Abstract
The morbidity and mortality associated with heart failure (HF) represents a significant public health challenge. Stage D HF identifies a distinct subgroup of advanced HF patients characterized by adverse clinical and hemodynamic factors which warrant evaluation for specialized advanced management strategies and/or consideration of palliative care in tandem with the same recommendations for goal-directed optimal medical therapy as earlier stages of HF. In fact, one of the inherent markers of progression to stage D disease is the need to withdraw previously tolerated neurohormonal agents in the setting of systemic circulatory limitations or renal dysfunction. Furthermore, the requirement for aggressive diuresis in the setting of borderline blood pressures and renal insufficiency is often complicated by worsening renal impairment. Assessment of the appropriate need for inotropic support, given the significant complications associated with their use, is also a frequently encountered challenge complicating the medical management of Stage D HF. This review outlines some of the most relevant challenges of pharmacological therapy in stage D HF and describes current and future strategies that may be employed to overcome some of these obstacles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
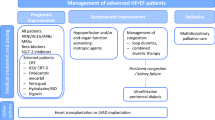
The morbidity and mortality associated with heart failure (HF) and in particular advanced HF, defined as persistent severe symptoms despite maximum goal-directed medical therapy (GDMT), continues to present a major public health challenge. The 2009 ACCF/AHA guidelines define Stage “D” HF patients as those with “truly refractory HF who might be eligible for specialized, advanced treatment strategies, such as mechanical circulatory support (MCS), procedures to facilitate fluid removal, continuous inotropic infusions, or cardiac transplantation or other innovative or experimental surgical procedures, or for end-of-life care, such as hospice” [1]. The Interagency\Registry for Mechanically Assisted Circulatory Support (INTERMACS) has described seven profiles to further stratify patients with advanced HF, ranging from the critical cardiogenic shock patient (profile 1) to the New York Heart Association (NYHA) Class III patient with activity limited to mild exertion (profile 7) [2]. In addition to symptomatic and hemodynamic categorization, there are several clinical clues that enable identification of a Stage D HF patient. These include increasing frequency of HF-related hospitalizations, cardiac cachexia, worsening renal function, and hyponatremia. Moreover, the need to significantly adjust and/or discontinue diuretic and neurohormonal medications can also herald the transition of a previously stable patient to stage D disease. This confluence of hostile factors—severe functional limitation, a high prevalence of adverse clinical and biochemical markers, and an inability to tolerate GDMT—are the hallmarks of Stage D HF. The management of these patients remains challenging as evidenced by the plateau in outcomes of end-stage HF using medical therapy alone, with no new drugs shown to improve survival. Therefore, it is increasingly important to consider non-pharmacological alternatives such as MCS and transplantation, or parallel strategies such as palliative care, for this final phase of the HF trajectory.
In the course of the current review, we will outline some of the most relevant challenges as they relate to pharmacological therapy in Stage D patients and discuss contemporary strategies that may be employed to overcome some of these difficulties.
