Abstract
Purpose of Review
The burden of heart failure in the United States is growing rapidly to epic proportions with serious clinical implications for patients and economic strain for healthcare systems. One of the most common reasons for hospitalization in acute decompensated heart failure (ADHF) is excess volume accumulation which leads to untoward symptoms including dyspnea,orthopnea, and edema.
Recent Findings
Over the past several decades, there has been great interest in exploring various decongestive strategies in order to achieve symptomatic improvement and favorable clinical outcomes. These include different modalities of loop diuretic administration, the adjunctive use of non-loop diuretics, and other diuretic sparing strategies.
Summary
Herein, we provide an appraisal of these decongestive strategies and discuss novel concepts predicting clinical outcomes based on diuretic response and decongestive adequacy while discussing commonly encountered problems such as worsening renal function in ADHF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The burden of heart failure in the USA is growing rapidly and has serious implications for patients and the economy. The anticipated prevalence of heart failure is estimated to rise by nearly 50% over the next two decades with total costs increasing by well over 100% [1, 2]. The main reason for hospitalization in patients with heart failure are signs and symptoms of worsening congestion and hypervolemia, which is responsible for over 90% of hospital admissions [3, 4]. As a result, this places a maxim to achieve adequate decongestion which may have ramifications on morbidity and mortality, as persistent congestion at hospital discharge has been associated with clinical deterioration [5]. Not surprisingly, the current heart failure treatment guidelines highly recommend the use of loop diuretics in the management of acute decompensated heart failure (ADHF) to relieve congestion [6,7,8]. However, these recommendations are based on a surprising dearth of evidence with regard to the effects of loop diuretic therapy on morbidity and mortality and provide little other guidance [1]. At the patient level, there may be unique clinical, hemodynamic, or metabolic characteristics and comorbidities that may influence inpatient diuretic therapy. Herein, we aim to review current diuretic strategies in ADHF based on a critical review of contemporary literature.
A Comparison of Loop Diuretic Therapies
Loop diuretic therapy is the mainstay of treatment for patients with ADHF. Loop diuretics currently available for use in the USA consist of furosemide, torsemide, bumetanide, and ethacrynic acid. Notably, ethacrynic acid is rarely used clinically given the high risk of ototoxicity as compared with other loop diuretics and will not be discussed here. The mechanism of action of the loop diuretics involves blockade of the sodium-potassium-chloride cotransporter in the thick ascending loop of Henle resulting in natriuresis and diuresis. Despite the widespread use of furosemide, the first Food and Drug Administration (FDA) approved loop diuretic in 1966, there are limited data to support its superiority to other available loop diuretics. Furosemide has a wide range of bioavailability (10–100%) with different inter- and intra-individual properties that can vary based on formulation and method of administration (IV versus oral). In fact, there is also a wide variation for urinary excretion of furosemide (25–42%) for differing diuretic formulations [9].
Torsemide and bumetanide, in contrast, have a much more reliable bioavailability that ranges consistently from 80–100% and torsemide has the longest half-life of the three loop diuretics (3–4 h) [10]. Torsemide, approved by the FDA in 1993, appears to have potential favorable effects on the renin angiotensin aldosterone system (RAAS) supported by both animal and human studies [10]. In rat models, torsemide and furosemide were shown to increase plasma renin and aldosterone levels [1]. In contrast to furosemide, torsemide may have additional favorable mineralocorticoid antagonizing properties. For example, torsemide inhibits the binding of aldosterone to its receptor in murine kidneys in a dose-dependent manner [11]. Additionally, torsemide may have antifibrotic effects in the myocardium [12, 13]. Torsemide may also have other clinical benefits over furosemide. The Torsemide In Congestive Heart Failure (TORIC) study was an open-label prospective cohort involving 1377 patients with New York Heart Association (NYHA) class II–III heart failure which compared torsemide to furosemide used for chronic diuretic therapy [14]. Subjects in the torsemide arm were not only more likely to have improved their NYHA functional class (p < 0.001) but also were observed to have lower mortality during follow-up (p < 0.05) supporting the chronic usage of torsemide over furosemide. Additionally, a meta-analysis of available studies comparing torsemide and furosemide, although limited to smaller cohorts with some single-center studies, also supported a comparable trend toward improvement in functional status and all-cause death with torsemide compared to furosemide [15]. Bumetanide is another highly potent and readily bioavailable loop diuretic. A small clinical trial suggested that bumetanide was as effective as furosemide in reducing edema [16]. However, studies informing its clinical usefulness and impact on outcomes are lacking.
