Abstract
Purpose of Review
Radiological studies can be helpful when evaluating patients with suspect esophageal disorders. From benign strictures to malignancy and motility disorders such as achalasia, imaging modalities play a significant role in diagnosis. This review explores the role of different imaging modalities in the most frequently encountered esophageal pathologies.
Recent Findings
Conventional barium esophagram has long been considered the primary imaging modality of the esophagus. In the same fashion, a timed barium esophagram is a valuable tool in the evaluation of achalasia and esophagogastric junction outlet obstruction. Over the last few decades there has been an increase in CT and MRI studies, which also play a role in the evaluation of esophageal pathologies. However, not infrequently, these newer imaging techniques can result in incidental esophageal findings. It is important that gastroenterologists appreciate the value of different modalities and recognize key imaging features.
Summary
The diagnosis and management of esophageal disorders is evolving. A basic understanding of esophageal radiology is essential to any gastroenterologist caring for patients with esophageal complaints.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radiographic testing is an important aspect in the work-up of many gastroenterological disorders. Testing ranges from simple X-rays to computed tomography (CT) or magnetic resonance imaging (MRI). Within the esophagus, many disorders can have substantial and impactful findings on different modalities. Barium esophagram is an often used test to evaluate the esophagus for a range of conditions. It frequently serves as an adjunct to other forms of testing such as endoscopy, or high-resolution esophageal manometry. Certain conditions may also benefit from CT or MRI in addition to the aforementioned studies. Gastroenterologists should understand the basic findings that can be seen on different radiographic modalities and how those may guide additional testing or management. Also, important to the discussion is the potential to have incidental findings on imaging and understanding what some of these findings may look like to pursue more definitive testing which may be endoscopy, high resolution manometry, or pH testing. We aim to highlight radiographic findings specific to the esophagus to show the importance and interplay between those tests and the work-up of the various disorders.
Normal Esophagus
The esophagus is a hollow tube, of approximately 25 cm in length, which connects the pharynx to the stomach. It begins superiorly at the upper esophageal sphincter (UES)—also referred to as the pharyngoesophageal segment (PE)—and extends inferiorly until the level of the lower esophageal sphincter (LES) [1]. The proximal esophagus is composed of skeletal muscle and the last two thirds consist of smooth muscle. Both UES and LES are in a state of tonic contraction at rest, but relax during the active phases of swallowing, in a coordinated fashion [1, 2].
There are three types of peristalsis that can occur in the esophagus. Primary peristalsis is initiated by the act of swallowing, and it is a propulsive type of peristalsis, being the primary wave that propels the ingested material through the esophagus towards the stomach. Secondary peristalsis is also a type of propulsive peristalsis; however, it does not occur in response to swallowing, rather it is a response to esophageal distension or irritation, such as refluxed gastric material. Tertiary peristalsis is a type of non-propulsive peristalsis and is a result of uncoordinated contractions. Though tertiary peristalsis can be seen in asymptomatic patients, it is frequently seen in motility disorders [1].
As previously mentioned, the UES normally relaxes during the act of swallowing, but this is generally not pronounced during an esophagram study in normal patients. When the UES is dysfunctional, a smooth indentation in the posterior aspect of the contrast-filled PE segment can be seen. The LES should also relax with the arrival of the bolus. When the LES is abnormal, a smooth tapering of the distal esophagus may be appreciated and in cases of obstruction, retained contrast or food debris may be seen on imaging.
Oropharyngeal Dysphagia
Oropharyngeal dysphagia refers to a disturbance in the oral and/or pharyngeal swallowing phases [3]. Symptoms vary from the sensation of food “sticking” or “holding up,” coughing, choking, regurgitation, aspiration, and repeated chest infections. It can be further classified as a disorder of swallow function versus a structural process. Disorders of swallowing function include absent swallowing reflex, decreased peristalsis, and coordination problems. Underlying medical problems such as neurological or prior surgical changes altering bolus movement through the oropharynx, as in head and neck cancer patients, are often implicated as etiologies for oropharyngeal dysphagia [4, 5•]. Structural etiologies include Zenker’s diverticulum (we will discuss further below), hypopharyngeal pouches, cricopharyngeal bars, and proximal webs. Often a complete history of present illness can help delineate which process may be causing the symptoms of oropharyngeal dysphagia. Barium fluoroscopy (video fluoroscopy or modified barium swallow study (MBSS)) has long been considered the primary modality to evaluate oropharyngeal dysphagia as it allows visualization of bolus flow and swallowing movement in real time.