Patient Characteristics and Outcomes in Stage D HF
The key challenge in optimizing the care of patients with Stage D HF is accurately identifying them in a timely manner in order to ensure the availability of a broad range of management options, including (but not limited to) advanced therapies such as MCS and cardiac transplantation. There are several clinical, biochemical, echocardiographic, and hemodynamic markers that can herald the onset of Stage D HF (Table 1). The Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM) was a multicenter registry designed to prospectively collect data on the characteristics and outcomes of Stage D patients [3••]. For the purpose of the registry, patients were classified as Stage D if they had NYHA III/IV symptoms for ≥60 consecutive days and either ≥2 hospitalizations requiring ≥2 days of intravenous diuretics, vasoactive agents, or inotropes within the past year, or ≥2 intravenous infusions of a vasoactive or inotropic agent, or ≥3 intravenous diuretic treatments over the preceding 60 days. Compared to patients with acute decompensated HF (ADHF), the 1433 stage D patients enrolled in ADHERE LM were younger, more likely to be male, had more severe left ventricular dysfunction, and had a higher prevalence of comorbidities including dyslipidemia, diabetes, coronary artery disease, chronic renal insufficiency, and arrhythmias. These characteristics are similar to findings from the Follow-up Serial Infusions of Nesiritide (FUSION I) [4] and the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) [5••] trials. Fatigue, rather than dyspnea, distinguished them from other patients with ADHF, and they were more likely to have a lower resting heart rate and systolic blood pressure. The majority of these patients were on chronic diuretic therapy (93 %), and 71 % received intravenous vasoactive therapy over the 2-year follow-up period. Overall, the registry confirmed that morbidity and mortality remains high in this contemporary, real-life cohort with an estimated 1-year freedom from hospitalization or death of 32.9 %, and worse outcomes in those who had been hospitalized within the past 6 months or had a prior history of arrhythmias. Challenges intrinsic to the medical management of these patients are discussed below.
Diuretic Therapy in Stage D HF
Diuretics remain the mainstay of therapy for symptomatic relief and optimization of volume status in all stages of HF. In the ADHERE LM registry, 93 % of stage D HF patients were receiving long-term oral diuretics and 73 % had received ≥1 intravenous diuretic treatment within the preceding 6 months [3••]. However, despite their ability to relieve symptoms, diuretics have not been shown to decrease mortality in patients with advanced HF. In fact, several studies have shown an independent association between higher doses of loop diuretics and impaired survival [6, 7]. It is difficult, however, to extract increased risk mediated by the higher diuretic dose alone from that related to its role as a potential marker of greater disease severity, including the higher prevalence of chronic renal insufficiency in patients with Stage D HF.
Renal dysfunction is itself a powerful predictor of adverse prognosis in advanced HF [8, 9]. Patients with chronic renal insufficiency usually require higher doses of loop diuretics to achieve adequate diuresis (diuretic resistance) and baseline renal impairment may in turn further worsen, even as the diuresis relieves symptoms (cardiorenal syndrome). Conventional strategies to overcome diuretic resistance include combination therapy with a thiazide diuretic [10] or transition to a more reliably bio-available loop diuretic such as torsemide or bumetanide [11]. Notably in the recent DOSE (Diuretic Optimization Strategies Evaluation) trial, where several intravenous diuretic strategies were evaluated in patients hospitalized with ADHF, no differences were seen across bolus or continuous loop diuretic strategies or among low- or high-dose groups in the primary efficacy endpoint incorporating HF symptoms and renal function [12•]. However, the high-dose intravenous furosemide strategy was associated with improved secondary outcomes including more diuresis and greater dyspnea relief at the expense of a transient, but not sustained, worsening of renal function. Although the DOSE trial enrolled all-comers with ADHF, baseline characteristics of the DOSE population (including the requirement for a baseline furosemide equivalent dose ≥80 mg) are consistent with a more advanced HF cohort, suggesting the general applicability of these findings to the stage D patient. Thus, based on the results of DOSE, high-dose diuretics can safely be used to try and restore fluid balance in patients with advanced HF and diuretic resistance. Non-diuretic strategies that have been evaluated unsuccessfully to overcome these considerable challenges to adequate decongestion without potentiating further renal dysfunction are shown in Table 2. Notably, none of the studies illustrated were specifically directed at stage D patients, but all included a significant proportion of patients with advanced HF, characterized by renal dysfunction, prior HF hospitalizations, and lower prescription of neurohormonal antagonists. Further studies are needed to identify novel strategies that can successfully relieve congestion in diuretic-resistant advanced HF patients without precipitating worsening renal function.