Decongestive Strategies
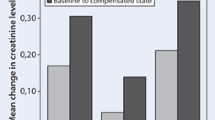
The primary method of diuretic administration to patients hospitalized with ADHF is intravenous (IV) given the need for a rapid onset of action and bypassing the possibility of impaired absorption due to gut edema. In clinical practice, the method of administering diuretics, whether by bolus dosing or continuous infusion, varies widely. Whether there were significant differences between these two decongestive strategies was the aim of the Diuretic Optimization Strategies Evaluation In Acute Heart Failure (DOSE-AHF) trial [17]. The DOSE-AHF trial used a 2 × 2 factorial design for the administration of furosemide as IV boluses or a continuous infusion and as either a low dose (IV dosing equivalent to home oral loop diuretic dosing) or a high dose (IV dosing 2.5 times higher than home oral loop diuretic dosing) in patients with ADHF. At 72 h after randomization, there were no differences in the co-primary endpoints of global assessment of symptoms and change in creatinine for either of the four strategies (p = 0.47 and p = 0.45, respectively). However, subjects in the high-dose arms had more favorable secondary outcomes including improved dyspnea, weight loss, and total fluid loss. These benefits were balanced by a higher incidence of transient worsening renal function (WRF), importantly, without a difference in 60-day outcomes. There has been a historical association between higher diuretic dosing linking WRF (classically defined as a >0.3 mg/dL increase in serum creatinine or >25% drop in estimated glomerular filtration rate) and clinical outcomes, although prognosis may depend on the clinical context in which WRF occurs and to what degree [18,19,20]. However, this association is confounded by patients who have greater severity of heart failure, more comorbidities, and persistent congestion despite diuresis [21].
In a post-hoc analysis of DOSE-AHF and the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF), plasma renin activity (PRA) and serum aldosterone levels were measured to explore the relationship between RAAS activation and decongestion strategy with clinical outcomes [22]. Patients who received a continuous infusion of furosemide had higher PRA levels. However, there was no significant difference in these biomarkers between high- and low-dose strategies. Additionally, the change in biomarkers from baseline to 72 or 96 h was not associated with differences in the outcomes of time to death or HF hospitalization. Taken in aggregate, acute and transient activation of RAAS may not be associated with post-discharge outcomes.
Recent studies suggest that transient WRF may not affect post-discharge outcomes [17, 23, 24]. This theory is re-demonstrated in both the Dopamine in Acute Decompensated Heart Failure (DAD-HF) and Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE-AHF) trials. In the DAD-HF trial, patients admitted for ADHF were randomized to receive either high-dose furosemide via continuous infusion (20 mg/h) or low-dose furosemide via continuous infusion (5 mg/h) along with low-dose dopamine (5 μg/kg/min) [25]. There were no differences in 60-day all-cause mortality or readmission rates despite a higher rate of WRF at 24 h in the high-dose furosemide group (p = 1 and p = 0.254, respectively). The DAD-HF II trial investigated differences in all-cause mortality or heart failure hospitalizations between three separate groups: high-dose furosemide (20 mg/h), low-dose furosemide (5 mg/h) with low-dose dopamine (5 μg/kg/min), and low-dose furosemide alone [26]. There was no significant difference between the groups with regards to all-cause mortality, heart failure rehospitalizations, or any additional benefit observed with the use of low-dose dopamine at day 60 (p = 0.74 and p = 0.55, respectively) or at 1 year of follow up (p = 0.84 and p = 0.40, respectively).