A MBBS is a dynamic continuous radiological examination of the anatomy and function of the oral cavity, pharynx and upper esophageal sphincter and is done in a collaborative fashion by a radiologist and a speech pathologist. Observation of the pharyngoesophageal bolus transit during the video fluoroscopy is considered one of the most important tools in evaluating direct aspiration.
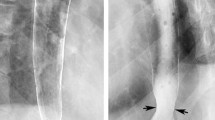
Pharyngeal anatomic findings such as cervical vertebral osteophytes and cricopharyngeal (CP) bars (a prominence of the cricopharyngeal fibers seen in the lateral view on MBSS) can be etiologies of dysphagia and globus sensation (Fig. 1). However, these findings can also be seen in asymptomatic patients. In a study that recruited adult patients with no history of dysphagia to undergo MBSS, spinal changes and CP bars were present in 46% and 8% of patients, respectively [6]. Both findings also tended to increase with age, with a CP bar being seen in more than 15% of patients over 70 years of age and in less than 3% of those 70 years or younger. Therefore, an individualized approach taking in consideration the history and physical findings of each patient should be taken in account while correlating with radiographic findings.
Double-contrasted barium esophagram demonstrates a cricopharyngeal bar (CP bar), a bar-like protrusion at the level of C5-C6 (arrow) that represents a prominent cricopharyngeal muscle. Usually relaxed on swallowing in asymptomatic individuals, the cricopharyngeus is not visible on a normal barium swallow. In this case, in addition to the CP bar, patient has also presence of cervical spine hardware
Cervical webs are thin mucosal folds most often found in the anterior wall of the lower hypopharynx and proximal cervical esophagus that can be a cause of dysphagia. Most often, they present as shelf-like filling defects, but can also be circumferential. Esophagrams can nicely demonstrate this condition though sometimes they can be mistaken with a post-cricoid defect, which is normal redundant mucosa along the anterior wall the hypopharynx [7].
Most of the etiologies mentioned here can be easily identified in an esophagram and sometimes on neck CTs. In some cases, further evaluation with a fiberoptic endoscopic evaluation of swallowing (FEES), esophageal and/or pharyngeal manometry, and functional lumen imaging probe (EndoFLIP™) may be used to fully define the etiology of the oropharyngeal dysphagia. These modalities, however, are beyond the scope of this review.
Esophageal Diverticulum
Proximal (Zenker’s, Killian-Jamieson and Laimer’s) and distal (epiphrenic) diverticula are usually solitary and frequently associated with an underlying esophageal disorder. Location, size, and degree of stasis can all be defined on a barium esophagram.
A Zenker’s diverticulum is a herniation of the hypopharynx through a defect in the Killian’s triangle, an area bound by the inferior pharyngeal constrictor muscles and the cricopharyngeal muscles [8]. It can usually be seen in a barium examination when pooling of contrast occurs anterior the C5 and C6 vertebrae. A Killian-Jamieson diverticula, which is an outpouching in the anterolateral wall at the pharyngoesophageal junction—inferior to the cricopharyngeal muscle—is often mistaken as a Zenker’s diverticula. Their differentiation is important as the management options are different. A barium contrast esophagram and axial neck CT can help differentiate both. During the barium studies, evaluating the anteroposterior and lateral images in order to identify the location of the diverticulum in relationship to the cricopharyngeal muscle is imperative: the Zenker’s will be above, and the Kilian-Jamieson will be below the cricopharyngeus muscle [9]. Although ultrasounds are not typically part of the modalities used to evaluate dysphagia and pharyngoesophageal diverticula, few Killian-Jamieson diverticula have been reported in ultrasounds mimicking thyroid nodules [10]. Another proximal diverticulum is the Laimer’s diverticula. It arises inferior to the cricopharyngeus in the Laimer’s triangle at the posterior aspect of the esophagus. This occurs in younger population and is a full thickness true diverticulum, differently from the Zenker’s and Killian-Jamieson. Only a handful of cases have been reported in the literature [11, 12].