Neurohormonal Therapy in Stage D HF
HF is a progressive syndrome characterized by activation of the renin-angiotensin system which facilitates adverse cardiac remodeling through angiotensin-II-mediated peripheral and efferent renal arteriolar vasoconstriction, aldosterone release, and worsening sympathetic stimulation. Neurohormonal therapy aimed at modifying the underlying pathophysiology of HF is therefore a critical component of HF disease management. Conversely, the inability to tolerate neurohormonal blockade at target doses, necessitating dose reduction and/or complete withdrawal of one or more of these agents is a significant marker of adverse outcomes [13, 9]. This commonly heralds the development of more advanced HF, where progressive circulatory compromise requires increased activation of the renin-angiotensin system to maintain adequate systemic and renal perfusion. In a single-center study of 259 consecutive patients admitted to a tertiary cardiomyopathy service, 23 % were found to be intolerant of angiotensin-converting enzyme inhibition (ACE-I) due to circulatory-renal limitations, defined as symptomatic hypotension, progressive renal dysfunction, and/or hyperkalemia [13]. These patients in turn were much less likely than those on ACE-I to receive beta-blockers at discharge, reflecting the coexistent perceived, or actual inability of these patients to also tolerate sympathetic nervous system inhibition. These findings are supported by more recent data from the ADHERE LM registry, which notably showed that only 77 % of patients were on beta-blockers and only 67 % were on an ACE-I or angiotensin receptor blocker (ARB) upon study entry. Mean serum creatinine among these patients was 1.8 mg/dL with 26 % having a value >2.0 mg/dL, and 84 % had required intravenous vasoactive or inotropic medications in the preceding 6 months [3••]. Thus, the inability to tolerate life-prolonging neurohormonal blockade is an important marker for the onset of stage D HF and represents a major challenge in the care of these patients.
In patients with ACE-I/ARB intolerance, especially those with serum creatinine >3 mg/dL, the combination of hydralazine and isordil can often be successfully substituted to maintain neurohormonal blockade [14•]. Hydralazine and nitrate therapy has been shown to improve outcomes in HF [15] and is recommended in the recently updated 2013 ACCF/AHA HF guidelines to reduce morbidity or mortality in patients with symptomatic systolic HF “who cannot be given an ACE-I or ARB because of drug intolerance, hypotension, or renal insufficiency.” [16••] Either hydralazine or nitrate therapy may also be used alone or in combination with tolerated doses of ACE-I and other neurohormonal agents if systemic vascular resistance is persistently elevated or to improve exertional symptoms through further reduction in filling pressures. Additionally, in patients with hypotension to long-acting ACE-I/ARB, transition to a shorter acting agent such as captopril may allow maintenance of low-dose neurohormonal blockade. Similarly, in patients with hypotension to beta-blockers, transition to a less vasodilating agent such as metoprolol succinate instead of carvedilol may be better tolerated. Lastly, aldosterone receptor antagonists remain indicated in all symptomatic HF patients with systolic dysfunction who are already on ACE-I/ARB or beta-blockers, including those in NYHA IV functional class and those with moderate renal dysfunction (estimated glomerular filtration rate [eGFR] >30 mL/min/1.73 m2) [16••]. No new trials of aldosterone antagonism have been carried out in the advanced HF population since the landmark Randomized Aldactone Evaluation Study (RALES) trial found that spironolactone was associated with a 30 % reduction in all-cause mortality together with reduced risk of sudden death and HF-hospitalization [17]. In practice, however, in the typical stage D patient with labile renal function and a related tendency to hyperkalemia, use of these agents is often contraindicated and rarely possible as alternative neurohormonal antagonism in those who have demonstrated sustained intolerance to ACE-I or beta-blockers. In summary, forced discontinuation of renin-angiotensin system antagonists and beta-blockers in HF represents a turning point towards a more advanced stage of disease in turn requiring assessment of more advanced treatment strategies.
