with relapsed and refractory lymphoma. Br J Cancer. 2005;92:1352–7.
Hawkes EA, Barton S, Cunningham D, et al. GEM-P chemotherapy is active in the treatment of relapsed Hodgkin lymphoma. Ann Hematol. 2014;93:827–34. Single institution study showing impressive ORR of 80% and 37% of CR with salvage outpatient chemotherapy regimen gemcitabine based, non cross toxic with first line chemotherapy drugs.
Brandwein JM, Callum J, Sutcliffe SB, et al. Evaluation of cytoreductive therapy prior to high dose treatment with autologous bone marrow transplantation in relapsed and refractory Hodgkin's disease. Bone Marrow Transplant. 1990;5:99–103.
Colwill R, Crump M, Couture F, et al. Mini-BEAM as salvage therapy for relapsed or refractory Hodgkin's disease before intensive therapy and autologous bone marrow transplantation. J Clin Oncol. 1995;13:396–402.
Falini B, Pileri S, Pizzolo G, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995;85:1–14.
Bartlett NL, Younes A, Carabasi MH, et al. A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood. 2008;111:1848–54.
Forero-Torres A, Leonard JP, Younes A, et al. A Phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol. 2009;146:171–9.
Fanale MA, Forero-Torres A, Rosenblatt JD, et al. A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin Cancer Res. 2012;18:248–55. The phase I trial that showed favorable safety profile and significant activity of Brentuximab in patient with relapsed/refractory CD30 positive lymphomas.
Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–21.
Younes A, Gopal AK, Smith SE, Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30:2183. The phase II trial that led to the accelerated FDA approval of Brentuximab in relapsed or refractory Hodgkin lymphoma.
Rothe A, Sasse S, Goergen H, et al. Brentuximab vedotin for relapsed or refractory CD30+ hematologic malignancies: the German Hodgkin Study Group experience. Blood. 2012;120:1470–2.
Sasse S, Rothe A, Goergen H, et al. Brentuximab vedotin (SGN-35) in patients with transplant-naive relapsed/refractory Hodgkin lymphoma. Leuk Lymphoma. 2013;54:2144–8.
Chen R FS, Palmer j et al Two-year follow up of patients with relapsed/refractory Hodgkin treated with brentuximab vedotin prior to reduced intensity allogeneic hematopoietic cell transplantation. Presented at the 12th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 19-22, 2013, 2013
Garciaz S, Coso D, Peyrade F, et al: Brentuximab vedotin followed by allogeneic transplantation as salvage regimen in patients with relapsed and/or refractory Hodgkin's lymphoma. Hematol Oncol, 2013 Brentuximab as a bridge to allogeneic transplant in patients with refractory disease.
Chen RW PJ, Siddiqi T, et al. : Brentuximab vedotin as first line salvage therapy in relapsed/refractory HL. ASH Annual Meeting Abstracts. 2012;120(21):3699, 2012
Moskowitz AJ SH, Gerecitano J, et al: PET-adapted sequential therapy with brentuximab vedotin and augmented-ICE induces FDG-PET normalization in 92% of patients with relapsed and refractory Hodgkin lymphoma, Presented at the 12th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 19–22, 2013.
Bartlett NL, Chen R, Fanale MA, et al. Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J Hematol Oncol. 2014;7:24. First study assessing efficacy and toxicity of brentuximab in the setting of retreatment.
Gopal AK, Ramchandren R, O'Connor OA, et al. Safety and efficacy of brentuximab vedotin for Hodgkin lymphoma recurring after allogeneic stem cell transplantation. Blood. 2012;120:560–8. First study assessing the safety and efficacy of brentuximab in the setting of relapse post allogeneic stem transplant.
Ozegowski W. KD: w-[bis-(chlorethyl)-amino-benzimidazolyl-(2)]-propionic or butyric acids as potential cytostatic agents. J Prakt Chem. 1963;20:178–86.
Ruffert KJH, Syrbe G, et al. Cytostasan (bendamustine) as an alternative therapeutic approach to treat malignant non-Hodgkin’s lymphoma. Z Klin Med. 1989;44:671–4.
Brockmann B. Therapy of the recurrence of malignant lymphoma. Z Aerztl Fortbild (Jena). 1992;86:843.