Epiphrenic diverticula account for less than 15% of all esophageal diverticula and are located in the distal 10 cm of the esophagus [13, 14]. They are mostly solitary, though a small percentage of patients may have two or more, and are false diverticula, containing only mucosa and submucosa. Epiphrenic diverticula have been shown to develop secondary to pulsion forces arising in setting of esophageal dysmotility, such as achalasia, distal esophageal spasms and less often, hypercontractile esophagus [15]. A barium swallow is probably the most important radiological test in this scenario (Fig. 2). The size may range from 1 to 14 cm, with a median size of 4–7 cm, and in 70% of the cases are located at the right side of the esophageal wall [13, 15].
Motility Disorders
Evaluation of esophageal motility disorders often involves multiple modalities. Although upper endoscopy, high-resolution esophageal manometry, and frequently EndoFLIP are necessary for the complete evaluation of esophageal disorders, imaging plays a significant role. Esophageal motility disorders are classified following a hierarchical scheme proposed by the Chicago Classification. In its most recent version, the Chicago Classification v4.0 (CCv4.0), classifies esophageal motility disorders as disorders of esophagogastric junction (EGJ) outflow and/or disorders of peristalsis [16••]. We will discuss radiological evaluation of these two categories below.
Disorders of EGJ Outflow
This category includes achalasia (types I, II and III) and EGJ outflow obstruction (EGJOO). Per CCv4.0, a manometric diagnosis of achalasia includes an abnormal median IRP and 100% absent peristalsis with either failed, peristalsis, panesophageal pressurizations, or premature contractions. EGJOO also is diagnosed by an elevated median IRP, which must be seen in the primary (supine) and secondary (upright) positions along with intrabolus pressurization. Contrary to achalasia, peristalsis is preserved. As per CCv4.0, the manometric diagnosis of EGJOO is always inconclusive and requires additional investigation supporting obstruction with a timed barium esophagram (TBE) and/or EndoFLIP [16••].
In patients with achalasia (either primary or secondary, also known as pseudoachalasia), dilation of the esophagus, tortuosity and degree of obstruction and stasis are readily evaluated by a barium esophagram. Radiographic findings also include absence of a normal peristaltic waves and retained food particles suggestive of poor esophageal emptying. A TBE is performed specifically to study the esophageal emptying. The protocol consists of drinking a low-density barium sulphate suspension within 15–20 s followed by left posterior oblique films taken 1, 2 and 5 min after ingestion. Barium empties the esophagus in most heathy patients within 1 min and in all healthy patients within 5 min [17]. Persistence of barium in the esophagus and incomplete or partial emptying over 5 min helps to diagnose achalasia (Fig. 3). Specifically, a barium height of ≥ 5 cm at one minute and ≥ 2 cm at 5 min are helpful cutoffs in differentiating achalasia from EGJOO and non-achalasia [18]. A typical radiological finding of smooth tapering of the distal aspect of the barium column, with an appearance known as “bird-beak,” can sometimes be seen in patients with disorders of the EGJ outflow. The smooth tapering can also be appreciated in cross-sectional imaging, sometimes even as an incidental finding, in patients without severe symptoms. A CT scan may also be able to show esophageal dilation, esophageal contents retention and it is also useful is differentiating primary form secondary achalasia. (Fig. 4). Patients with secondary achalasia caused by malignant process may have evident findings of nodularity, ulceration, findings of tumor at the cardia, an irregular narrowing at the level of the GE junction and are more likely to have a thickened wall (thickness defined in > 5 mm) [19].
Disorders of Peristalsis
This CCv4.0 category includes absent contractility, ineffective esophageal motility (IEM), distal esophageal spams (DES) and hypercontractile esophagus (HE).
Absent contractility is a manometric diagnosis that is made when the median IRP is normal but there is 100% failed peristalsis [16••]. This condition can be seen in patients with scleroderma. Absent or decreased primary peristalsis can be seen on esophagram and often, reflux can be observed on barium studies related to an incompetent LES. Similar findings can also be seen in patients with IEM, but they are non-diagnostic.