Inotropic Therapy in Stage D HF
Inotropes enhance myocardial contractility and are usually considered for stage D HF patients with a refractory clinical course characterized by borderline systemic blood pressure, low cardiac output, and end-organ hypoperfusion. The three major currently available inotropes are dopamine and dobutamine—B-adrenergic agonists with direct effects on myocardial contractility as well as vascular and chronotropic effects—and milrinone, a phosphodiesterase inhibitor more appropriately classified as an inodilator due to its use of cyclic adenosine monophosphate (cAMP) as a secondary messenger. However, the main challenge surrounding the use of these agents for stage D HF centers around the struggle between the associated effective increase in cardiac output and end-organ function, and the risk of serious adverse events, including arrhythmias, ischemia, and death. Although shown to provide short-term improvement in cardiac output and related hemodynamic parameters, no major trial of inotropes in advanced HF has demonstrated a survival benefit [18–21]. Indeed, in recent studies evaluating outcomes in inotrope-dependent patients, 6-month mortality has exceeded 40 % [20, 21]. These findings are similar to those observed in the medically treated arm of the REMATCH trial where 72 % of patients were on intravenous inotropic therapy with a mortality approaching 100 % after 2 years of follow-up [5••]. In a post hoc analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, which was a randomized multicenter study looking at pulmonary-artery catheter-guided versus clinically guided assessment in 433 patients with severe ADHF, use of an intravenous inotrope was associated with a significantly increased risk of mortality and the combined endpoint of death and re-hospitalization, independent of patient and hemodynamic-related risk factors [22]. Choice of one agent over another does not appear to modify the adverse prognosis associated with inotrope use in stage D patients. A recent retrospective analysis of 112 inotrope-dependent non-transplant candidate patients found no significant mortality difference between patients treated with milrinone or dobutamine [21]. As in other studies, the prognosis of these end-stage inotrope-dependent HF patients was extremely poor, with 76 % dying over a mean follow-up of 130 days. Therefore, the key to making optimal therapeutic decisions surrounding inotropes in the Stage D patient is ensuring that they are prescribed in the appropriate setting, given the inherent risks associated with both this stage of the disease and this class of drugs.
The 2013 ACCF/AHA guidelines recommend that the use of intravenous inotropes remain limited to symptom relief and support of end-organ function in those with reduced LVEF, LV dilatation, and advanced HF [16••]. Practically speaking, the use of inotropes should be restricted to short-term “bridging therapy” in stage D candidates eligible or potentially eligible for advanced therapy with MCS and/or cardiac transplantation. This could include a bridge to reduction of filling pressures and support of worsening end-organ function in refractory decompensated patients or temporary stabilization of hemodynamic status due to rapid progressive circulatory collapse or comorbid conditions such as sepsis in acutely deteriorated patients. Use of chronic continuous infusions of inotropes to preserve or augment systemic perfusion and secondary end-organ function in stage D patients listed for transplantation also remains supported in the current guidelines [16••]. However, in the contemporary era of evolving ventricular assist device (VAD) technologies with post-implant 1-year survival rates for bridge-to-transplant candidates upwards of 80 % [23], the significant risks associated with longer-term inotropic therapy favor an earlier device insertion strategy. In addition to arrhythmic and ischemic complications, the morbidity associated with the use of indwelling central venous catheters for inotrope delivery, principally the risk of infection, is being increasingly recognized [24•, 25]. In a recent study of 129 stage D HF patients awaiting cardiac transplantation on chronic milrinone, 27 % experienced a serious adverse event, primarily driven by infections (91 %). These infections in turn led to a high rate of associated events and complications including increased hospitalizations, defibrillator removal (9 %), and a particularly high rate (30 %) of temporary inactivation from the transplant list [24•]. These findings raise further concern over the intermediate and long-term risks associated with inotrope use in transplant-eligible patients, but need to be individually weighed against the potential for significant operative risk and morbidity related to LVAD complications. Lastly, the final setting in which to consider inotropes in stage D heart failure is as palliative therapy for symptom control in a selected group of patients with end-stage disease who are ineligible for either transplantation or VAD as destination therapy [16••, 20]. However, prior to initiation of inotropes, it is important that all possible clinical options and goals of care are reviewed with the individual and their families to determine the most appropriate care plan for this final stage of their disease. In general, current guidelines also recommend documentation of the need/benefit of inotropic therapy to support cardiac output and end-organ perfusion with invasive hemodynamic monitoring prior to committing patients to chronic inotrope use [16••].