Herold M. KK, Anger G, et al: Risk-adapted combined radiotherapy and chemotherapy for Hodgkin’s disease – results of a pilot study. Onkologie. 1992;15:501–5.
Moskowitz AJ, Hamlin Jr PA, Perales MA, et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol. 2013;31:456–60. First prospective phase II trial evaluating efficacy of bendamustine in heavily pretreated Hodgkin lymphoma patients.
Ghesquieres H, Stamatoullas A, Casasnovas O, et al. Clinical experience of bendamustine in relapsed or refractory Hodgkin lymphoma: a retrospective analysis of the French compassionate use program in 28 patients. Leuk Lymphoma. 2013;54:2399–404. Recent retrospective study confirming acitvity of bendamustine in heavily pretreated patients with HL.
Corazzelli G, Angrilli F, D'Arco A, et al. Efficacy and safety of bendamustine for the treatment of patients with recurring Hodgkin lymphoma. Br J Haematol. 2013;160:207–15. Recent retrospective study confirming acitvity of bendamustine in heavily pretreated patients with HL.
Dutton A, Reynolds GM, Dawson CW, et al. Constitutive activation of phosphatidyl-inositide 3 kinase contributes to the survival of Hodgkin's lymphoma cells through a mechanism involving Akt kinase and mTOR. J Pathol. 2005;205:498–506.
Johnston PB, Inwards DJ, Colgan JP, et al. A Phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol. 2010;85:320–4.
Oki Y, Buglio D, Fanale M, et al. Phase I study of panobinostat plus everolimus in patients with relapsed or refractory lymphoma. Clin Cancer Res. 2013;19:6882–90. Phase I study suggesting synergy of the combination of HDACI panobinostat with mTOR inhibitor everolimus, although limited by the development of significative thrombocytopenia in more than half of the patients.
Buglio D, Georgakis GV, Hanabuchi S, et al. Vorinostat inhibits STAT6-mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood. 2008;112:1424–33.
Younes A, Sureda A, Ben-Yehuda D, et al. Panobinostat in patients with relapsed/refractory Hodgkin's lymphoma after autologous stem-cell transplantation: results of a phase II study. J Clin Oncol. 2012;30:2197–203. The largest phase II trial conducted with the panobinostat, which demonstrated to be the most effective HDACI in Hodgkin lymphoma.
Younes A, Oki Y, Bociek RG, et al. Mocetinostat for relapsed classical Hodgkin's lymphoma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2011;12:1222–8.
Kirschbaum MH, Goldman BH, Zain JM, et al. A phase 2 study of vorinostat for treatment of relapsed or refractory Hodgkin lymphoma: Southwest Oncology Group Study S0517. Leuk Lymphoma. 2012;53:259–62.
Harrison SJ, Hsu AK, Neeson P, et al. Early thymus and activation-regulated chemokine (TARC) reduction and response following panobinostat treatment in patients with relapsed/refractory Hodgkin lymphoma following autologous stem cell transplant. Leuk Lymphoma. 2014;55:1053–60.
Fehniger TA, Larson S, Trinkaus K, et al. A phase 2 multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood. 2011;118:5119–25. The largest phase II trial of lenalidomide in Hodgkin lymphoma.
Sawas AC-GS, Neylon E. A Case Series Of Continuous Low Dose Lenalidomide In Patients With Relapsed Or Refractory Classical Hodgkin Lymphoma. Blood. 2013;122:5134.
Kuruvilla JTD, Wang L. Phase II Trial of Lenalidomide in Patients with Relapsed or Refractory Hodgkin Lymphoma. Blood. 2008;112:3052. ASH Annual Meeting Abstracts.
Younes A, Romaguera J, Hagemeister F, et al. A pilot study of rituximab in patients with recurrent, classic Hodgkin disease. Cancer. 2003;98:310–4.
Oki Y, Pro B, Fayad LE, et al. Phase 2 study of gemcitabine in combination with rituximab in patients with recurrent or refractory Hodgkin lymphoma. Cancer. 2008;112:831–6.
Hahn T, McCarthy Jr PL, Hassebroek A, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013;31:2437–49.