DES is a rare condition that can manifest as intermittent chest pain and dysphagia. On esophageal manometry, it is diagnosed in patients with normal IRP and ≥ 20% of premature contractions (distal latency < 4.5 s). On barium esophagram, it is possible to see intermittently absent primary peristalsis and frequent tertiary (non-peristaltic) contractions. The later are frequently repetitive and simultaneous giving the esophagus, on an esophagram, the appearance of a “rosary bead” or “corkscrew” [1]. The LES functions normally and the esophagus is usually normal caliber. However, frequently, the findings are nonspecific and must be correlated with clinical and manometry findings before a diagnosis can be made.
Hypercontractile esophagus is also a rare motility disorder that is characterized manometrically by ≥ 20% of swallows having hypercontractile features, i.e., a distal contractile integral (DCI) greater than 8,000 mmHg*cm*s, in the absence of mechanical obstruction [16••]. The radiological findings in patients with HE are nonspecific, with tertiary contractions sometimes seen. If there is concern for a possible outflow obstruction causing a secondary hypercontractility, TBE can be considered as an adjunct to manometry [20•].
Gastroesophageal Reflux Disease
Gastroesophageal reflux disease (GERD) is the most prevalent gastrointestinal disorder in the United States. Worldwide, it is estimated that one in 10 adults experience reflux symptoms [21]. The initial diagnosis is often clinical, based on a combination of symptoms, endoscopic evaluation of the mucosa, reflux monitoring and response to therapeutic intervention. No gold standard test for the diagnosis of GERD has been established [22••]. Reflux can often be seen in barium radiography but this finding should not be considered diagnostic for GERD as it has poor sensitivity and specificity for GERD [22••]. In a study comparing the esophagram findings with endoscopic and/or histological evidence of reflux, the findings in the barium study had only a 77.2% sensitivity and a 56.7% specificity for reflux [23]. In another study including patients who had an esophagram and subsequently underwent pH testing, no difference was seen in the proportion of patients with a positive pH test among those with (68%) or without (65%) spontaneous reflux on a barium study [24]. Presence of barium above the thoracic inlet increases the sensitivity for reflux but not sufficient to make a diagnosis of GERD and specificity remains low [23]. Esophageal thickening can be seen on CT scans and is usually a nonspecific finding. Mural thickening can be diffuse, segmental, or focal and is often seen in esophagitis [25]. Endoscopic correlation is needed due to the low specificity of this finding.
Although there is limited role of imaging to diagnose GERD, there is a clear role in the evaluation of hiatal hernias, and in the assessment of GERD complications such as peptic strictures and adenocarcinoma. A barium upper GI series can be a good tool to delineate hernia type and size (Fig. 5). Frequently, CT scans are used for surgical planning of complex hernia repairs.
Esophageal imaging also plays a role in the pre-operative evaluation anti-reflux surgery. A barium esophagram is considered required prior to anti-reflux surgery by the American College of Surgeons [26]. In some centers, a modified barium study called the Marshmallow Swallow Study (MSS) is used to screen patients for motility abnormality and risk of post-operatory dysphagia. Though not widely used, it was initially described over 50 years ago [27]. The protocol consists of having patient swallow a single large bolus of thin barium with a piece of marshmallow (approximately half normal marshmallow) followed by fluoroscopy and video recordings in two different positions (prone flat position and in the prone at 15° Trendelenburg). The radiologist then watches for evidence of peristaltic wave and counts the number of waves it takes to pass the bolus through the GE junction. Two or more peristaltic waves in the Trendelenburg position are considered a failed MSS test. A recent retrospective study evaluated the accuracy on the MSS in predicting post-surgical dysphagia in patients who also underwent HRM and found that the positive predictive value (PPV) for a pass on the MSS and no post-operative dysphagia was 0.833. The authors concluded that MSS may be an effective screening tool to rule out major esophageal motility disorder prior to anti-reflux surgery and may alleviate the need for HRM [28].
Benign Strictures
Benign esophageal strictures are a frequent cause of dysphagia. Distal esophageal strictures are most often reflux-induced (or peptic). Proximal and mid esophagus stricture are often caused by ingestion of caustic substances, congenital esophageal stenosis, skin diseases such as pemphigus or esophageal intramural pseudo diverticulosis. Other unusual causes include Crohn’s disease, Candida esophagitis, graft-versus-host disease, eosinophilic esophagitis, esophageal lichen planus, Bechet’s disease, and prior endoscopic sclerotherapy for esophageal varices [29, 30]. Esophageal strictures are best evaluated by a biphasic esophagography that includes both single- and double- contrast spot images. When a stricture is seen on esophagram, it may be classified in benign or malignant. Malignant strictures are discussed further in the text.