An ideal strategy to reduce the increased morbidity and mortality associated with inotropes is to develop an agent that supports cardiac output and thereby end-organ perfusion without increasing heart rate or myocardial oxygen demand. Levosimendan, a calcium sensitizer touted to have these properties, failed to show a benefit relative to dobutamine [26] and, although provided more rapid and durable symptomatic relief than placebo, was associated with an increased risk of adverse cardiovascular events [27]. Recently, a new agent, omecamtiv mecarbil, a selective cardiac myosin activator with no effect on intracellular calcium or cAMP—thereby capable of increasing myocardial contractility without increasing myocardial oxygen consumption—has emerged. In a phase II trial of 45 patients with chronic systolic HF receiving intravenous infusions of omecamtiv mecarbil or placebo, concentration-dependent increases in LVEF and stroke volume were seen in the treated group for up to 72 h. However, ischemia was noted at higher plasma concentrations [28]. A multi-center, randomized, double-blind, placebo-controlled trial of omecamtiv mecarbil in 600 acute HF patients (ATOMIC-AHF, ClinicalTrials.gov NCT01300013) is currently underway, with preliminary results indicating no change in the primary efficacy endpoint of dyspnea response on a Likert scale, although an improvement was seen in the cohort receiving the highest dose and in those with highest plasma concentrations. Notably, the myocardial ischemia seen in the early study at higher doses was not seen, although a small increase in troponin was observed, raising the concern that the prolongation of systolic ejection time inherent to the mechanism of action for this agent may be at the expense of shortened diastolic time and compromised coronary perfusion. It is also important to note that this trial includes patients with ADHF who have a systolic blood pressure >90 mmHg and a mean eGFR of 50 mL/min/1.73 m2 and is thus not necessarily representative of the stage D HF population [29].
Other Medical Therapies for Stage D HF
Digoxin remains primarily recommended in HF patients who remain symptomatic despite optimal neurohormonal and diuretic therapy [16••]. In the Digitalis Investigation Group (DIG) trial, digoxin was shown to reduce the risk of HF re-hospitalization, with the greatest benefit in those at highest risk (lower LVEF, greater cardiac enlargement, NYHA III-IV) [30]. Although there are no specific trials of digoxin in stage D HF patients intolerant of standard recommended neurohormonal agents, in practice, many advanced HF patients, particularly those with concomitant atrial fibrillation, receive digoxin unless contraindicated due to renal failure or conduction disease. This was confirmed in the ADHERE LM registry, where 45 % of stage D patients, compared to 23 % with ADHF, were receiving this therapy [3••].