Anderlini P, Saliba R, Acholonu S, et al. Fludarabine-melphalan as a preparative regimen for reduced-intensity conditioning allogeneic stem cell transplantation in relapsed and refractory Hodgkin's lymphoma: the updated M.D. Anderson Cancer Center experience. Haematologica. 2008;93:257–64.
Sureda A, Robinson S, Canals C, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin's lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26:455–62.
Sureda A, Canals C, Arranz R, et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin's lymphoma. Results of the HDR-ALLO study - a prospective clinical trial by the Grupo Espanol de Linfomas/Trasplante de Medula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2012;97:310–7. The largest prospective trial so far on the role of RIC allo-sct in Hodgkin lymphoma.
Sobol U, Rodriguez T, Smith S, et al: Seven-year follow-up of allogeneic transplant using BCNU, etoposide, cytarabine and melphalan chemotherapy in patients with Hodgkin lymphoma after autograft failure: importance of minimal residual disease. Leuk Lymphoma, 2013.
Anderlini P SR, Ledesma C: Reduced-Intensity Conditioning (RIC) and Allogeneic Stem Cell Transplantation (allo-SCT) For Relapsed/Refractory Hodgkin Lymphoma (HL) In The Brentuximab Vedotin Era: Favorable Overall and Progression-Free Survival (OS/PFS) With Low Transplant-Related Mortality (TRM) Blood 2013 122:410, 2013.
Proctor SJ, Wilkinson J, Jones G, et al. Evaluation of treatment outcome in 175 patients with Hodgkin lymphoma aged 60 years or over: the SHIELD study. Blood. 2012;119:6005–15.
Evens AM, Helenowski I, Ramsdale E, et al. A retrospective multicenter analysis of elderly Hodgkin lymphoma: outcomes and prognostic factors in the modern era. Blood. 2012;119:692–5.
Kelley TW, Pohlman B, Elson P, et al. The ratio of FOXP3+ regulatory T cells to granzyme B + cytotoxic T/NK cells predicts prognosis in classical Hodgkin lymphoma and is independent of bcl-2 and MAL expression. Am J Clin Pathol. 2007;128:958–65.
Halbsguth TV, Boll B, Borchmann P, et al. The unique characteristics and management of patients over 60 years of age with classic Hodgkin lymphoma. Curr Hematol Malig Rep. 2011;6:164–71.
Boll B, Goergen H, Arndt N, et al. Relapsed hodgkin lymphoma in older patients: a comprehensive analysis from the German hodgkin study group. J Clin Oncol. 2013;31:4431–7. Largest retrospective study on safety and efficacy of second line treatment modalities in older patients with relapsed or refractory Hodgkin Lymphoma.
Puig N, Pintilie M, Seshadri T, et al. High-dose chemotherapy and auto-SCT in elderly patients with Hodgkin's lymphoma. Bone Marrow Transplant. 2011;46:1339–44.
Gopal AK, Bartlett NL, Forero-Torres A, et al: Brentuximab vedotin in patients aged 60 years or older with relapsed or refractory CD30-positive lymphomas: a retrospective evaluation of safety and efficacy. Leuk Lymphoma, 2014 Largest retrospective analysis evaluating the safety and efficacy of brentuximab in older patients (60 years and older) with relapsed or refractory Hodgkin lymphoma or Anaplastic Large Cell lymphoma.
Acknowledgments
CD is supported by a Clinical Investigator Career Development Award from the Lymphoma Research Foundation.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Dr. Francesca Montanari declares no potential conflicts of interest.
Dr. Catherine Diefenbach is a consultant for Seattle Genetics.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Montanari, F., Diefenbach, C. Relapsed Hodgkin Lymphoma: Management Strategies. Curr Hematol Malig Rep 9, 284–293 (2014). https://doi.org/10.1007/s11899-014-0220-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-014-0220-7
Keywords
- Hodgkin lymphoma
- Relapsed disease
- Refractory disease
- Risk stratification
- Autologous stem cell transplant
- Reduced-intensity conditioning allogeneic stem cell transplant
- Brentuximab vedotin
- Bendamustine
- Histone deacetylase inhibitors
- Everolimus
- Panobinostat
- Lenalidomide
- Rituximab
- Immunochemotherapy
- Elderly patients