In the ROSE-AHF trial, the investigators explored the hypothesis that low-dose dopamine or low-dose nesiritide in addition to loop diuretic therapy would enhance decongestion while preserving renal function [27]. Patients admitted with ADHF and renal dysfunction (GFR 15–60 mL/min/1.73 m2) were randomized to receive low-dose dopamine or nesiritide, in addition to standard diuretic therapy, compared to a placebo group. There were no differences between the two groups when compared to placebo for the endpoints of enhanced diuresis or preservation of renal function at 72 h (cumulative urine volume for dopamine vs placebo p = 0.59 and for nesiritide vs placebo p = 0.49; change in cystatin C level for dopamine vs placebo p = 0.72 and for nesiritide vs placebo p = 0.36).
Diuretic Resistance and the Role of Adjunctive Therapies
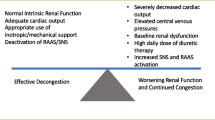
Persistent signs and symptoms of congestion at hospital discharge have been associated with high morbidity and mortality [5]. Inadequate diuresis could be the result of insufficient diuretic dosing or due to true diuretic resistance. Electrolyte concentrations in the urine may yield important clues in monitoring for adequate decongestion. Interestingly, Verbrugge et al. studied the urinary composition of sodium and chloride ions in patients undergoing diuresis for ADHF [28]. The authors found that the 24 h concentration of urinary sodium and chloride dropped significantly as patients became effectively diuresed, suggesting that persistently elevated levels of these ions may indicate that euvolemia has not been achieved.
In ADHF, the diuretic response curve is shifted downward and to the right, reflecting the need for higher diuretic doses over time [29, 30]. Potential mechanisms for diuretic resistance include the “braking phenomenon” (acute decrease in response to loop diuretics after repeated dosing), post-diuretic effect (post-diuretic sodium retention after therapy has worn off), rebound effect (chronic loop diuretic use leading to increased distal nephron sodium reabsorption), or renal adaptation (hypertrophy and hyperfunction of distal tubule cells resulting in increased distal sodium uptake and aldosterone excretion after long-term exposure to loop diuretics) [1, 31, 32]. Patients who are effectively diuretic resistant, or have reduced diuretic efficiency (DE), have been shown to have lower survival [33, 34]. Diuretic efficiency is a novel metric that appears to provide additional prognostic information for patients admitted with decompensated heart failure [35]. Diuretic dose alone may not be an appropriate marker for true diuretic resistance. Diuretic efficiency, on the other hand, is an estimate of net fluid output produced per unit of furosemide equivalents. Testani and colleagues evaluated the association of DE with clinical variables and outcomes in two independent cohorts of patients admitted with ADHF. The authors concluded that DE provided distinct prognostic information in addition to total diuretic dose and total urine output. Their findings highlighted an association between low DE and worsened survival after adjusting for in-hospital diuretic dose, fluid output, and baseline characteristics. Additionally, diuretic responsiveness was evaluated in 4379 patients from the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial [36]. The authors found comparable results and demonstrated that diuretic unresponsiveness was associated with an increased risk of death or rehospitalization early after discharge.
There are several adjunctive therapies that have been used or considered in the setting of loop diuretic resistance. These include therapies with mechanisms of action at the level of the kidney and include combination therapy with acetazolamide and thiazide diuretics, aldosterone antagonists, and vasopressin antagonists among others. There is an overall paucity of data regarding the use of acetazolamide for clinical heart failure. In the early 1950s, shortly after the introduction of carbonic anhydrase inhibitors as potential novel treatments for ADHF, a series of case reports were published detailing successful diuresis with the addition of acetazolamide [37]. Most recently, a small study involving 54 patients hospitalized for ADHF used protocol-driven diuretic strategies including acetazolamide to assess for decongestion and natriuretic response [38]. Those patients who received acetazolamide in addition to standard loop diuretic therapy had significantly higher natriuretic responses as compared to those receiving loop diuretics alone or with adjunctive thiazide diuretics. Significant side effects with the use of carbonic anhydrase inhibitors include metabolic acidosis, as the mechanism of action involves renal loss of bicarbonate in addition to sodium, potassium, and water. Thiazide diuretics, another choice, are theoretically useful by means of sequential nephron blockade and the inhibition of distal tubule sodium resorption. Although there have been many studies evaluating the use of combination therapy with thiazide and loop diuretics, these studies are limited by small samples sizes, lack of control groups, cohorts with limited generalizability, inconsistency of diuretic regimens, and lack of focus on clinical outcomes [32].