Post-Surgical Changes
Certain post-surgical changes or complications can also present with obstructive symptoms such as dysphagia and regurgitation. A tight fundoplication, for example, in a patient presenting with dysphagia, can be visualized on esophagram and the delayed contrast passage can be a clue. Patients with a history of laparoscopic adjustable gastric banding (LAGB) can sometimes present with dysphagia if the band is overinflated. Esophageal dilation has been described as a complication in up to 71% of LAGB patients [31]. Again, the esophagram can be a great tool. In more severe cases, a CT scan may be able to show a dilated esophagus with retained contents, distal esophageal thickening and position of band or prior other interventions (Fig. 6).
Patient with a history of laparoscopic adjustable gastric banding (LAGB) presenting with dysphagia and regurgitation in setting of an overinflated band. a Esophagram shows a band projecting over the left upper quadrant with a Phi angle of 34 degrees (within normal limits). b Contrast passes through the gastroesophageal junction during esophagram without difficulty, however, almost no contrast is seen passing through the LAGB and the esophagus is moderately dilated. c CT scan shows a dilated esophagus filled with debris. d Upper endoscopy shows a dilated esophagus and (e) the endoscopic appearance of the LAGB on retroflexion
Esophageal Malignancies
Esophageal cancer is a relatively uncommon gastrointestinal malignancy with an incidence rate in the United States of 449.4 cases per 100,000 [32]. It carries a poor prognosis unless diagnosed at an early stage. Barium studies, CT scans, positron emission tomography (PET), PET/CT scans, and endoscopic ultrasounds are the most important and often utilized imaging modalities for diagnosis and staging of esophageal cancer. Resectability is determined by the stage of the disease and accurate staging is crucial to ensure proper therapeutic planning. The proximal and distal extent of the tumor, its location in relation to the carina and whether or not there is gastric cardia involvement, factors that are important for surgical planning [33].
Esophageal cancers are not infrequently found on imaging used to evaluate patients with dysphagia. Double contrast barium studies have been found to be a sensitive technique for detection of carcinomas of the esophagus and esophagogastric junction with a predictive value of 42% [34]. Several features of esophageal cancer can be identified on barium esophagram. Malignant strictures typically cause an asymmetric narrowing with abrupt, shelf-like margins and irregular contours. Esophageal tumors can appear as polypoid, infiltrative, or ulcerative lesions on imaging (Fig. 7). Superficial spreading tends to show a nodular pattern without a well-defined mass and on occasion, the findings may be described by the radiologist as an irregularity that is suspicious for malignancy. Complications such as tracheoesophageal fistula formation from locally advanced disease can also be seen on barium studies.
CT is complimentary to barium esophagram and endoscopy. It helps to define the local extent of the tumor by showing the extent of involvement of the esophageal wall and tumor invasion of peri-esophageal fat. Of note, CT cannot reliably distinguish between T1 (invasion of mucosa or submucosa) and T2 (invasion of muscularis propria) and for this purpose an endoscopic ultrasound is the best alternative [35]. Infiltration of the peri-esophageal fat seen on CT denotes a T3 tumor and adversely affects prognosis. Infiltration of adjacent mediastinal structures such as the aorta or tracheobronchial tree denotes a T4 lesion which is considered inoperable. The sensitivity/specificity of CT scan for detecting T3 and T4 disease are 75%/78% and 75%/86%, respectively [36]. Invasion of adjacent structures is sometimes difficult to predict on CT scans, but the loss of fat planes between the tumor and adjacent structures, demonstration of tracheobronchial fistula, displacement of the airway and thickening of the wall of the tracheobronchial tree all suggest invasion [34]. Nodal disease and distant metastasis can be seen on CT and MRI.