Intravenous vasodilators (nitroglycerin, nitroprusside, nesiritide) are indicated in patients hospitalized with HF as an adjunct to diuretic therapy in order to accelerate improvement in congestive symptoms in the absence of symptomatic hypotension [16••]. Nesiritide, a recombinant form of human B-type natriuretic peptide, has been specifically studied in a combined stage C/D population. In the randomized, double-blind placebo-controlled Second Follow-up Serial Infusions of Nesiritide (FUSION II) trial (n = 911), serial outpatient nesiritide infusions showed no difference in the primary endpoint of time to all-cause mortality or cardiovascular or renal hospitalization at 12 weeks compared to placebo [31]. There was a higher rate of hypotension in the treatment group although importantly, this did not translate into a higher rate of predefined worsening renal function or adverse events overall. Notably, nesiritide was administered as a bolus of 2 ųg/kg followed by an infusion at 0.01 ųg/kg/min thereafter, while other studies in an ADHF cohort have shown less hypotension and even reno-protective effects at lower doses (≤0.005 ųg/kg/min) and with the avoidance of a bolus dose [32]. The Renal Optimization Strategies Evaluation (ROSE) acute HF trial (Table 2) evaluated the efficacy of low-dose nesiritide (0.005 ųg/kg/min) without a bolus in a population of hospitalized ADHF patients with renal dysfunction (eGFR 15–60 mL/min/1.73 m2) and found no difference in the co-primary endpoints of decongestion and renal function at 72 h in those patients treated with low-dose nesiritide versus placebo [33]. Interestingly, in subgroup analyses, 72-h cumulative urine volume was higher in the group treated with low-dose nesiritide compared to placebo in patients with lower baseline blood pressure or lower LVEF, both potential surrogates of more advanced HF. Further investigation is needed using primary analyses in this population to confirm these findings. For the present time, use of intravenous vasodilators, including nesiritide, is not routinely recommended for stage D patients but is limited to dyspnea relief in those hospitalized ADHF patients with sufficient blood pressure to tolerate them. Despite their well-accepted effectiveness in relieving symptoms rapidly, their potential to induce significant hypotension remains a major challenge to their widespread use in stage D patients who frequently present with borderline systemic pressures. In some instances, invasive hemodynamic monitoring with observed high systemic vascular resistance and low cardiac output may allow careful use of intravenous vasodilators even in the presence of borderline systemic pressures [14•].
Cost of Medical Management of Stage D HF
The existing and projected worsening economic burden posed by HF is one of the greatest challenges facing those who govern healthcare resource utilization. The triad of an aging population, increased survival of patients with cardiac comorbidities in the setting of ongoing advances in the treatment of ischemic and valvular disease, and the continued growth and success of novel, but expensive (pharmacological, percutaneous and surgical) HF therapies, mean that advanced HF in particular presents a massive economic challenge to the adequate provision of, and appropriate utilization of health care resources. Given that the majority of patients with stage D HF are treated with medical management alone, it is important to consider the cost associated with these therapies in this population, in order to offset and/or modify some of these economic challenges. A recent study specifically examining these issues in 47 patients from the medically treated arm of the REMATCH trial found that costs and resource use increased as overall disease burden progressed [34]. The estimated mean total cost of medical therapy per patient with advanced HF in the final 2 years of life was $156,168, with over 50 % expended in the last 6 months. Consistent with studies in other chronic diseases, a trend to lower costs was shown in those patients who died in hospice care compared to those who died as inpatients, providing further support for a palliative care strategy in tandem with current recommendations for its expanded and earlier use to improve quality of life in stage D patients [16••].
Conclusions
Development of, or progression to, stage D HF identifies a clinically, biochemically, and hemodynamically distinct subgroup of HF patients with high morbidity and mortality. Therapeutic decisions based on data collected predominantly in stable or ADHF patients may not be applicable to these patients. In addition to classic hemodynamic profiles, the need to withdraw neurohormonal therapy, in the setting of prohibitive circulatory and/or renal limitations, represents both a defining characteristic of, and the dominant challenge to the medical management of this patient population. These findings should herald prompt assessment for advanced pathways of care, including not only eligibility for advanced therapies such as MCS and transplantation, but also consideration of a parallel or primary strategy of palliative care. Given the lack of evidence suggesting a survival benefit as well as increasing recognition of the associated comorbidities and complications, use of inotropes should be limited to “bridging therapy” for patients eligible for advanced therapies or in rare cases, for symptom relief as part of a palliative strategy. Overall, as the burden of advanced HF continues to increase over the coming decades, challenges surrounding optimal pharmacological therapy in Stage D HF are likely to continue to prevail. One of the most important counter-acting strategies will be to ensure that additional research targeting stage D HF patients using currently available and/or novel HF therapies continues to be performed. It is hoped that a concerted and focused effort may someday lead to a “paradigm” shift for these end-stage patients, just as it has for their stable, less advanced counterparts [35].