Aldosterone antagonists have been previously studied in small-scale trials. In a study of 21 patients deemed diuretic resistant, high-dose spironolactone in addition to high-dose bumetanide and angiotensin converting enzyme inhibitor (ACE-i) therapy resulted in significant natriuresis and diuresis [39]. In a recent prospective, single-blind pilot study of 100 patients admitted with ADHF, those who received high-dose spironolactone had lower N-terminal pro-brain natriuretic peptide (NT-proBNP) levels, less signs and symptoms of congestion, and no difference in serum potassium levels at 3 days of follow up [40]. The Aldosterone Targeted Neurohormonal Combined with Natriuresis Therapy in Heart Failure (ATHENA-HF) trial was a randomized, double-blind, placebo-controlled trial that was designed to assess the safety and efficacy of 100 mg/day of spironolactone therapy in 360 patients hospitalized with ADHF [41, 42]. The authors found no significant difference in the primary outcome of NT-proBNP levels at 96 h between the high-dose spironolactone group and placebo (p = 0.76). Additionally, there were no significant differences in the secondary outcomes of dyspnea, urine output, or change in potassium between the two groups.
Vasopressin antagonists have also been studied as both independent and adjunctive therapies to loop diuretics. Arginine vasopressin (AVP) has been found to be inappropriately elevated in acute and chronic HF and is associated with poor outcomes [43]. Pharmacologic blockade of V2 receptors results in increased renally-mediated free water excretion. The Effects of Oral Tolvaptan in Patients Hospitalized for Worsening Heart Failure (EVEREST) trial was a large study involving over 4000 patients admitted with ADHF who were randomized to receive tolvaptan, a V2 receptor antagonist, or placebo in addition to standard medical therapy for at least 60 days [44]. The median follow-up time was 10 months. The co-primary endpoints were all-cause mortality and cardiovascular death or hospitalization for heart failure, for which there was no difference between the two groups (p = 0.68 and p = 0.55, respectively). However, benefits were seen with regards to increased serum sodium levels and decongestion in the short term. Tolvaptan was re-evaluated in a recently published study, Targeting Acute Congestion With Tolvaptan in Congestive Heart Failure (TACTICS-HF), where the authors hypothesized that select ADHF patients with severe heart failure symptoms or hyponatremia may benefit from upfront adjunctive tolvaptan therapy in addition to loop diuretics. This hypothesis was largely based on post-hoc analyses from the EVEREST trial showing an improvement in the secondary endpoints of patient-assessed dyspnea (day 1) and body weight reduction (days 1 and 7) [45]. In this trial, 257 patients with ADHF were randomized to receive tolvaptan with a fixed-dose furosemide regimen and the primary endpoint was the proportion of patients with at least moderate improvement in dyspnea within 24 h. There was no significant difference between the tolvaptan and placebo groups with respect to the primary endpoint or secondary endpoints including in-hospital or post-discharge outcomes up to 30 days (p = 0.32 for the primary endpoint). Again, although theoretically promising, tolvaptan does not have a firmly established role for the treatment of ADHF [46].