PET scan is also used for staging of patients with esophageal malignancy (Fig. 8). PET can detect distant metastasis but has limited sensitivity for identifying locoregional disease. The accuracy for nodal staging has been reported to be between 48 and 90% [34, 37, 38]. Overall, a combined PET/CT performs better than PET alone for localization of disease [39].
a An 18F-FDG-PET scan shows uptake in the distal esophagus and cardia (arrowhead) at a site of known tumor. b PET scan also shows a lesion in segment 6 of the liver (arrowhead). c Uptake demonstrated in proximal stomach and segment 6 of liver in patient with distal esophageal adenocarcinoma and cardia involvement
Eosinophilic Esophagitis
Eosinophilic Esophagitis (EoE) is a chronic, immune, antigen-mediated disease that often presents with esophageal dysphagia and is associated with an eosinophilic predominant inflammation on histology [40]. An upper endoscopy with biopsies is required for diagnosis which relies on histopathological criteria. Endoscopic findings include fixed strictures, linear furrows, narrowing of the lumen, strictures, mucosal edema, decreased vascularity, white plaques or exudates, a fragile “crepe-paper” mucosa and firmness noted during biopsy termed the “tug sign” [41]. Currently, the EoE Endoscopic Reference Score (EREFS) is applied for a homogeneous nomenclature on endoscopic reports and a score > 2 is highly predictive of EoE [42]. Recently, EndoFLIP has been gaining space in the evaluation of EoE, but this is outside of the scope of this review.
Despite the need for endoscopy for a diagnosis, imaging can offer valuable information. Frequently, an esophagram is done prior to endoscopic evaluation as part of the dysphagia work-up and it may show findings that range from rings to a narrow caliber esophagus (Fig. 9) [43•]. Radiographic imaging can also be utilized to plan for an upcoming endoscopy to ensure the appropriate equipment and location for the procedure is available: adult vs pediatric endoscope, or access to intra-procedural fluoroscopy.
Conclusion
The evaluation of patients with esophageal disorders requires multiple modalities. Radiological imaging is an essential part of the evaluation of such patients and is often complimentary. Although a barium esophagram is traditionally considered the main imaging modality of the esophagus, different techniques can be used. The importance of obtaining a high-quality and thorough history cannot be over emphasized, as this will help guide the clinician to determine the best radiographic or non-radiographic test to obtain to work-up the patient’s symptoms. While a targeted approach based on history during the patient encounter can narrow the testing that is performed, often patients will have undergone a variety of tests prior to their visit. Understanding radiographic tests and findings as well as how they relate to non-radiographic tests is an important skill that all gastroenterologists need to maintain to provide comprehensive care.
Data Availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Summerton SL. Radiographic evaluation of esophageal function. Gastrointest Endosc Clin N Am. 2005;15(2):231–42.
Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J Clin Gastroenterol. 2008;42(5):610–9.
Rommel N, Hamdy S. Oropharyngeal dysphagia: manifestations and diagnosis. Nat Rev Gastroenterol Hepatol. 2016;13(1):49–59.
Panebianco M, Marchese-Ragona R, Masiero S, Restivo DA. Dysphagia in neurological diseases: a literature review. Neurol Sci. 2020;41(11):3067–73.
Cristofaro MG, Barca I, Ferragina F, Novembre D, Ferro Y, Pujia R, et al. The health risks of dysphagia for patients with head and neck cancer: a multicentre prospective observational study. J Transl Med. 2021;19(1):472. This retrospective observational multicenter study evaluates the clinical impact of dysphagia in head and neck cancer patients. It demonstrated a much higher weight loss rate and incidence of malnutrition in the subgroup of patients who experienced dysphagia and highlights the importance of timely diagnosis and treatment.
Yin T, Jardine M, Miles A, Allen J. What is a normal pharynx? a videofluoroscopic study of anatomy in older adults. Eur Arch Otorhinolaryngol. 2018;275(9):2317–23.
Grant PD, Morgan DE, Scholz FJ, Canon CL. Pharyngeal dysphagia: what the radiologist needs to know. Curr Probl Diagn Radiol. 2009;38(1):17–32.
Le Mouel J-P, Fumery M. Zenker’s diverticulum. N Engl J Med. 2017;377(22): e31.
Stewart KE, Smith DRK, Woolley SL. Simultaneously occurring zenker’s diverticulum and Killian-Jamieson diverticulum: case report and literature review. J Laryngol Otol. 2017;131(8):661–6.