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53(15):e1–e90.
Stevenson LW, Pagani FD, Young JB, Jessup M, Miller L, Kormos RL, et al. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant : Off Publ Int Soc Heart Transplant. 2009;28(6):535–41.
Costanzo MR, Mills RM, Wynne J. Characteristics of “Stage D” heart failure: insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM). Am Heart J. 2008;155(2):339–47. Large multicenter registry study providing prospectively collected data on the characteristics and outcomes of 1,433 Stage D patients.
Yancy CW, Saltzberg MT, Berkowitz RL, Bertolet B, Vijayaraghavan K, Burnham K, et al. Safety and feasibility of using serial infusions of nesiritide for heart failure in an outpatient setting (from the FUSION I trial). Am J Cardiol. 2004;94(5):595–601.
Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W et al. Long-term use of a left ventricular assist device for end-stage heart failure. The New England journal of medicine. 2001;345(20):1435–43. Landmark trial showing a marked survival benefit for left ventricular assist device therapy as destination therapy compared to optimal medical management for stage D patients ineligible for cardiac transplantation.
Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol. 2006;97(12):1759–64.
Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, et al. The Seattle heart failure model: prediction of survival in heart failure. Circulation. 2006;113(11):1424–33.
Mahon NG, Blackstone EH, Francis GS, Starling 3rd RC, Young JB, Lauer MS. The prognostic value of estimated creatinine clearance alongside functional capacity in ambulatory patients with chronic congestive heart failure. J Am Coll Cardiol. 2002;40(6):1106–13.
Teuteberg JJ, Lewis EF, Nohria A, Tsang SW, Fang JC, Givertz MM, et al. Characteristics of patients who die with heart failure and a low ejection fraction in the new millennium. J Card Fail. 2006;12(1):47–53.
Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56(19):1527–34.
Spannheimer A, Goertz A, Dreckmann-Behrendt B. Comparison of therapies with torasemide or furosemide in patients with congestive heart failure from a pharmacoeconomic viewpoint. Int J Clin Pract. 1998;52(7):467–71.
Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR et al. Diuretic strategies in patients with acute decompensated heart failure. The New England journal of medicine. 2011;364(9):797–805. Prospective randomized trial of several diuretic strategies in acute decompensated heart failure which found no significant differences in global assessment of symptoms or change in renal function according to high- or low-dose or bolus or continuous infusion intravenous furosemide dosing.
Kittleson M, Hurwitz S, Shah MR, Nohria A, Lewis E, Givertz M, et al. Development of circulatory-renal limitations to angiotensin-converting enzyme inhibitors identifies patients with severe heart failure and early mortality. J Am Coll Cardiol. 2003;41(11):2029–35.
Nohria A, Lewis E, Stevenson LW. Medical management of advanced heart failure. JAMA : the journal of the American Medical Association. 2002;287(5):628–40. Classic review covering the spectrum of pharmacological therapies in advanced heart failure.
Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325(5):303–10.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr., Drazner MH et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-239. Most recent ACCF/AHA guidelines for the management of heart failure.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–17.
Fonarow GC. Pharmacologic therapies for acutely decompensated heart failure. Rev Cardiovasc Med. 2002;3 Suppl 4:S18–27.
Cuffe MS, Califf RM, Adams Jr KF, Benza R, Bourge R, Colucci WS, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA : J Am Med Assoc. 2002;287(12):1541–7.
Hauptman PJ, Mikolajczak P, George A, Mohr CJ, Hoover R, Swindle J, et al. Chronic inotropic therapy in end-stage heart failure. Am Heart J. 2006;152(6), 1096 e1-8.
Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E, Starling RC. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail. 2009;2(4):320–4.