Non-diuretic Means of Decongestion
Additional means of fluid removal include direct, mechanical removal of fluid from the body or by means of increasing renal blood flow, cardiac output, or decreasing systemic vascular resistance. Ultrafiltration (UF) was thought to be a promising option both as an alternative to loop diuretics and for use in patients with diuretic resistance given the ability to remove fluid at a rate of up to 500 mL/h [29]. The Relief for Acutely Fluid Overloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) study was a small proof of concept trial designed to assess the safety and efficacy of UF in patients with ADHF [47]. A total of 40 patients were assigned to an 8 h session of UF or usual care. The primary endpoint was weight loss after 24 h and was not significantly different between the two groups (p = 0.24). Subsequently, the Ultrafiltration versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Congestive Heart Failure (UNLOAD) trial randomized 200 patients with ADHF to receive UF or standard IV diuretic therapy [48]. The primary endpoints were weight loss, which was significantly different between the two groups (p = 0.001), and dyspnea, which was no different between the two groups, at 48 h. There was also a significant reduction in HF rehospitalization (p = 0.04); however, the rehospitalizations were investigator reported thus limiting the validity of these findings. Additionally, these trials were unblinded with small sample sizes and a short duration of follow up [31].
Lastly, the CARRESS-HF trial randomized 188 patients with ADHF, persistent congestion, and WRF to receive UF or pharmacologic therapy [49]. Unlike the RAPID-CHF and UNLOAD trials, this study specified a rate of fluid removal in the UF arm at 200 mL/h and targeted a urine output of 3 to 5 L/day in the medical therapy arm. Patients in the previous studies underwent UF or IV diuretic therapy based on physician discretion without discrete goals for fluid removal. The primary endpoint was the bivariate change in serum creatinine from baseline and body weight assessed at 4 days. UF was inferior to pharmacologic therapy due to a significant increase in creatinine in the UF group (p = 0.003) and more patients in the UF group had a serious adverse event (p = 0.03). Although conceptually an encouraging means of volume removal, UF receives a class IIb recommendation from the ACCF/AHA and ESC guidelines for patients with refractory congestion not responding to medical therapy or as a consideration for patients with obvious volume overload to alleviate congestive symptoms and fluid weight [6, 7]. Indeed, a pooled analysis of patients with ADHF and WRF (acute cardiorenal syndrome) showed that those who received an aggressive approach to decongestion via an algorithmic stepped pharmacologic approach had greater weight change and net fluid loss (p < 0.001 for both) along with improved renal function (p = 0.03) [50].
Other means of achieving decongestion in patients with ADHF, largely measured by a reduction in dyspnea with treatment, include several novel vasoactive medications. Seralaxin, or recombinant human relaxin-2, has shown promise in the Serelaxin in Acute Heart Failure (RELAX-AHF) trial involving 1161 patients admitted with ADHF [51]. The co-primary outcome involved two different measures of dyspnea including a visual analogue scale and a seven-level Likert scale, the former of which was significantly improved after receiving 48 h of treatment with serelaxin (p = 0.007). Although there was no improvement in change in bodyweight from baseline through day 14, there were improvements in measures of worsening heart failure, length of hospital stay, and a reduction in mortality reported at day 180. The Randomized Evaluation of Intravenous Levosimendan Efficacy (REVIVE I and II) trials evaluating the effects of levosimendan, a drug with inotropic and vasodilator properties, reported improvement in the overall clinical status reported by patients during treatment [52]. Unfortunately, this clinical improvement was heavily counterbalanced by an increased risk of hypotension, dangerous cardiac arrhythmias, and a higher rate of death in those receiving the treatment. Other studies involving IV vasodilator therapies including human recombinant BNP (nesiritide), endothelin-1 receptor antagonists (tezotentan), or adenosine receptor antagonists (rolofylline) have been largely neutral [51]. Additionally, ularitide, a synthetic form of the human natriuretic peptide urodilantin with natriuretic, diuretic, and vasodilatory properties, was studied in the Trial of Ularitide’s Efficacy and safety in patients with Acute Heart Failure (TRUE-AHF study). The authors found no significant difference in cardiovascular mortality during a median follow up time of 15 months (p = 0.75) [53]. The group receiving ularitide had lower NT-proBNP levels as compared to placebo (p < 0.001); however, they experienced significantly more hypotension (p < 0.001).