Kim HK, Lee JI, Jang HW, Bae SY, Lee JH, Kim YS, et al. Characteristics of Killian-Jamieson diverticula mimicking a thyroid nodule. Head Neck. 2012;34(4):599–603.
Kumoi K, Ohtsuki N, Teramoto Y. Pharyngo-esophageal diverticulum arising from laimer’s triangle. Eur Arch Otorhinolaryngol. 2001;258(4):184–7.
Ujiie N, Taniyama Y, Sato C, Kamei T. Surgical intervention for Laimer's diverticulum, a rare type of pharyngoesophageal diverticulum: a case report. OTO Open. 2019;3(2):2473974x19847670.
Varghese TK, Jr., Marshall B, Chang AC, Pickens A, Lau CL, Orringer MB. Surgical treatment of epiphrenic diverticula: a 30-year experience. Ann Thorac Surg. 2007;84(6):1801–9; discussion 01–9.
Soares R, Herbella FA, Prachand VN, Ferguson MK, Patti MG. Epiphrenic diverticulum of the esophagus. from pathophysiology to treatment. J Gastrointest Surg. 2010;14(12):2009–15.
Nehra D, Lord RV, DeMeester TR, Theisen J, Peters JH, Crookes PF, et al. Physiologic basis for the treatment of epiphrenic diverticulum. Ann Surg. 2002;235(3):346–54.
Yadlapati R, Kahrilas PJ, Fox MR, Bredenoord AJ, Prakash Gyawali C, Roman S, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0(©). Neurogastroenterol Motil. 2021;33(1):e14058. This study includes an updated classification scheme for esophageal motility disorders using metrics form high-resolution esophageal manometry (HRM): the Chicago Classification v4.0 (CCv4.0). It summarizes a more rigorous and expansive HRM manometry protocol, provingin standardized criteria for diagnosis of disorders of peristalsis and obstruction at the esophagogastric junction.
Neyaz Z, Gupta M, Ghoshal UC. How to perform and interpret timed barium esophagogram. J Neurogastroenterol Motil. 2013;19(2):251–6.
Blonski W, Kumar A, Feldman J, Richter JE. Timed barium swallow: diagnostic role and predictive value in untreated achalasia, esophagogastric junction outflow obstruction, and non-achalasia dysphagia. Am J Gastroenterol. 2018;113(2):196–203.
Licurse MY, Levine MS, Torigian DA, Barbosa EM. Utility of chest CT for differentiating primary and secondary achalasia. Clin Radiol. 2014;69(10):1019–26.
Chen JW, Savarino E, Smout A, Xiao Y, de Bortoli N, Yadlapati R, et al. Chicago classification update (v4.0): technical review on diagnostic criteria for hypercontractile esophagus. Neurogastroenterol Motil. 2021;33(6):e14115. This study is a technical review on diagnostic criteria for hypercontractile esophagus (HE). It emphasizes the Chicago Classification v4.0 criteria for HE and expands on diagnostic criteria for clinically relevant HE.
Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54(5):710–7.
Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2022;117(1):27–56. This revised ACG Guideline provides updated, evidence-based recommendations and practical guidance for diagnosis and management of GERD. It includes important advances in the endoscopic and surgical management of GERD and also updates in the data for pharmacological and lifestyle management of reflux disease.
Dane B, Doshi A, Khan A, Megibow A. Utility of water siphon maneuver for eliciting gastroesophageal reflux during barium esophagography: correlation with histologic findings. AJR Am J Roentgenol. 2018;211(2):335–9.
Johnston BT, Troshinsky MB, Castell JA, Castell DO. Comparison of barium radiology with esophageal pH monitoring in the diagnosis of gastroesophageal reflux disease. Am J Gastroenterol. 1996;91(6):1181–5.
Berkovich GY, Levine MS, Miller WT. CT findings in patients with esophagitis. Am J Roentgenol. 2000;175(5):1431–4.
Jobe BA, Richter JE, Hoppo T, Peters JH, Bell R, Dengler WC, et al. Preoperative diagnostic workup before antireflux surgery: an evidence and experience-based consensus of the esophageal diagnostic advisory panel. J Am Coll Surg. 2013;217(4):586–597.
McNally EF, Del Gaudio W. The radiopaque esophageal marshmallow bolus. Am J Roentgenol. 1967;101(2):485–9.