Elkayam U, Tasissa G, Binanay C, Stevenson LW, Gheorghiade M, Warnica JW, et al. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J. 2007;153(1):98–104.
Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant : Off Publ Int Soc Heart Transplant. 2014;33(6):555–64.
Haglund NA, Cox ZL, Lee JT, Song Y, Keebler ME, DiSalvo TG et al. Are peripherally inserted central catheters associated with increased risk of adverse events in status 1b patients awaiting transplantation on continuous intravenous milrinone? Journal of cardiac failure. 2014. Recent study highlighting the potential significant risks, predominantly infection, associated with chronic inotrope infusion therapy in stage D patients eligible for cardiac transplantation.
Gorodeski EZ, Kim A. Implications Of Central Venous Catheters In Patients With Stage D Heart Failure Who Are Stable But Inotrope Dependent. Journal of cardiac failure. 2014.
Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA : J Am Med Assoc. 2007;297(17):1883–91.
Packer M, Colucci W, Fisher L, Massie BM, Teerlink JR, Young J, et al. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail. 2013;1(2):103–11.
Cleland JG, Teerlink JR, Senior R, Nifontov EM, Mc Murray JJ, Lang CC, et al. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet. 2011;378(9792):676–83.
Valentova M, von Haehling S. An overview of recent developments in the treatment of heart failure: update from the ESC Congress 2013. Expert Opin Invest Drugs. 2014;23(4):573–8.
Investigation G. Digitalis. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336(8):525–33.
Yancy CW, Krum H, Massie BM, Silver MA, Stevenson LW, Cheng M, et al. Safety and efficacy of outpatient nesiritide in patients with advanced heart failure: results of the Second Follow-Up Serial Infusions of Nesiritide (FUSION II) trial. Circ Heart Fail. 2008;1(1):9–16.
Riter HG, Redfield MM, Burnett JC, Chen HH. Nonhypotensive low-dose nesiritide has differential renal effects compared with standard-dose nesiritide in patients with acute decompensated heart failure and renal dysfunction. J Am Coll Cardiol. 2006;47(11):2334–5.
Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA : J Am Med Assoc. 2013;310(23):2533–43.
Russo MJ, Gelijns AC, Stevenson LW, Sampat B, Aaronson KD, Renlund DG, et al. The cost of medical management in advanced heart failure during the final two years of life. J Card Fail. 2008;14(8):651–8.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004.
Russell SD, Miller LW, Pagani FD. Advanced heart failure: a call to action. Congestive Heart Fail. 2008;14(6):316–21.
Metra M, Ponikowski P, Dickstein K, McMurray JJ, Gavazzi A, Bergh CH, et al. Advanced chronic heart failure: a position statement from the study group on advanced heart failure of the heart failure association of the European society of cardiology. Eur J Heart Fail. 2007;9(6–7):684–94.
Konstam MA, Gheorghiade M, Burnett Jr JC, Grinfeld L, Maggioni AP, Swedberg K, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA : J Am Med Assoc. 2007;297(12):1319–31.
Voors AA, Dittrich HC, Massie BM, DeLucca P, Mansoor GA, Metra M, et al. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: results from PROTECT (placebo-controlled randomized study of the selective adenosine A1 receptor antagonist rolofylline for patients hospitalized with acute decompensated heart failure and volume overload to assess treatment effect on congestion and renal function). J Am Coll Cardiol. 2011;57(19):1899–907.
Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367(24):2296–304.
Compliance with Ethics Guidelines
Conflict of Interest
Emer Joyce and Anju Nohria declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Pharmacologic Therapy
Rights and permissions
About this article
Cite this article
Joyce, E., Nohria, A. Therapeutic Adjustments in Stage D Heart Failure: Challenges and Strategies. Curr Heart Fail Rep 12, 15–23 (2015). https://doi.org/10.1007/s11897-014-0240-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-014-0240-6