Conclusion
Decongestion with loop diuretics is a mainstay for the treatment of ADHF. Despite little guidance in the heart failure treatment guidelines, their use is highly endorsed. As a result, there have been several recent studies attempting to clarify the differences in various approaches to loop and adjunctive diuretic use, renal protective strategies, and non-loop diuretic strategies. Although providing important insights into the treatment of congestion, results from these studies have largely been neutral leaving little influence on practice. In contrast, promising findings from goal-directed decongestion strategies, including targeted urine output volumes or goal urine electrolyte composition, might be useful. Regardless, because of the increasing public health and economic burden related to ADHF hospitalizations, the improvement of decongestion strategies is clearly warranted and starts with an enhanced understanding of diuretic response and the development of novel therapies with a favorable influence on outcomes.
References
Buggey J, Mentz RJ, Pitt B, et al. A reappraisal of loop diuretic choice in heart failure patients. Am Heart J. 2015;169:323–33.
Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292.
Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–6.
Adams Jr KF, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–16.
Ambrosy AP, Pang PS, Khan S, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835–43.
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239.
Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200.
Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–194.
Murray MD, Haag KM, Black PK, Hall SD, Brater DC. Variable furosemide absorption and poor predictability of response in elderly patients. Pharmacotherapy. 1997;17:98–106.
Wargo KA, Banta WM. A comprehensive review of the loop diuretics: should furosemide be first line? Ann Pharmacother. 2009;43:1836–47.
Uchida T, Yamanaga K, Nishikawa M, Ohtaki Y, Kido H, Watanabe M. Anti-aldosteronergic effect of torasemide. Eur J Pharmacol. 1991;205:145–50.
Lopez B, Gonzalez A, Beaumont J, Querejeta R, Larman M, Diez J. Identification of a potential cardiac antifibrotic mechanism of torasemide in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:859–67.
Lopez B, Querejeta R, Gonzalez A, Sanchez E, Larman M, Diez J. Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. J Am Coll Cardiol. 2004;43:2028–35.
Cosin J, Diez J. Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail. 2002;4:507–13.
Bikdeli B, Strait KM, Dharmarajan K, et al. Dominance of furosemide for loop diuretic therapy in heart failure: time to revisit the alternatives? J Am Coll Cardiol. 2013;61:1549–50.
Konecke LL. Clinical trial of bumetanide versus furosemide in patients with congestive heart failure. J Clin Pharmacol. 1981;21:688–90.
Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805.
Schrier RW. Role of diminished renal function in cardiovascular mortality: marker or pathogenetic factor? J Am Coll Cardiol. 2006;47:1–8.
Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–30.
Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015;36:1437–44.
Metra M, Davison B, Bettari L, et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart fail. 2012;5:54–62.
Mentz RJ, Stevens SR, DeVore AD, et al. Decongestion strategies and renin-angiotensin-aldosterone system activation in acute heart failure. JACC Heart fail. 2015;3:97–107.
Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–72.
van der Meer P, Postmus D, Ponikowski P, et al. The predictive value of short-term changes in hemoglobin concentration in patients presenting with acute decompensated heart failure. J Am Coll Cardiol. 2013;61:1973–81.
Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the Dopamine in Acute Decompensated Heart Failure (DAD-HF) Trial. J Card Fail. 2010;16:922–30.
Triposkiadis FK, Butler J, Karayannis G, et al. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: the Dopamine in Acute Decompensated Heart Failure II (DAD-HF II) trial. Int J Cardiol. 2014;172:115–21.
Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310:2533–43.
Verbrugge FH, Nijst P, Dupont M, Penders J, Tang WH, Mullens W. Urinary composition during decongestive treatment in heart failure with reduced ejection fraction. Circ Heart fail. 2014;7:766–72.
Felker GM, Mentz RJ. Diuretics and ultrafiltration in acute decompensated heart failure. J Am Coll Cardiol. 2012;59:2145–53.