Caudell CW, Covil EP, Gilpin JW, Hodgens B, Ewing A, Kothari SN. Can the marshmallow esophagram replace high-resolution manometry as an appropriate screening for esophageal motility prior to anti-reflux surgery? Am J Surg. 2022;224(6):1366–9.
Decker A, Schauer F, Lazaro A, Monasterio C, Schmidt AR, Schmitt-Graeff A, et al. Esophageal lichen planus: current knowledge, challenges and future perspectives. World J Gastroenterol. 2022;28(41):5893–909.
Boregowda U, Goyal H, Mann R, Gajendran M, Patel S, Echavarria J, et al. Endoscopic management of benign recalcitrant esophageal strictures. Ann Gastroenterol. 2021;34(3):287–99.
DeMaria EJ, Sugerman HJ, Meador JG, Doty JM, Kellum JM, Wolfe L, et al. High failure rate after laparoscopic adjustable silicone gastric banding for treatment of morbid obesity. Ann Surg. 2001;233(6):809–18.
Jemal A, Siegel R, Kratzer TB. American cancer Society. Cancer statistics center. 2023. http://cancerstatisticscenter.cancer.org. Accessed 22 May 2023
Dempsey DT. Barium upper GI series in adults: a surgeon’s perspective. Abdom Radiol (NY). 2018;43(6):1323–8.
Iyer R, Dubrow R. Imaging of esophageal cancer. Cancer Imaging. 2004;4(2):125–32.
Thakkar S, Kaul V. Endoscopic ultrasound stagingof esophageal cancer. Gastroenterol Hepatol (N Y). 2020;16(1):14–20.
Pongpornsup S, Posri S, Totanarungroj K. Diagnostic accuracy of multidetector computed tomography (MDCT) in evaluation for mediastinal invasion of esophageal cancer. J Med Assoc Thai. 2012;95(5):704–11.
Choi JY, Lee KH, Shim YM, Lee KS, Kim JJ, Kim SE, et al. Improved detection of individual nodal involvement in squamous cell carcinoma of the esophagus by FDG PET. J Nucl Med. 2000;41(5):808–15.
Muijs CT, Beukema JC, Pruim J, Mul VE, Groen H, Plukker JT, et al. A systematic review on the role of FDG-PET/CT in tumour delineation and radiotherapy planning in patients with esophageal cancer. Radiother Oncol. 2010;97(2):165–71.
Collins CD. PET/CT in oncology: for which tumours is it the reference standard? Cancer Imaging. 2007;7 Spec No A(Special issue A):S77–87.
Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3–20 e6; quiz 21–2.
Muller S, Puhl S, Vieth M, Stolte M. Analysis of symptoms and endoscopic findings in 117 patients with histological diagnoses of eosinophilic esophagitis. Endoscopy. 2007;39(4):339–44.
Dellon ES, Cotton CC, Gebhart JH, Higgins LL, Beitia R, Woosley JT, et al. Accuracy of the eosinophilic esophagitis endoscopic reference score in diagnosis and determining response to treatment. Clin Gastroenterol Hepatol. 2016;14(1):31–9.
Hernandez PV, Amer S, Lam-Himlin DM, DiSantis DJ, Menias CO, Horsley-Silva JL. Eosinophilic esophagitis: imaging features with endoscopic and pathologic correlation. Abdom Radiol (NY). 2020;45(3):591–600. This review highlights imaging features of eosinophilic esophagitis on fluoroscopy and correlates imaging features with endoscopic and pathology findings.
Acknowledgements
Megan Affeldt, PA-C for assisting in identifying radiographic images.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Literature review and material preparation were performed by Catiele Antunes and Joshua A. Sloan. The first draft of the manuscript was written by Catiele Antunes and Joshua A. Sloan was involved in all revisions and edits. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Competing Interests
Catiele Antunes: no relevant financial or non-financial interests to disclose.
Joshua A. Sloan: Speakers Bureau and Advisory Board for Sanofi-Regeneron. Consult for Medtronic.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Antunes, C., Sloan, J.A. Esophageal Radiography Interpretation: a Primer for the Gastroenterologist. Curr Gastroenterol Rep 25, 363–373 (2023). https://doi.org/10.1007/s11894-023-00903-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11894-023-00903-7