Michael Felker G. Diuretic management in heart failure. Congest Heart Fail (Greenwich, Conn). 2010;16 Suppl 1:S68–72.
Mentz RJ, Kjeldsen K, Rossi GP, et al. Decongestion in acute heart failure. Eur J Heart Fail. 2014;16:471–82.
Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56:1527–34.
Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol. 2006;97:1759–64.
Neuberg GW, Miller AB, O’Connor CM, et al. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144:31–8.
Testani JM, Brisco MA, Turner JM, et al. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart fail. 2014;7:261–70.
ter Maaten JM, Dunning AM, Valente MA, et al. Diuretic response in acute heart failure-an analysis from ASCEND-HF. Am Heart J. 2015;170:313–21.
Belsky H. Use of a new oral diuretic, diamox, in congestive heart failure. N Engl J Med. 1953;249:140–3.
Verbrugge FH, Dupont M, Bertrand PB, et al. Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol. 2015;70:265–73.
van Vliet AA, Donker AJ, Nauta JJ, Verheugt FW. Spironolactone in congestive heart failure refractory to high-dose loop diuretic and low-dose angiotensin-converting enzyme inhibitor. Am J Cardiol. 1993;71:21a–8a.
Ferreira JP, Santos M, Almeida S, Marques I, Bettencourt P, Carvalho H. Mineralocorticoid receptor antagonism in acutely decompensated chronic heart failure. Eur J Intern Med. 2014;25:67–72.
Butler J, Hernandez AF, Anstrom KJ, et al. Rationale and design of the ATHENA-HF Trial: aldosterone targeted neurohormonal combined with natriuresis therapy in heart failure. JACC Heart Fail. 2016;4:726–35.
Butler J. Aldosterone Targeted NeuroHormonal CombinEd With Natriuresis TherApy in Heart Failure (ATHENA-HF) Trial. American Heart Association Scientific Sessions 2016 Accessed 12 Dec 2016, 2016.
Goldsmith SR, Gheorghiade M. Vasopressin antagonism in heart failure. J Am Coll Cardiol. 2005;46:1785–91.
Konstam MA, Gheorghiade M, Burnett Jr JC, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. JAMA. 2007;297:1319–31.
Felker GM, Mentz RJ, Cole R, et al., Efficacy and Safety of Tolvaptan in Patients Hospitalized with Acute Heart Failure, J Am Coll Cardiol. 2016; doi:10.1016/j.jacc.2016.09.004
Starling RC, Young JB. Tolvaptan in Acute Heart Failure: Time to Move On. J Am Coll Cardiol. 2016; doi:10.1016/j.jacc.2016.09.005
Bart BA, Boyle A, Bank AJ, et al. Ultrafiltration versus usual care for hospitalized patients with heart failure: the Relief for Acutely Fluid-Overloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) trial. J Am Coll Cardiol. 2005;46:2043–6.
Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–83.
Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–304.
Grodin JL, Stevens SR, de Las FL, et al. Intensification of medication therapy for cardiorenal syndrome in acute decompensated heart failure. J Card Fail. 2016;22:26–32.
Teerlink JR, Cotter G, Davison BA, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet (London, England). 2013;381:29–39.
Packer M, Colucci W, Fisher L, et al. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail. 2013;1:103–11.
Packer M. Short- and Long-Term Effect of Immediate Vasodilator Therapy in Acutely Decompensated Heart Failure:Results of the TRUE-AHF Trial. American Heart Association Scientific Sessions 2016 Accessed 12 Dec 2016, 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
E. Ashley Hardin and Justin L. Grodin declare that they have no conflict of interest
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pharmacologic Therapy
Rights and permissions
About this article
Cite this article
Hardin, E.A., Grodin, J.L. Diuretic Strategies in Acute Decompensated Heart Failure. Curr Heart Fail Rep 14, 127–133 (2017). https://doi.org/10.1007/s11897-017-0319-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-017-0319-y